Abstract
Borrelia burgdorferi outer surface protein OspB is expressed by spirochetes in the Ixodes scapularis gut. ospB is transcribed from a bicistronic operon with ospA, a known spirochete adhesion gene in the tick gut. Here we examine whether OspB also has a specific function in ticks. OspB specifically binds to a protein or protein complex within the tick gut. We also assessed whether selected nonborreliacidal OspB antibodies or F(ab)2 fragments interfere with B. burgdorferi-tick attachment in vivo. We examined engorged ticks that fed on B. burgdorferi N40-infected scid mice that had been treated with OspB F(ab)2 fragments. Control F(ab)2 fragments did not interfere with B. burgdorferi colonization of the tick gut, whereas OspB F(ab)2 fragments significantly inhibited the attachment of spirochetes to the tick gut. These studies show that nonbactericidal OspB antibodies interfere with B. burgdorferi colonization of I. scapularis, highlighting a specific role for OspB in spirochete- arthropod interactions and suggesting new antibody-mediated strategies for interfering with B. burgdorferi transmission.
Borrelia burgdorferi is responsible for Lyme disease, the most common vector-borne disease in the United States (16, 22). The bacterium is maintained in an enzootic life cycle, which primarily involves Ixodes scapularis ticks and mice (3). Ixodes ticks can feed on a wide range of vertebrate hosts, including humans, and deposit spirochetes into the skin during engorgement. The bacteria soon spread, often causing a skin rash known as erythema migrans in humans, and after several weeks may disseminate to distant organs, resulting in arthritis, carditis, and neurological disease (22). Laboratory mice infected with B. burgdorferi develop symptoms that are reminiscent of the human disease, including inflammation of the joints and heart. Inbred mice therefore serve as a reliable model for the study of experimental Lyme borreliosis (1).
B. burgdorferi is exquisitely adapted to survive in diverse host locations. Differential gene expression by the spirochete is thought to contribute to this adaptive process. For example, B. burgdorferi synthesizes BBK32 (6), a fibronectin-binding protein, and DbpA (11), a decorin-binding protein, early in mammalian infection, and these proteins are believed to be involved in spirochete pathogenicity. On the other hand, OspA, a lipoprotein, is primarily expressed by B. burgdorferi in ticks and generally is downregulated in mammals (20). Spirochetes swiftly upregulate OspA when entering ticks from an infected host and continue to produce abundant OspA within the resting tick (4). This preferential expression of ospA within I. scapularis suggests that OspA has a function within the vector. A recent study showing that OspA mediates spirochete adherence within the tick gut by binding to an I. scapularis protein supports this contention (18). Furthermore, nonbactericidal OspA antibodies can inhibit B. burgdorferi attachment to the tick gut (19), highlighting the importance of OspA in spirochete-tick interactions in vivo and indicating how stage-specific gene expression contributes to the maintenance of the natural cycle of the spirochete.
The ospA and ospB genes are organized into a single operon under the control of a common promoter (12). Most studies have focused on OspA, and less information is available on the role of OspB during the life cycle of B. burgdorferi. Several reports, however, have indicated that OspB is present on the surface of B. burgdorferi within unfed ticks (2, 17, 23). Certain OspB antibodies, either as whole immunoglobulin G (IgG) or as Fab fragments, can be bactericidal in vitro, and studies have demonstrated that vaccination with OspB can protect mice from B. burgdorferi infection (5, 7, 13, 14). Because OspB is located on the surface of B. burgdorferi, expressed by spirochetes within ticks, and encoded on a bicistronic operon with ospA, we examined the role of OspB in B. burgdorferi-I. scapularis interactions.
MATERIALS AND METHODS
B. burgdorferi and I. scapularis.
A low-passage-number clonal isolate of B. burgdorferi N40 that is infectious to mice was used throughout the study (18). Adult female I. scapularis ticks were collected in Connecticut. The egg mass was laid in the laboratory. Hatched larvae were allowed to feed on uninfected C3H mice to produce pathogen-free nymphs. All tick rearing was performed in an incubator at 26°C in 85% relative humidity with a 12-h light-dark cycle.
Enzyme-linked immunosorbent assay (ELISA) and confocal microscopy to assess protein binding to TGE.
