Abstract
Background: The human IGF2-P4 and IGF2-P3 promoters are highly active in a variety of human cancers, while existing at a nearly undetectable level in the surrounding normal tissue. Thus, a double promoter DTA-expressing vector was created, carrying on a single construct two separate genes expressing the diphtheria toxin a-fragment (DTA), from two different regulatory sequences, selected from the cancer-specific promoters IGF2-P4 and IGF2-P3. Methods: The therapeutic potential of the double promoter toxin vector P4-DTA-P3-DTA was tested in different cancer cells (pancreatic cancer, ovarian cancer and HCC). Results: The double promoter vector P4-DTA-P3-DTA exhibited superior inhibition activity in different cancer cell lines, compared to the single promoter expression vectors activity. Conclusions: Our findings suggest that administration of P4-DTA-P3-DTA has the potential to reach and eradicate tumor cells and thus may help reduce tumor burden, improve the quality of life of the patients; and prolong their life span.
Keywords: IGF2, pancreatic cancer, ovarian cancer, glioblastoma, HCC, targeted cancer therapy
Introduction
The IGF system plays an important role in normal growth and development as well as in pathological situations, particularly tumorigenesis (reviewed by [1-5]).
The 67-aa IGF2 is a member of the insulin like growth factor family that is involved in cell proliferation and differentiation [6]. The IGF2 gene is transcribed from four different promoters (P1–P4) and is transcriptionally regulated in a development-dependent and tissue-specific manner. The P3 and P4 promoters are the major IGF2 promoters during embryogenesis and tumor development [6,7], while P1 is exclusively active in adult liver tissue and P2 activity is rarely detected in adult human [6,8].
Increased expression of IGF2 is a common feature of both pediatric and adult malignancies [9], and mounting evidence implicates IGF2 as a major factor contributing to oncogenesis [10]. Cancer cells with a strong tendency to metastasize have higher expression of IGF2 [5].
Enhanced levels of IGF2 have been detected in many mouse and human tumors, including Wilm’s tumor [11,12], hepatocellular carcinoma (HCC) [13,14], breast cancer, prostate cancer, bladder carcinoma [15,16], colon carcinoma and gastrointestinal tumors [17-19], adrenal cancer [20,21], lung cancer [22,23], renal cell carcinoma [24], ovarian cancer [25] and in many other tumors. These findings suggest that transcriptional up-regulation of IGF2 activity may be importantly involved in the tumorigenesis of several cancers [18,26-31].
Based on theses findings and on further studies of our group, the transcriptional regulatory sequences of the IGF2 gene emerged as candidates for targeted cancer therapy.
We have recently shown that IGF2-P4 or IGF2-P3 are significantly expressed in the majority of human bladder carcinomas [8,32]. Our group has previously reported the construction of a double promoter vector expressing diphtheria toxin A-chain (DTA) gene, under the control of IGF2-P4 and IGF2-P3 regulatory sequences (P4-DTA-P3-DTA). We showed that this construct was able to selectively kill bladder tumor cells and inhibit tumor growth in vitro and in vivo in accordance to the transcriptional activity of the above-mentioned regulatory sequences [8]. The use of a double promoter DTA-expressing vector, carrying on a single construct two separate genes expressing DTA, from two different regulatory sequences (IGF2-P4 and IGF2-P3; ‘P4-DTA-P3-DTA’ vector) is highly novel. This novel approach, create a new family of plasmids regulated by two regulatory sequences.
In this study the therapeutic potential of the double promoter vector (‘P4-DTA-P3-DTA’) was further tested in tumor cells of spectrum of solid tumors (pancreatic cancer, ovarian cancer and HCC). The results show very high inhibition activity of the double promoter vector in all tested cancer cell lines, as well as positive expression of IGF2-P4 and IGF2-P3 in the cells. Thus, indicating that the P4-DTA-P3-DTA construct has a high therapeutic potential and therefore could be a promising candidate for targeted cancer therapy in a broad spectrum of tumors expressing IGF2-P4, IGF2-P3 or both.
Materials and methods
Cell culture
The human ovarian carcinoma cell lines (ES-2), human pancreatic cancer cell lines (CRL-1469), human HCC cell lines (Hep3B) and the glioma cell lines: human glioblastoma (U87 and A172) and mouse glioblastoma (GL261), were obtained and grown as previously described [8,32,33].
RNA isolation, cDNA synthesis and PCR
RNA extraction from cells or frozen tissue blocks, cDNA synthesis and PCR protocol were all conducted as previously described [8,32,33].
Plasmid construction
The DTA (P3-DTA and P4-DTA) or luciferase single promoter vectors were designed as described [34]. We constructed double promoter expression plasmids, carrying on a single construct two separate genes expressing DTA from two different regulatory sequences: IGF2-P3 + IGF2-P4 (hereinafter: “P4-DTA-P3-DTA”) (cloned by GENEARTTM (Germany)). A double promoter control construct was created, using the same strategy, expressing the luciferase reporter gene (‘P4-Luc-P3-Luc’).
Transfection luciferase activity
Cells were harvested and luciferase activity was determined as previously described [8,32,33]. LucSV40 was used as a positive control for the efficiency of transfection, while Luc-1 that lacks any regulatory sequences was used as a negative control. In-vitro jetPEITM transfection reagent was used as described [8,32,33]. Cells were cotransfected with 2μg of the LucSV40 control vector and with the indicated amounts of DTA expressing vector (P3-DTA, P4-DTA or the DTA double promoter expressing vector (P4-DTA-P3-DTA). Cytotoxic activity was determined by calculating the % of decrease of LucSV40 activity in cotransfected cells compared to cells transfected with LucSV40 alone.
