Abstract
Dermatomyositis represents an idiopathic inflammatory connective-tissue disease, characterized by inflammation of the muscles and the skin. There is a high incidence of malignancy in patients with dermatomyositis. The main purpose of the present paper is to describe and underline the clinical significance of dermatomyositis manifestations as a precursor and early clinical signs of small cell lung cancer. A physical examination, laboratory tests, anti-Jo-1 antibody and muscle biopsy were performed. The most important findings were SGOT 284 IU/L, CPK 11083 IU/L, aldolase 76.3 IU/L (normal values <7.6). The patient was treated with chemotherapy and a significant improvement of clinical and laboratory findings were noted. The diagnosis of lung cancer could be correlated with the clinical existence of dermatomyositis. Increased awareness is needed regarding the association of dermatomyositis with malignancies in order to achieve correct and timely diagnosis of the underling cancer.
Keywords: Dermatomyositis, early manifestation, precursor, lung cancer
Introduction
Dermatomyositis is an idiopathic inflammatory connective-tissue disease related to polymyositis that is characterized by inflammation of the muscles and the skin. In 1975, Bohan and Peter, in a paper published in New England Journal of Medicine, introduced the diagnostic criteria for the diagnosis of dermatomyositis and polymyositis [1]. Four out of the five criteria involve the muscles: a) gradually exacerbated, symmetrical, proximal muscle weakness, b) increased enzymes related to the muscles, c) abnormal electromyogram, d) histological confirmation of myositis in muscle biopsy and e) skin manifestations. The above mentioned authors proposed the following classification for myositis: a) dermatomyositis, b) polymyositis, c) myositis associated to malignancy, d) juvenile dermatomyositis - polymyositis and e) myositis partially covered by other collagen disease. Dermatomyositis differs from polymyositis only in the associated skin rash and the higher incidence of malignancy [1]. The pathogenesis of dermatomyositis is definitely associated to autoimmune mechanisms. Anti-nuclear antibodies or antibodies against nuclear antigen (ENA) are often positive in patients with myositis but this is not specific as they are also positive in other diseases. However, a series of other auto-antibodies are almost exclusively found in myositis, such as anti-Jo-1, anti-PL-7, anti-PL-12, anti-EJ and anti-OJ. These antibodies target a series of enzymes that help in binding an amino acid to specific transfer RNA. In addition, auto-antibodies against various other intracellular proteins or elements (anti-SRP, anti-Mi-2) may be present in myositis (the majority of patients with anti-SRP have been reported to have polymyositis and anti-Mi-2 auto-antibodies are supposed to be very specific for dermatomyositis) [2]. Histologically there is an inflammatory infiltration of the muscles by B-lymphocytes and the increased CD4+ / CD8+ T-lymphocyte ratio. This infiltration can be perivascular, around and inside the muscle bundles, and it may even involve solitary muscle cells [2]. The first hypothesis that there is a relation of dermatomyositis with malignancy was raised in 1916, when the simultaneous occurrence of dermatomyositis and stomach cancer was reported, and it was confirmed in 1976 in a review that included 258 cases of dermatomyositis associated with different kinds of cancer [3]. However, the high incidence of malignancy in patients with dermatomyositis was reinforced after the publication of two large epidemiological studies. In 1992, a well-designed population study from Sweden reported that in 392 patients with dermatomyositis the incidence of malignancy was found to be 15%. The relative risk in women was 3.4 and in men 2.4, whereas malignancy was the major cause of death in 40% of these patients [4]. In 2001, a large study from Australia were enrolled 537 patients with biopsy proven dermatomyositis or polymyositis and reported that the occurrence of malignancy before, during or after the diagnosis of myositis was higher in patients with dermatomyositis in comparison to those with polymyositis (42% vs. 18%). After correction for age and gender, a six-fold risk for malignancy was found in patients with dermatomyositis compared to the general population. Moreover, the relative risk for malignant disease in dermatomyositis compared with polymyositis was found to be 2.4 [5]. In other studies the incidence of malignancy varies between 15%-25%. In 1/3 of patients, malignancy is found before the diagnosis of dermatomyositis, whereas in 1/3 of patients they present simultaneously and in the last 1/3 of patients malignancy presents months or years after the diagnosis of dermatomyositis. The time frame between the manifestation of malignancy and dermatomyositis is about two years, irrespective to which disease presents first. In few patients, dermatomyositis presents after the recurrence of malignancy, whereas in other patients with known dermatomyositis, it may relapse after the diagnosis of cancer [6,7]. The type of malignancy diagnosed in patients with dermatomyositis or polymyositis is in accordance with the distribution of the diagnosis of malignancy in the general population, with possible exceptions the increased incidence of cervical, lung, ovarian, pancreatic, bladder, and stomach cancers [2,4,8,9].