Recombinant OspB and ErpT (a representative control protein) from B. burgdorferi N40 were expressed and purified in their nonlipidated forms (18). OspB and ErpT were expressed either without a fusion partner or as fusion proteins with glutathione transferase (after which the fusion partner was cleaved by use of a protease), as previously described (18). OspB, ErpT, or bovine serum albumin (BSA) was labeled with fluorescein isothiocyanate (FITC) from Molecular Probes (Eugene, Oreg.). The extent of conjugation of FITC per molecule of protein was determined according to the manufacturer's instructions. One microgram of each FITC-labeled protein represents 35 pmol of OspB, 31 pmol of ErpT, and 15 pmol of BSA. One picomole of OspB, ErpT, and BSA bound to 2, 2.1, and 6.5 pmol of FITC, respectively. Guts from flat nymphal I. scapularis ticks were dissected in phosphate-buffered saline (PBS) and homogenized on ice with a Kontes microhomogenizer (VWR Scientific Products, West Chester, Pa.) as described previously (18). One gut extract equivalent (0.5 μg) of protein extract per well was used to coat microtiter plates (ICN Biomedical Incorporated, Costa Mesa, Calif.). Protein concentrations were determined by using the Bio-Rad protein assay kit (Bio-Rad, Hercules, Calif.). One hundred microliters of each extract (5 μg/ml) in PBS was used to coat the wells. As controls, plates were coated (100 μl/well) with 10 μg of fetal bovine serum/ml in a similar fashion. Plates were incubated overnight at 4°C, with the plates tightly covered with cellophane to prevent evaporation. Plates were then washed three times in PBS with 0.05% Tween 20 (PBS-Tween 20). Nonspecific sites were blocked by incubating the tick gut extract (TGE)-coated wells with 15% normal fetal calf serum for 2 h at 37°C. Plates were then incubated with 100 μl of FITC-labeled OspB, ErpT, or BSA (10 μg/ml) at 37°C for 1 h. The plates were washed three times with PBS-Tween 20. Binding was detected by using anti-FITC IgG-horseradish peroxidase (Amersham Pharmacia Biotech, Piscataway, N.J.) as a secondary reagent, and TMB microwell peroxidase substrate (KPL, Gaithersburg, Md.) was used for color development. The optical density (OD) was read at 450 nm at 15 min.
The assessment of protein binding by confocal microscopy was performed as previously described (19). Five to 10 mid-guts were dissected from nymphal ticks in 100 μl of PBS. The organs were cut into two pieces and placed on sialylated glass slides (PGC Scientific, Gaithersburg, Md.) to enhance attachment. Slides were washed twice with PBS, incubated with PBS-Tween 20 with 5% fetal calf serum for 30 min at room temperature, and then incubated for 1 h at room temperature with FITC-labeled OspB, ErpT, or BSA (50 μl of 10-μg/ml FITC-labeled protein). Samples were subsequently stained with propidium iodide (50 μl of a 10-μg/ml solution) for 3 min at room temperature, washed three times with PBS-Tween 20, and mounted in glycerol for examination. The tissues were viewed under a Zeiss LSM 510 scanning laser confocal microscope equipped with an argon-krypton laser.
Treatment of the tick gut with lipase, glycosidase, or trypsin.
TGE was prepared as described above, and equal aliquots were incubated with either heat-inactivated (95°C for 10 min) or active lipase or glycosidases as follows: wheat germ lipase was used at 10 U/ml (Sigma) for 1 h at 37°C, and O-glycosidase, PNGase F, α2(3,6,8,9)-neuraminidase, β-N-acetylglucosaminidase, and β(1-4)-galactosidase treatment was done with a glycoprotein deglycosylation kit (Sigma). An equal aliquot of TGE was also incubated with trypsin for 1 h at 37°C at 10 μg/ml (Sigma) in the presence or absence of 20 μg of soybean trypsin inhibitor (Sigma)/ml. After enzyme incubation, the TGE and enzyme-treated TGE were used to coat microtiter plates and were probed with labeled OspB as described for ELISAs.
Antibodies and generation and characterization of F(ab)2 fragments.