Results
The expression of IGF2-P4 and IGF2-P3 in cell lines of different cancers determined by RT-PCR
A possible indication for IGF2-P4 and IGF2-P3 promoter activity can be the expression of their specific transcripts. To evaluate the possible use of IGF2-P4 and IGF2-P3 regulatory sequences for targeted therapy in different solid tumors, we determined the expression of IGF2-P4 and IGF2-P3 transcripts by in a broad spectrum of cancer cell lines. Total RNA was extracted from the cell cultures and the expression of IGF2-P4 and IGF2-P3 transcripts was detected by RT-PCR analysis in a broad spectrum of cancer cell lines: Hep3B, ES-2, CRL-1469, A172, U87 and GL261. The expression levels are shown in Figure 1.
Figure 1.
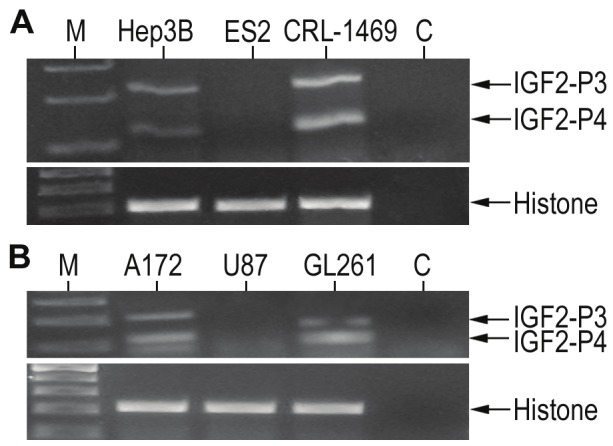
The expression of IGF2-P3 and IGF2-P4 in different cancer cell lines determined by RT-PCR: Shown are RT-PCR analyses of IGF2-P3 (upper band) and IGF2-P4 (lower band) in human cancer cell lines of (A): Hep3B (hepatocellular carcinoma), ES-2 (ovarian cancer) and CRL-1469 (pancreatic cancer), and in human glioma cell lines (B): A172 ((glioblastoma), U87 (glioblastoma) and GL261 (mouse glioblastoma). ‘M’, 100bp DNA ladder, ‘C’, negative control. Lower figures, are RT-PCR product of histone internal control.
Enhanced in vitro activity of the double promoter P4-DTA-P3-DTA in different cancer cells
The activity of the double promoter construct P4-DTA-P3-DTA was tested in vitro by determining its ability to lyse different human carcinoma cell lines, relative to the activity of expression vectors carrying either sequence alone (single promoter constructs: P4-DTA or P3-DTA), in order to examine its activity in a broad spectrum of tumor cells.
Consequently the protein synthesis inhibition activity of the P4-DTA-P3-DTA construct was tested in vitro in the following human cancer cells lines: Hep3B, ES-2 and CRL-1469.
Anti-tumor therapeutic activity was determined by measuring the inhibition of luciferase activity following co-transfection with LucSV40. Each cell line was co-transfected with 2μg of LucSV40 and P4-DTA, P3-DTA, or P4-DTA-P3-DTA in a dose-response manner at different concentrations (0.005μg - 0.05μg).
As seen in Figure 2, the double promoter expression vector P4-DTA-P3-DTA exhibited far enhanced efficiency in lysing spectrum of tumor cells, relative to each of the single promoter constructs carrying either sequence alone.
Figure 2.
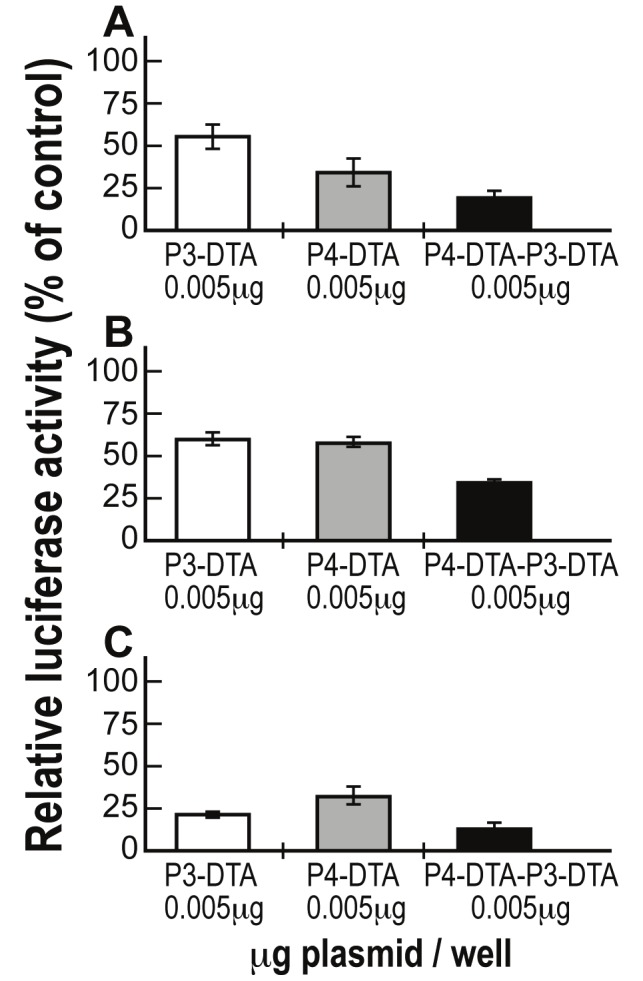
Enhanced activity of P4-DTA-P3-DTA vector in spectrum of tumor cells: Protein synthesis inhibition activity of the P3-DTA (white), P4-DTA (gray) and P4-DTA-P3-DTA (black) vectors in Hep3B (A), ES-2 (B) and CRL-1469 (C) cells, was measured as a reduction of LucSV40 activity. Cells were cotransfected with 2μg of LucSV40 and with different concentrations (0.005μg - 0.05μg /well) of the DTA expressing vectors or with LucSV40 alone. Transfection experiments were stopped after 48 hours and luciferase activity was assessed. The decrease in LucSV40 activity was determined by comparison to the same cell type transfected with LucSV40 alone as a measure of cytotoxicity. The diverse effect of each vector at the lowest plasmid transfected concentration (0.005μg) is indicated.