Case report
A 60-year-old man was admitted to our department with the diagnosis of small cell lung cancer (limited disease). About three months before admission, the patient started to have muscle weakness, pain in the shoulders, and dry cough. The history also revealed that two years before these symptoms, the patient had a skin rash, mainly over the sternum (upper front thoracic wall) accompanied with fluctuating pruritus, treated as allergic rash with corticosteroid and antihistaminic drugs. During the year before diagnosis, the skin rash and the pruritus became persistent without any change. It is worth mentioning that the patient was a smoker (80py) and a frequent drinker, while his family history was positive for malignancy (his mother suffered from breast cancer, his father, grandfather and 3 uncles had been diagnosed with colon cancer). After admission to our department, the physical examination revealed a purple skin rash on the face and neck, oedema of the right upper lid, nail disorders, and muscle weakness, concerning mostly the upper limbs (Figures 1, 2, 3 and 4). Taking into account the above symptoms and signs, the clinical suspicion of dermatomyositis was confirmed by the laboratory findings: SGOT 284 IU/L, CPK 11083 IU/L, and aldolase 76.3 IU/L (normal values <7.6). Anti-Jo-1 antibody test was negative and muscle biopsy was non-diagnostic. During hospitalization, the eyelid oedema, the skin rash and the muscle weakness became gradually worse and swallowing difficulties, mainly at the initiation of the attempt, were added to the symptoms. The patient was treated with chemotherapy (cisplatin 80mg/m2 d1 and etoposide 100mg/m2 d1-3) and prednisolone (75mg daily). A significant improvement was noted, both clinical and laboratory. Before the second cycle of chemotherapy, the biochemical profile was SGOT 52 IU/L, CPK 643 IU/L, and aldolase 9.1 IU/L. The patient was administered 6 cycles of chemotherapy in total, while he suffered from several frequent dermatomyositis recurrences with skin rash, difficulties in swallowing, and proximal muscle weakness, treated with corticosteroids. In addition, the patient underwent focal radiotherapy to the lung and prophylactic radiotherapy to the brain. Unfortunately, the patient developed liver metastases and was subsequently treated with 1st line chemotherapy using topotecan. Finally, the patient passed away after the 1st cycle of the 1st line chemotherapy (11-month survival).
Figure 1.
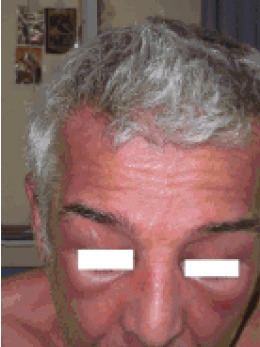
Facial purple skin rash- oedema of the eyelids.
Figure 2.
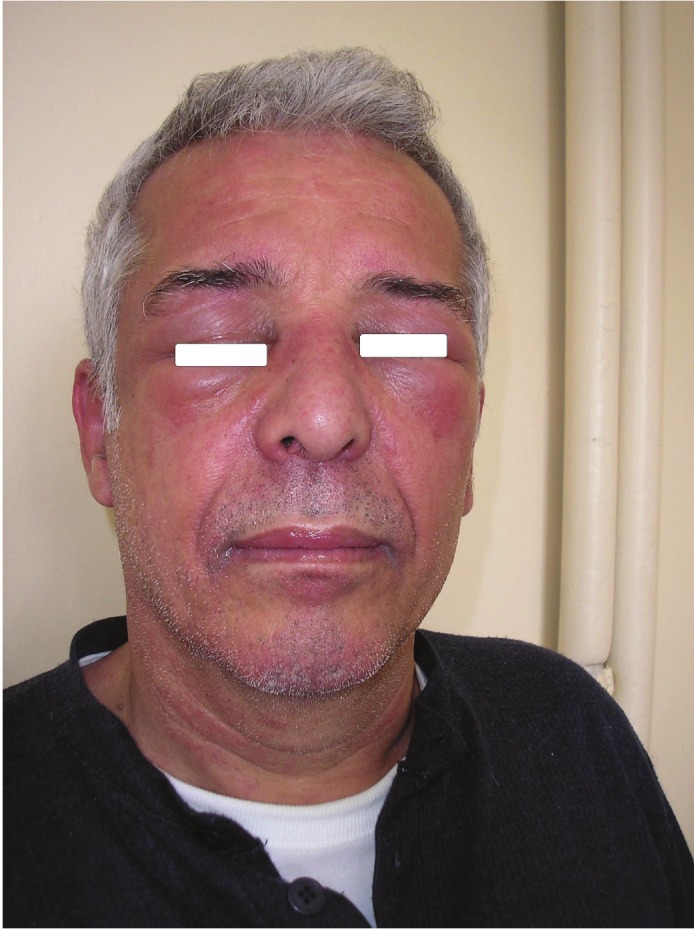
Cervical - Facial purple skin rash- oedema of the eyelids.