The generation of OspB polyclonal or monoclonal antibodies (MAbs) B22J (B22) and B27G (B27) against B. burgdorferi N40 has been described previously (7). Ten milliliters of normal rabbit sera or polyclonal antisera was passed over a 0.5-ml protein A column (Bio-Rad Laboratories) that was then washed twice with 20 ml of PBS, pH 7.4. The bound IgG was eluted in 1 ml of 0.1 M glycine, pH 3.0. The Ab was then concentrated and desalted in a spin column (Amicon, Beverly, Mass.), and the protein concentration was determined by use of a Bio-Rad protein assay kit (Bio-Rad Laboratories), with BSA (American Bioanalyticals, Natick, Mass.) as the standard. We used immobilized pepsin to generate F(ab)2 fragments from the whole IgG fraction of normal rabbit sera or OspB antisera (ImmunoPure F(ab)2 preparation kit; Pierce, Rockford, Ill.). The cleaved F(ab)2 fragments were then separated from Fc fragments or undigested IgG F(ab)2 fragments and were concentrated in a spin column according to the manufacturer's instructions. The purity of the F(ab)2 fragments was checked by running an aliquot through a sodium dodecyl sulfate-12% polyacrylamide gel. The binding of OspB F(ab)2 to B. burgdorferi lysates and whole spirochetes was analyzed by a standard ELISA and immunofluorescence, respectively, as described previously (19).
Bactericidal assay.
Antibodies or F(ab)2 fragments were tested for their bactericidal activity against B. burgdorferi N40 by dark-field microscopy as described previously (19). Briefly, spirochetes (5 × 106/ml) were incubated in Barbour-Stoenner-Kelly (BSK) medium with 20% OspB polyclonal sera, control sera, or OspB-producing hybridoma culture supernatants as well as 50 μg of purified F(ab)2 fragments/ml for 48 h at 33°C. The percentage of viable spirochetes was determined by dark-field microscopic observation of the loss of spirochete motility and refractivity from 10 random fields in a double-blinded manner. In addition, 50-μl aliquots from each control or antibody-treated group were removed and incubated with 500 μl of BSK medium at 33°C for 5 days. The B. burgdorferi cells were then counted, and these results were compared with the initial viability by dark-field microscopy.
In vivo infection and adherence studies.
Pathogen-free NCr immunodeficient mice (NCr-SCID) from the National Institutes of Health (Bethesda, Md.) were infected with B. burgdorferi N40 (105 spirochetes/mouse, three animals per group) by intradermal injection into the back. After 3 weeks, selected F(ab)2 fragments (100 μg/mouse) or MAbs (200 μl/mouse) were administered to groups of mice (100 μl intraperitoneally and 100 μl subcutaneously). Twenty-four hours later, 10 I. scapularis nymphs were placed on each mouse. The animals again were treated with F(ab)2 fragments or antibodies on the next day. The nymphs were allowed to feed to repletion and detach from the mice, which usually occurred at 72 to 96 h. Guts from each group of nymphs were dissected under a microscope in PBS (20 ml/gut) and were examined 24, 48, and 72 h after tick detachment. Five-microliter aliquots were examined for viable spirochetes by dark-field microscopy.
Organs from nymphal ticks were prepared for microscopy, as described previously, by the dissection of gut diverticula in PBS (20 ml/gut). The lumen of each gut diverticulum was exposed by a vertical incision with a fine blade so that individual diverticula were separated from each other, and both ends were opened to facilitate the outflow of blood. The organs were washed three times under a dissecting microscope until the cessation of a visible flow of blood from open diverticula. The isolated organs were placed on sialylated glass slides (PGC Scientific) to enhance attachment, allowed to dry, and fixed with acetone for 5 min. Acetone-fixed slides were rinsed twice with PBS and incubated for 30 min with PBS-Tween 20 and 5% normal goat serum at room temperature. Organs were incubated with an affinity-purified FITC-labeled goat anti-B. burgdorferi Ab (Kirkegaard & Perry Laboratories) at a dilution of 1/50 in PBS-Tween 20 with 5% normal goat serum at room temperature for 1 h. The samples were counterstained with propidium iodide (50 ml of a 10-mg/ml solution) for 3 min at room temperature, washed three times with PBS-Tween 20, and mounted in glycerol for examination. The tissues were viewed with a Zeiss LSM 510 scanning laser confocal microscope equipped with an argon-krypton laser. The distribution of spirochetes in the gut was determined by scanning the entire organ from end to end and throughout its depth at each point.
Statistical analysis.
Results are expressed as means ± standard errors. The significance of the differences between the mean values of the groups was evaluated by Student's t test or repeated-measure analysis of variance with the Fisher protected least significant difference test by use of Statview software (SAS Institute, Cary, N.C.).