Augmented-than-additive protein synthesis inhibition activity of the P4-DTA-P3-DTA vector in different carcinoma cell lines
The presence of an augmented-than-additive protein synthesis inhibition activity of the double promoter construct P4-DTA-P3-DTA was tested in Hep3B, ES-2 and CRL-1469 cells. The cells were co-transfected with 2μg of LucSV40 and either (a) the indicated concentrations (Figure 3) of the single-promoter constructs P4-DTA + P3-DTA in combination, or (b) P4-DTA-P3-DTA alone. The total amount of DNA cotransfected in samples receiving both single promoter constructs was therefore twice (0.005μg + 0.005μg) than the cells transfected only with P4-DTA-P3-DTA (only 0.005μg). Luciferase activity was determined and compared to that of cells transfected with LucSV40 alone. The double-promoter construct P4-DTA-P3-DTA exhibited enhanced efficiency in lysing the cancer cell lines, relative to the combined activity of both single promoter constructs (P4-DTA + P3-DTA), in Hep3B human HCC cells (Figure 3A). Very similar results were obtained in ES-2 human ovarian cancer cells (Figure 3B) and CRL-1469 human pancreatic cancer cells (Figure 3C).
Figure 3.
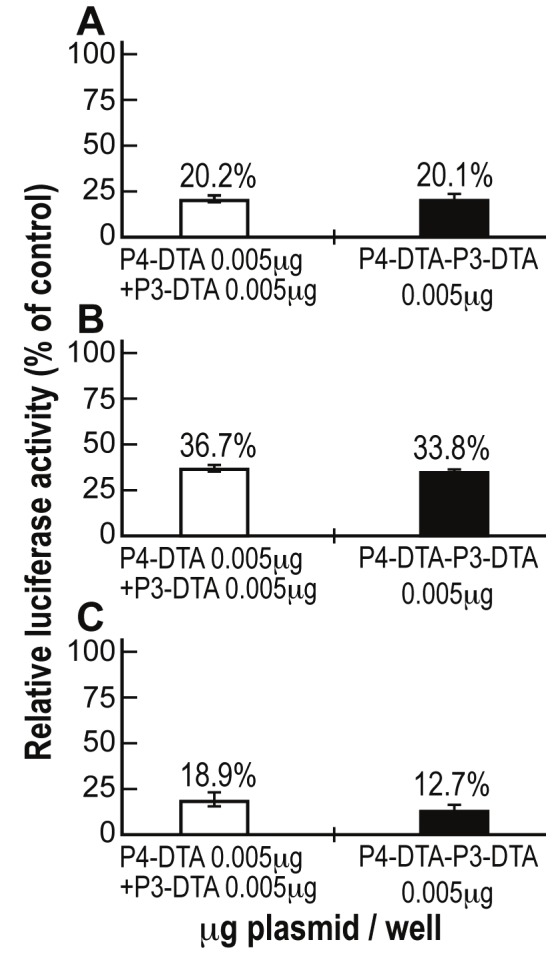
Augmented-than-additive activity of P4-DTA-P3-DTA in different human cancer cell lines: The additive effect of P4-DTA-P3-DTA vector was tested in Hep3B (A), ES-2 (B) and CRL-1469 (C) cells, transfected with only 0.005μg P4-DTA-P3-DTA (black) and compared to combination transfection of both vectors (white) P3-DTA + P4-DTA (which the total transfected concentration was therefore twice (0.005μg + 0.005μg)).
Thus, P4-DTA-P3-DTA double promoter expression vector exhibited augmented-than-additive protein synthesis inhibition activity relative to expression vectors carrying either sequence alone when tested in a broad spectrum of tumor cells.
Discussion
The present study shows the successful use of a double promoter expressing vector, carrying on a single construct two separate DNA sequences expressing DTA from two different cancer-specific regulatory sequences, IGF2-P4 and IGF2-P3. This construct was used to transfect and to eradicate tumor cells of different solid tumors.