Figure 3.
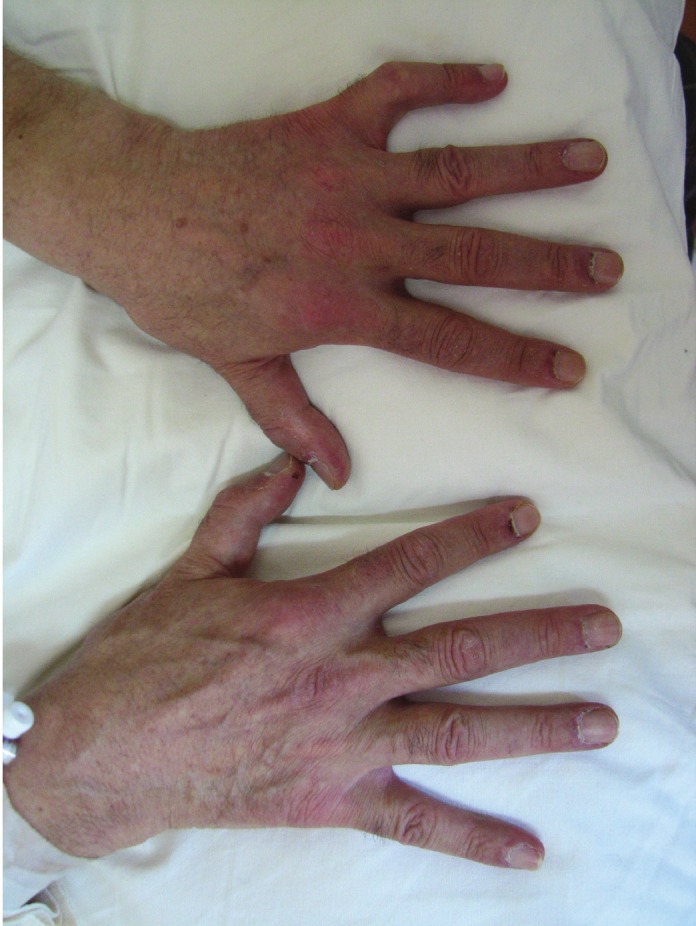
Nail disorders- Skin rash.
Figure 4.
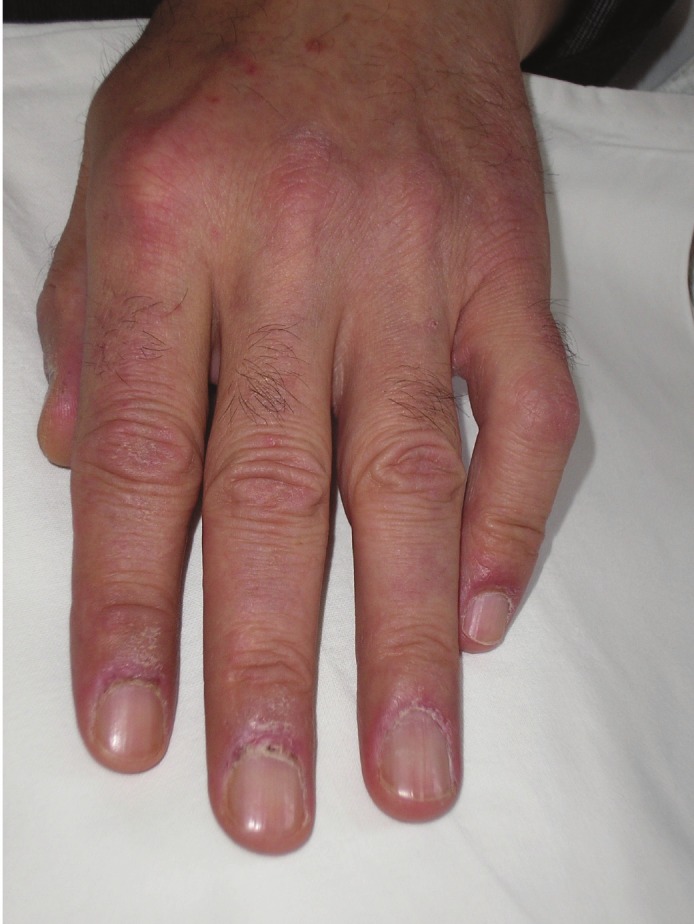
Nail disorders- Skin rash.
Discussion
Dermatomyositis and polymyositis may present at any age, with a higher incidence in the age of 50s and 60s and a woman/man ratio of 2:1. It is also well known that there is an increased incidence of malignancy in adults with dermatomyositis. Almost one out of four patients will be diagnosed with malignancy, simultaneously, before, or after the diagnosis of dermatomyositis. Ovarian, lung, pancreatic, stomach, and colon cancers, as well as non-Hodgkin’s lymphomas, are the most frequent types of malignancy associated with dermatomyositis [9]. However, nasopharyngeal cancer is more frequent in South-eastern Asia patients [10]. The presence of malignancy does seem to modify neither the clinical findings of dermatomyositis nor the response to treatment. Thus, the severity or the distribution of the muscle weakness, the duration of the symptoms until the diagnosis of dermatomyositis, the CPK level, and the existence of dysphagia do not seem to be influenced by the presence or not of malignancy. The successful treatment of the neoplasm may sometimes treat the myopathy as well, whereas in other cases myopathy does not respond to cancer treatment.
In the present case, dermatomyositis and lung cancer were diagnosed simultaneously. The patient was initially treated with corticosteroids having a significant response, and a few days later he was administered chemotherapy. The clinical manifestations of dermatomyositis responded rapidly to treatment and this allowed us to taper corticosteroids. Since chemotherapy was administered a week after the initiation of corticosteroids, we cannot be certain whether chemotherapy had contributed to the improvement of dermatomyositis symptoms. In some patients, dermatomyositis presents as a paraneoplastic syndrome and has a parallel clinical course with the neoplasm. In other cases, it exhibits a different clinical course. There are studies showing improvement of myositis after surgical excision of the neoplasm and others that do not confirm this finding. Different types of malignancy may be diagnosed in patients with dermatomyositis. In women, the most frequent types of malignancy are ovarian, cervical and breast cancers. In patients with dermatomyositis and lung cancer, the most frequent type is that of the small-cell lung cancer, followed by squamous cell carcinoma, whereas adenocarcinoma is relatively rare [11]. In the present case, the diagnosis of a small cell lung cancer was made. The presence of malignancy is more frequent in patients older than 45 years of age in comparison to patients 15-45 years old. The type of cancer is usually related to the patients’ age. Younger males with dermatomyositis may present testicular cancer, while older patients are more likely to develop colon cancer. In cases where the malignancy is diagnosed before the dermatomyositis, the interval is usually less than 2 years. On the contrary, in cases where the malignancy is diagnosed after the dermatomyositis, it is still more likely to occur within 2 years of the manifestation of dermatomyositis, but the risk remains high even after 5 years [9]. Therefore, patients with dermatomyositis are advised to undergo malignancy screening during the first three years after the diagnosis of dermatomyositis, except for ovarian cancer that may present even 5 years following the diagnosis of dermatomyositis. Thus, patients are advised to Ca-125 testing every 6 months [12]. In the presence of certain additional risk factors, such as the sudden onset of dermatomyositis, the manifestation of general symptoms, the absence of Raynaud’s syndrome, and the presence of specific auto-antibodies, a thorough work-up for malignancy with CT or MRI should be undertaken [13]. Finally, it is worth-mentioning that although there is no doubt that dermatomyositis is associated with an increased incidence of malignancy, the underlying causes and pathophysiologic mechanisms remain unclear.