Among IGFs’ several physiologic roles, their role in the pathogenesis of malignancies is of central importance. The IGF system role in the pathogenesis of malignancies has been established in vitro and by in vivo animal models. In addition, growing body of epidemiological studies confirm the correlation between the serum levels of IGF system components and cancer risk and survival in humans [35]. IGF2 is a strong mitogen for a wide variety of cancer cell lines, including sarcoma, leukemia, and cancers of the bladder, prostate, breast, lung, colon, stomach, esophagus, liver, pancreas, kidney, thyroid, brain, ovary, uterus [36-41] and many other cancers [5]. Increased expression of IGF2 as a result of the loss of its imprinting is frequently seen in a variety of human tumors [42-44]. In addition, abnormal signal transduction and/or promoter activation was reported as a major mechanism for IGF2 over-expression in a variety of tumors [45-48].
Hepatocellular carcinoma (HCC) is one of the most common cancers, representing a major international health problem because its incidence is increasing in many countries. Many genes and gene products, such as p53, nm23, telomerase and vascular endothelial growth factor, have been identified as being associated with the metastatic potential of HCC [49]. Recently, epigenetic dysregulation of the 11p15 locus has attracted attention as one of several pathways in hepatocarcinogenesis [50,51]. Among several genes localized to 11p15, epigenetic abnormalities in IGF2 and H19 genes have been observed in HCC. It has been reported that, during hepatocarcinogenesis, increased expression of the IGF2 gene is associated with loss of adult-type promoter (P1) transcription, reimprinting of the fetal-type promoters (P3-P4) and expression of both alleles of the H19 gene [49]. Biallelic expression of the IGF2 gene in HCC has also been observed [52].
Pancreatic cancer has an exceptionally high mortality rate, making it the fourth most common cause of cancer deaths in the United States. Insulin resistance and inflammation may be the biological mechanisms shared by obesity and diabetes in promoting pancreatic carcinogenesis [53]. The abnormality of IGF-axis confers insulin resistance and plays an important role in pancreatic cancer development [54,55].
Ovarian cancer is the leading cause of death from gynecologic malignancies because the majority of cases are not detected until the disease has metastasized. Ovarian tumors are end results of a complex pathway involving multiple oncogenes and tumor suppressor genes [56]. Gene expression profiles obtained from these cancers indicated that more than half exhibited a high level of expression of IGF2 gene [10]. High IGF2 expression was recently shown to be a predictor of poor prognosis in epithelial ovarian cancers and was associated with the most aggressive forms of this disease [25]. Recent study has demonstrated that IGF2 represents a therapeutic target in ovarian cancer, particularly in the setting of taxol resistance [57].
These findings indicate that IGF2 dysregulation should be considered as an important independent marker for cancer risk, and as a potential target for novel antineoplastic therapies and/or preventative strategies in high-risk groups [4,35]. As a result of these findings, intensive effort is being directed towards investigating the utility of the IGF2 system as both a diagnostic marker and a therapeutic target in cancer therapy.
Based on early studies of our group and others, the transcriptional regulatory sequences of the IGF2 gene emerged as candidates for cancer targeted therapy. IGF2 (the human P3 and P4 promoters) is an onco-fetal gene and oncogene [58,59], expressed in the fetus and in a broad spectrum of tumors, but rarely in normal adult tissues [8,32,34]. Therefore over plurality of cancer specific promoters, IGF2-P3 and IGF2-P4 regulatory sequences were selected for targeting cancer cells. The IGF2-P3 and P4 regulatory sequences are expected to be good candidates for specifically inducing the expression of DTA in target tumor cells but not in cells of normal tissue. They are known to be differentially over-activated in various tumor types and to show no or minimum activity in the surrounding normal tissue [15,16]. This is in addition to the known autocrine/paracrine mode of IGF2 mitogen action in the development of a wide range of human malignancies. Accordingly, destruction of the IGF2 expressing tumor cells not only will eliminate part of the tumor but will also diminish the supply of mitogenic IGF2 to neighboring tumor cells and may lead to arrest in tumor growth and prevent following metastases process.
The use of a double promoter DTA-expressing vector is highly novel. Tumor cells can express high levels of IGF2-P3, IGF2-P4, or both. That way, majority of tumor cells could efficiently express DTA. Thus the use of such tumor markers, by expressing toxin only in cancer cells, presents distinguish targeted therapy approach [60].
Subunit A of the diphtheria toxin (DTA), a highly potent poison, was chosen as an effector molecule. When only the cDNA coding for the A-fragment is expressed, the released DT-A toxin from the lysed cells will not be able to enter neighboring cells in the absence of the DT-B fragment [61]. This approach not only will insure high killing activity but will be of great advantage against any unintended toxicity to non-target normal cells. Moreover, introduction of DTA DNA sequence under the control of regulatory sequences of genes differentially expressed in tumors but not in adjacent non-tumor cells will selectively favor the specificity of the treatment.
In vitro superior therapeutic effect in a broad spectrum of cancers
In order to determine if the double promoter strategy could be applicable to a broader spectrum of cancer indications we further studied the expression of IGF2-P4 and IGF2-P3 in a broad spectrum of tumor cells. Furthermore we tested the protein synthesis inhibition activity of the double promoter vector, P4-DTA-P3-DTA, in different cancer cells.
Superior activity of the double promoter construct, P4-DTA-P3-DTA (Figure 2) relative to the single promoter constructs (P4-DTA, or P3-DTA), was demonstrated in the following human cancer cells lines: Hep3B (HCC), ES-2 (ovarian cancer), and CRL-1469 (pancreatic cancer). Thus, P4-DTA-P3-DTA expression vector consistently exhibited significantly superior activity when tested in different tumor cells, relative to expression vectors carrying either sequence alone. The consistency of these results in the different cancer cell lines demonstrates the superior ability of the P4-DTA-P3-DTA construct in broad spectrum of tumor cells expressing IGF2-P4, IGF2-P3, or both.