Conclusions
Dermatomyositis has an incidence of about 5-10 new cases per 1.000.000 population yearly. It is more frequent among women and in 25% of cases it is related to malignancy, such as ovarian, breast, colon cancer, melanoma, non-Hodgkin’s lymphomas, lung cancer, myelo-hyperplastic syndromes, and nasopharyngeal cancer. In 1/3 of cases dermatomyositis precedes malignancy, in 1/3 they present simultaneously, and in the remaining 1/3 of cases it occurs after the diagnosis of malignancy. The relative risk for the development of a neoplasm in patients with dermatomyositis is 2.4 in men and 3.4 in women. In the present case, the diagnosis of lung cancer contributed to the diagnosis of dermatomyositis that had been missed for about two years due to misdiagnosis of its symptoms and signs as well as the administration of steroids. Increased awareness is needed of the association of dermatomyositis with malignancies in order to achieve correct and timely diagnosis.
Acknowledgements
All authors disclose any sponsorship, funding arrangements or conflicts of interest relating to the present paper.
References
- 1.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 3.Barnes BE, Mawr B. Dermatomyositis and malignancy. A review of the literature. Ann Intern Med. 1976 Jan;84:68–76. doi: 10.7326/0003-4819-84-1-68. [DOI] [PubMed] [Google Scholar]
- 4.Sigurgeirsson B, Lindelöf B, Edhag O, Allander E. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med. 1992;326:363–7. doi: 10.1056/NEJM199202063260602. [DOI] [PubMed] [Google Scholar]
- 5.Buchbinder R, Forbes A, Hall S, Dennett X, Giles G. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann Intern Med. 2001;134:1087–95. doi: 10.7326/0003-4819-134-12-200106190-00008. [DOI] [PubMed] [Google Scholar]
- 6.Callen JP. The value of malignancy evaluation in patients with dermatomyositis. J Am Acad Dermatol. 1982;6:253–9. doi: 10.1016/s0190-9622(82)70018-0. [DOI] [PubMed] [Google Scholar]
- 7.Cox NH, Lawrence CM, Langtry JA, Ive FA. Dermatomyositis. Disease associations and an evaluation of screening investigations for malignancy. Arch Dermatol. 1990;126:61–5. doi: 10.1001/archderm.126.1.61. [DOI] [PubMed] [Google Scholar]
- 8.Stockton D, Doherty VR, Brewster DH. Risk of cancer in patients with dermatomyositis or polymyositis, and follow-up implications: a Scottish population-based cohort study. Br J Cancer. 2001;85:41–5. doi: 10.1054/bjoc.2001.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, Evans SR, Felson DT. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96–100. doi: 10.1016/S0140-6736(00)03540-6. [DOI] [PubMed] [Google Scholar]
- 10.Ang P, Sugeng MW, Chua SH. Classical and amyopathic dermatomyositis seen at the National Skin Centre of Singapore: a 3-year retrospective review of their clinical characteristics and association with malignancy. Ann Acad Med Singapore. 2000;29:219–23. [PubMed] [Google Scholar]
- 11.Fujita J, Tokuda M, Bandoh S, Yang Y, Fukunaga Y, Hojo S, Ueda Y, Dobashi N, Dohmoto K, Ishida T, Takahara J. Primary lung cancer associated with polymyositis/dermatomyositis, with a review of the literature. Rheumatol Int. 2001;20:81–4. doi: 10.1007/s002960000070. [DOI] [PubMed] [Google Scholar]
- 12.Whitmore SE, Rosenshein NB, Provost TT. Ovarian cancer in patients with dermatomyositis. Medicine (Baltimore) 1994;73:153–60. doi: 10.1097/00005792-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Sparsa A, Liozon E, Herrmann F, Ly K, Lebrun V, Soria P, Loustaud-Ratti V, Bouyssou-Gauthier ML, Boulinguez S, Bédane C, Jauberteau MO, Vidal E, Bonnetblanc JM. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885–90. doi: 10.1001/archderm.138.7.885. [DOI] [PubMed] [Google Scholar]