The protein synthesis inhibition activity of the DTA expressing vectors (Figure 2), does correlate with IGF2-P4 and IGF2-P3 RNA expression levels (Figure 1) in the cells, except ES-2 cell line in which RNA could not be detected. This exception could be explained by possible inhibitory elements upstream the endogenous regulatory sequences which do not appear in the cloned promoters and therefore their activity is not correlated.
Similarly in our previous studies, H19 RNA could not be detected in T24P (human bladder cancer cell lines). However, H19 expression was detected in tumors developed in nude mice following inoculation of T24P cells, correspondingly to the case of ES-2 cells. This occurrence may results from the major role IGF2 and H19 play in tumor development. Both genes contribute to the initiation of neoplasia and for tumor growth directly or via secondary routes such as angiogenesis, uncontrolled cells proliferation and inhibition of apoptosis. Moreover, IGF2 exerts its effects in autocrine and paracrine manner [3,62] which contributes to its significant expression in vivo, in tumors.
In vitro augmented-than-additive activity
In vitro additive activity of the double promoter vector P4-DTA-P3-DTA (Figure 3) was exhibited in Hep3B, ES-2 and CRL-1469 human cancer cells lines. Thus, P4-DTA-P3-DTA vector exhibited superior efficiency in lysing the cancer cell lines, relative to the combined activity of both single promoter constructs (P4-DTA + P3-DTA). It should be stressed that the superior activity was demonstrated even though the total amount of DNA co-transfected in samples receiving both single promoter constructs (P4-DTA + P3-DTA) was therefore twice (0.005μg + 0.005μg) than the cells transfected only with P4-DTA-P3-DTA (0.005μg).
Overall, the double promoter vector P4-DTA-P3-DTA exhibited augmented-than-additive anticancer activity relative to single promoter expression vectors carrying either DTA sequence alone, when tested in a broad spectrum of tumor cells. The consistency of these results across each of the different cancer cell lines demonstrates the superior anti-tumor activity of P4-DTA-P3-DTA double promoter construct in cancer in spectrum of solid tumors. As IGF2 is expressed at very high levels in a broad spectrum of different cancers, therefore we propose a double promoter expression approach for treating a variety of tumors expressing IGF2-P4, IGF2-P3, or both. According to this approach patients may be treated with a double promoter expression toxin vector which is under the control of the IGF2-P4 and IGF2-P3 regulatory sequences, differentially expressed in those cancers.
As the majority of the tumor cells express IGF2-P4, IGF2-P3, or both, therefore the use of prerequisite diagnostic test (such as in-situ hybridization) will be unnecessary.
Although this is a preliminary study, our working hypothesis is that administration of P4-DTA-P3-DTA has the potential to reach tumor cells, deliver its intracellular toxin without targeting normal tissues, and thus may help reduce tumor burden, improve the quality of life of the patients; and prolong their life span. This suggested approach should be further studied in appropriate animal models.
Abbreviations
- DTA
diptheria toxin A
- IGF2
insulin like growth factor 2
- HCC
hepatocellular carcinoma.
References
- 1.Chao W, D’Amore PA. IGF2: epigenetic regulation and role in development and disease. Cytokine Growth Factor Rev. 2008;19:111–120. doi: 10.1016/j.cytogfr.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeRoith D, Roberts CT Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 3.Pavelic K, Bukovic D, Pavelic J. The role of insulin-like growth factor 2 and its receptors in human tumors. Mol Med. 2002;8:771–780. [PMC free article] [PubMed] [Google Scholar]
- 4.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 5.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 6.Engstrom W, Shokrai A, Otte K, Granerus M, Gessbo A, Bierke P, Madej A, Sjolund M, Ward A. Transcriptional regulation and biological significance of the insulin like growth factor II gene. Cell Prolif. 1998;31:173–189. doi: 10.1111/j.1365-2184.1998.tb01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Pagter-Holthuizen P, Jansen M, van Schaik FM, van der Kammen R, Oosterwijk C, Van den Brande JL, Sussenbach JS. The human insulin-like growth factor II gene contains two development-specific promoters. FEBS Lett. 1987;214:259–264. doi: 10.1016/0014-5793(87)80066-2. [DOI] [PubMed] [Google Scholar]
- 8.Amit D, Tamir S, Birman T, Gofrit ON, Hochberg A. Development of targeted therapy for bladder cancer mediated by a double promoter plasmid expressing diphtheria toxin under the control of IGF2-P3 and IGF2-P4 regulatory sequences. Int J Clin Exp Med. 2011;4:91–102. [PMC free article] [PubMed] [Google Scholar]
- 9.Foulstone E, Prince S, Zaccheo O, Burns JL, Harper J, Jacobs C, Church D, Hassan AB. Insulin-like growth factor ligands, receptors, and binding proteins in cancer. J Pathol. 2005;205:145–153. doi: 10.1002/path.1712. [DOI] [PubMed] [Google Scholar]
- 10.Murphy SK, Huang Z, Wen Y, Spillman MA, Whitaker RS, Simel LR, Nichols TD, Marks JR, Berchuck A. Frequent IGF2/H19 domain epigenetic alterations and elevated IGF2 expression in epithelial ovarian cancer. Mol Cancer Res. 2006;4:283–292. doi: 10.1158/1541-7786.MCR-05-0138. [DOI] [PubMed] [Google Scholar]
- 11.Reeve AE, Eccles MR, Wilkins RJ, Bell GI, Millow LJ. Expression of insulin-like growth factor-II transcripts in Wilms’ tumour. Nature. 1985 Sep 19-25;317:258–60. doi: 10.1038/317258a0. [DOI] [PubMed] [Google Scholar]
- 12.Scott J, Cowell J, Robertson ME, Priestley LM, Wadey R, Hopkins B, Pritchard J, Bell GI, Rall LB, Graham CF, et al. Insulin-like growth factor-II gene expression in Wilms’ tumour and embryonic tissues. Nature. 1985;317:260–262. doi: 10.1038/317260a0. [DOI] [PubMed] [Google Scholar]
- 13.Cariani E, Lasserre C, Seurin D, Hamelin B, Kemeny F, Franco D, Czech MP, Ullrich A, Brechot C. Differential expression of insulin-like growth factor II mRNA in human primary liver cancers, benign liver tumors, and liver cirrhosis. Cancer Res. 1988 Dec 1;48:6844–9. [PubMed] [Google Scholar]
- 14.Cariani E, Seurin D, Lasserre C, Franco D, Binoux M, Brechot C. Expression of insulin-like growth factor II (IGF-II) in human primary liver cancer: mRNA and protein analysis. J Hepatol. 1990;11:226–231. doi: 10.1016/0168-8278(90)90118-b. [DOI] [PubMed] [Google Scholar]
- 15.Fichera E, Liang S, Xu Z, Guo N, Mineo R, Fujita-Yamaguchi Y. A quantitative reverse transcription and polymerase chain reaction assay for human IGF-II allows direct comparison of IGF-II mRNA levels in cancerous breast, bladder, and prostate tissues. Growth Horm IGF Res. 2000 Apr;10:61–70. doi: 10.1054/ghir.2000.0141. [DOI] [PubMed] [Google Scholar]
- 16.Li SL, Goko H, Xu ZD, Kimura G, Sun Y, Kawachi MH, Wilson TG, Wilczynski S, Fujita-Yamaguchi Y. Expression of insulin-like growth factor (IGF)-II in human prostate, breast, bladder, and paraganglioma tumors. Cell Tissue Res. 1998 Mar;291:469–79. doi: 10.1007/s004410051016. [DOI] [PubMed] [Google Scholar]
- 17.Hassan AB, Howell JA. Insulin-like growth factor II supply modifies growth of intestinal adenoma in Apc(Min/+) mice. Cancer Res. 2000;60:1070–1076. [PubMed] [Google Scholar]
- 18.Tricoli JV, Rall LB, Karakousis CP, Herrera L, Petrelli NJ, Bell GI, Shows TB. Enhanced levels of insulin-like growth factor messenger RNA in human colon carcinomas and liposarcomas. Cancer Res. 1986;46:6169–6173. [PubMed] [Google Scholar]
- 19.Wang S, Souza RF, Kong D, Yin J, Smolinski KN, Zou TT, Frank T, Young J, Flanders KC, Sugimura H, Abraham JM, Meltzer SJ. Deficient transforming growth factor-beta1 activation and excessive insulin-like growth factor II (IGFII) expression in IGFII receptor-mutant tumors. Cancer Res. 1997;57:2543–2546. [PubMed] [Google Scholar]
- 20.Gicquel C, Bertagna X, Schneid H, Francillard-Leblond M, Luton JP, Girard F, Le Bouc Y. Rearrangements at the 11p15 locus and overexpression of insulin-like growth factor-II gene in sporadic adrenocortical tumors. J Clin Endocrinol Metab. 1994;78:1444–1453. doi: 10.1210/jcem.78.6.7911125. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Kahri AI, Heikkila P, Ilvesmaki V, Voutilainen R. H19 and insulin-like growth factor-II gene expression in adrenal tumors and cultured adrenal cells. J Clin Endocrinol Metab. 1995;80:492–496. doi: 10.1210/jcem.80.2.7531713. [DOI] [PubMed] [Google Scholar]
- 22.Moorehead RA, Sanchez OH, Baldwin RM, Khokha R. Transgenic overexpression of IGF-II induces spontaneous lung tumors: a model for human lung adenocarcinoma. Oncogene. 2003;22:853–857. doi: 10.1038/sj.onc.1206188. [DOI] [PubMed] [Google Scholar]
- 23.Takanami I, Imamuma T, Hashizume T, Kikuchi K, Yamamoto Y, Yamamoto T, Kodaira S. Insulin-like growth factor-II as a prognostic factor in pulmonary adenocarcinoma. J Surg Oncol. 1996;61:205–208. doi: 10.1002/(SICI)1096-9098(199603)61:3<205::AID-JSO8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 24.Nonomura N, Nishimura K, Miki T, Kanno N, Kojima Y, Yokoyama M, Okuyama A. Loss of imprinting of the insulin-like growth factor II gene in renal cell carcinoma. Cancer Res. 1997;57:2575–2577. [PubMed] [Google Scholar]
- 25.Sayer RA, Lancaster JM, Pittman J, Gray J, Whitaker R, Marks JR, Berchuck A. High insulin-like growth factor-2 (IGF-2) gene expression is an independent predictor of poor survival for patients with advanced stage serous epithelial ovarian cancer. Gynecol Oncol. 2005;96:355–361. doi: 10.1016/j.ygyno.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Lambert S, Carlisi A, Collette J, Franchimont P, Gol-Winkler R. Insulin-like growth factor II in two human colon-carcinoma cell lines: gene structure and expression, and protein secretion. Int J Cancer. 1992;52:404–408. doi: 10.1002/ijc.2910520313. [DOI] [PubMed] [Google Scholar]
- 27.Lamonerie T, Lavialle C, de Galle B, Binoux M, Brison O. Constitutive or inducible overexpression of the IGF-2 gene in cells of a human colon carcinoma cell line. Exp Cell Res. 1995;216:342–351. doi: 10.1006/excr.1995.1043. [DOI] [PubMed] [Google Scholar]
- 28.Schneid H, Holthuizen PE, Sussenbach JS. Differential promoter activation in two human insulin-like growth factor-II-producing tumor cell lines. Endocrinology. 1993;132:1145–1150. doi: 10.1210/endo.132.3.8382597. [DOI] [PubMed] [Google Scholar]
- 29.Singh P, Dai B, Given RL, Lu X, Holthuizen PE. Differential activation of IGF-II promoters P3 and P4 in Caco-2 cells during growth and differentiation. Gastroenterology. 1998;114:1221–1229. doi: 10.1016/s0016-5085(98)70428-7. [DOI] [PubMed] [Google Scholar]
- 30.Toretsky JA, Helman LJ. Involvement of IGF-II in human cancer. J Endocrinol. 1996;149:367–372. doi: 10.1677/joe.0.1490367. [DOI] [PubMed] [Google Scholar]
- 31.Werner H, LeRoith D. The role of the insulin-like growth factor system in human cancer. Adv Cancer Res. 1996;68:183–223. doi: 10.1016/s0065-230x(08)60354-1. [DOI] [PubMed] [Google Scholar]
- 32.Amit D, Hochberg A. Development of targeted therapy for bladder cancer mediated by a double promoter plasmid expressing diphtheria toxin under the control of H19 and IGF2-P4 regulatory sequences. J Transl Med. 2010;8:134. doi: 10.1186/1479-5876-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amit D, Matouk IJ, Lavon I, Birman T, Galula J, Abu-Lail R, Schneider T, Siegal T, Hochberg A, Fellig Y. Transcriptional targeting of glioblastoma by diphtheria toxin-A driven by both H19 and IGF2-P4 promoters. Int J Clin Exp Med. 2012;5:124–135. [PMC free article] [PubMed] [Google Scholar]
- 34.Ayesh B, Matouk I, Ohana P, Sughayer MA, Birman T, Ayesh S, Schneider T, de Groot N, Hochberg A. Inhibition of tumor growth by DT-A expressed under the control of IGF2 P3 and P4 promoter sequences. Mol Ther. 2003 Apr;7:535–41. doi: 10.1016/s1525-0016(03)00056-x. [DOI] [PubMed] [Google Scholar]
- 35.Moschos SJ, Mantzoros CS. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology. 2002;63:317–332. doi: 10.1159/000066230. [DOI] [PubMed] [Google Scholar]
- 36.Frostad S, Bruserud O. In vitro effects of insulin-like growth factor-1 (IGF-1) on proliferation and constitutive cytokine secretion by acute myelogenous leukemia blasts. Eur J Haematol. 1999;62:191–198. doi: 10.1111/j.1600-0609.1999.tb01743.x. [DOI] [PubMed] [Google Scholar]
- 37.LeRoith D, Baserga R, Helman L, Roberts CT Jr. Insulin-like growth factors and cancer. Ann Intern Med. 1995;122:54–59. doi: 10.7326/0003-4819-122-1-199501010-00009. [DOI] [PubMed] [Google Scholar]
- 38.Macaulay VM. Insulin-like growth factors and cancer. Br J Cancer. 1992;65:311–320. doi: 10.1038/bjc.1992.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oku K, Tanaka A, Yamanishi H, Nishizawa Y, Matsumoto K, Shiozaki H, Mori T. Effects of various growth factors on growth of a cloned human esophageal squamous cancer cell line in a protein-free medium. Anticancer Res. 1991;11:1591–1595. [PubMed] [Google Scholar]
- 40.Singh P, Dai B, Yallampalli U, Lu X, Schroy PC. Proliferation and differentiation of a human colon cancer cell line (CaCo2) is associated with significant changes in the expression and secretion of insulin-like growth factor (IGF) IGF-II and IGF binding protein-4: role of IGF-II. Endocrinology. 1996;137:1764–1774. doi: 10.1210/endo.137.5.8612513. [DOI] [PubMed] [Google Scholar]
- 41.Yaginuma Y, Nishiwaki K, Kitamura S, Hayashi H, Sengoku K, Ishikawa M. Relaxation of insulin-like growth factor-II gene imprinting in human gynecologic tumors. Oncology. 1997;54:502–507. doi: 10.1159/000227610. [DOI] [PubMed] [Google Scholar]
- 42.Morison IM, Reeve AE. Insulin-like growth factor 2 and overgrowth: molecular biology and clinical implications. Mol Med Today. 1998;4:110–115. doi: 10.1016/s1357-4310(97)01197-0. [DOI] [PubMed] [Google Scholar]
- 43.Ohlsson R, Franklin G. Normal development and neoplasia: the imprinting connection. Int J Dev Biol. 1995;39:869–876. [PubMed] [Google Scholar]
- 44.Wu HK, Squire JA, Catzavelos CG, Weksberg R. Relaxation of imprinting of human insulin-like growth factor II gene, IGF2, in sporadic breast carcinomas. Biochem Biophys Res Commun. 1997;235:123–129. doi: 10.1006/bbrc.1997.6744. [DOI] [PubMed] [Google Scholar]
- 45.Bae SK, Bae MH, Ahn MY, Son MJ, Lee YM, Bae MK, Lee OH, Park BC, Kim KW. Egr-1 mediates transcriptional activation of IGF-II gene in response to hypoxia. Cancer Res. 1999;59:5989–5994. [PubMed] [Google Scholar]
- 46.Lu L, Katsaros D, Wiley A, Rigault de la Longrais IA, Puopolo M, Schwartz P, Yu H. Promoter-specific transcription of insulin-like growth factor-II in epithelial ovarian cancer. Gynecol Oncol. 2006;103:990–995. doi: 10.1016/j.ygyno.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Mineo R, Fichera E, Liang SJ, Fujita-Yamaguchi Y. Promoter usage for insulin-like growth factor-II in cancerous and benign human breast, prostate, and bladder tissues, and confirmation of a 10th exon. Biochem Biophys Res Commun. 2000;268:886–892. doi: 10.1006/bbrc.2000.2225. [DOI] [PubMed] [Google Scholar]
- 48.Sohda T, Yun K, Iwata K, Soejima H, Okumura M. Increased expression of insulin-like growth factor 2 in hepatocellular carcinoma is primarily regulated at the transcriptional level. Lab Invest. 1996;75:307–311. [PubMed] [Google Scholar]
- 49.Iizuka N, Oka M, Tamesa T, Hamamoto Y, Yamada-Okabe H. Imbalance in expression levels of insulin-like growth factor 2 and H19 transcripts linked to progression of hepatocellular carcinoma. Anticancer Res. 2004;24:4085–4089. [PubMed] [Google Scholar]
- 50.Schwienbacher C, Gramantieri L, Scelfo R, Veronese A, Calin GA, Bolondi L, Croce CM, Barbanti-Brodano G, Negrini M. Gain of imprinting at chromosome 11p15: A pathogenetic mechanism identified in human hepatocarcinomas. Proc Natl Acad Sci U S A. 2000;97:5445–5449. doi: 10.1073/pnas.090087497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 52.Kim KS, Lee YI. Biallelic expression of the H19 and IGF2 genes in hepatocellular carcinoma. Cancer Lett. 1997;119:143–148. doi: 10.1016/s0304-3835(97)00264-4. [DOI] [PubMed] [Google Scholar]
- 53.Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, Abbruzzese JL. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. Jama. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong X, Li Y, Tang H, Chang P, Hess KR, Abbruzzese JL, Li D. Insulin-like growth factor axis gene polymorphisms modify risk of pancreatic cancer. Cancer Epidemiol. 2011;36:206–211. doi: 10.1016/j.canep.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 55.Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339–353. doi: 10.1016/s1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 56.Kim HT, Choi BH, Niikawa N, Lee TS, Chang SI. Frequent loss of imprinting of the H19 and IGF-II genes in ovarian tumors. Am J Med Genet. 1998;80:391–395. doi: 10.1002/(sici)1096-8628(19981204)80:4<391::aid-ajmg16>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 57.Huang GS, Brouwer-Visser J, Ramirez MJ, Kim CH, Hebert TM, Lin J, Arias-Pulido H, Qualls CR, Prossnitz ER, Goldberg GL, Smith HO, Horwitz SB. Insulin-like growth factor 2 expression modulates Taxol resistance and is a candidate biomarker for reduced disease-free survival in ovarian cancer. Clin Cancer Res. 2010;16:2999–3010. doi: 10.1158/1078-0432.CCR-09-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matouk IJ, DeGroot N, Mezan S, Ayesh S, Abulail R, Hochberg A, Galun E. The H19 non-coding RNA is essential for human tumor growth. PLoS ONE. 2007;2:e845. doi: 10.1371/journal.pone.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao X, Hu JF, Daniels M, Shiran H, Zhou X, Yan H, Lu H, Zeng Z, Wang Q, Li T, Hoffman AR. A methylated oligonucleotide inhibits IGF2 expression and enhances survival in a model of hepatocellular carcinoma. J Clin Invest. 2003;111:265–273. doi: 10.1172/JCI15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biran H, Ariel I, de Groot N, Shani A, Hochberg A. Human imprinted genes as oncodevelopmental markers. Tumour Biol. 1994;15:123–134. doi: 10.1159/000217882. [DOI] [PubMed] [Google Scholar]
- 61.Maxwell IH, Glode LM, Maxwell F. Expression of diphtheria toxin A-chain in mature B-cells: a potential approach to therapy of B-lymphoid malignancy. Leuk Lymphoma. 1992;7:457–462. doi: 10.3109/10428199209049802. [DOI] [PubMed] [Google Scholar]
- 62.Kawamoto K, Onodera H, Kan S, Kondo S, Imamura M. Possible paracrine mechanism of insulin-like growth factor-2 in the development of liver metastases from colorectal carcinoma. Cancer. 1999;85:18–25. doi: 10.1002/(sici)1097-0142(19990101)85:1<18::aid-cncr3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]