RESULTS
OspB binds to the I. scapularis gut.
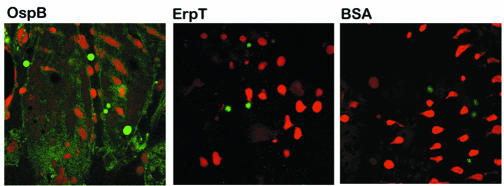
We first assessed whether OspB bound to a TGE by using an ELISA-based assay. Wells were coated with TGE and probed with FITC-labeled recombinant OspB from B. burgdorferi N40, a prototypic infectious B. burgdorferi sensu stricto isolate. We detected significant binding of OspB to TGE in comparison to other control proteins, such as BSA or B. burgdorferi ErpT, which adhered poorly to the TGE (Fig. 1). OspB only bound weakly to antigens in the fetal bovine serum (Fig. 1). The capacity of OspB to adhere to the tick gut was further examined by confocal microscopy. FITC-labeled OspB bound to the intact unfixed tick gut (Fig. 2), while FITC-labeled ErpT or FITC-labeled BSA did not bind to the tick gut (Fig. 2).
FIG. 1.
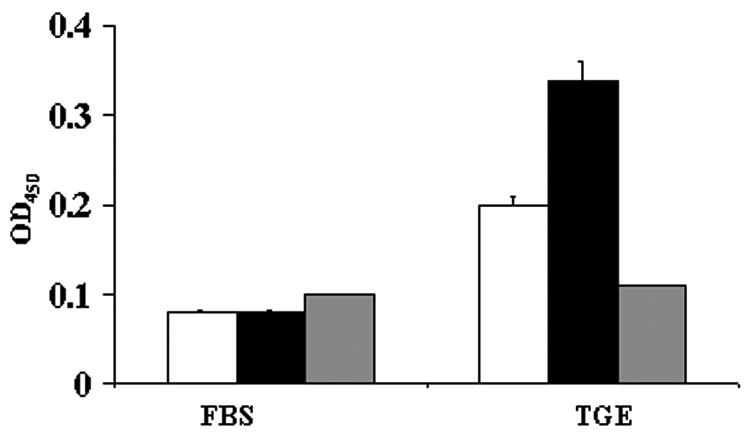
OspB binds to an I. scapularis TGE. FITC-labeled OspB (black bars), ErpT (white bars) from B. burgdorferi N40, and BSA (gray bars) were used to probe either fetal bovine serum (FBS)- or TGE-coated wells. Bars represent the OD450 values at 15 min (means ± standard deviations) from three experiments. The difference between the binding of OspB to TGE and that of either BSA (P < 0.001) or ErpT (P < 0.005) was significant.
FIG. 2.
OspB directly binds to the I. scapularis gut. The direct binding of FITC-labeled OspB to the intact unfixed tick salivary gland was detected by confocal microscopy. FITC-labeled ErpT and FITC-labeled BSA were used as controls. After probing of the tick gut with FITC-labeled protein (green), the tissues were stained with propidium iodide to localize the nuclei of the gut cells (red). The FITC and propidium iodide images were examined at ×630 magnification and are presented as a single image for clarity.
OspB binding to the tick gut is abolished by trypsin treatment.
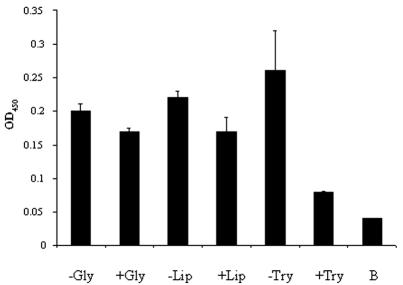
The OspB receptor in ticks was next partially characterized. For an understanding of the biochemical nature of the OspB binding moiety, the TGE was treated with wheat germ lipase, a variety of glycosidases [O-glycosidase, PNGase F,α2(3,6,8,9)-neuraminidase, β-N-acetylglucosaminidase, andβ(1-4)-galactosidase], or trypsin. Heat-denatured enzyme (in the case of lipase or glycosidases) and protease in the presence of a specific inhibitor (soybean trypsin inhibitor) served as controls. Soybean trypsin inhibitor alone also served as a control and did not alter the binding of OspB to the TGE (not shown). While glycosidases or lipase treatment did not affect OspB binding to the TGE, pretreatment of the TGE with trypsin markedly diminished OspB adherence (Fig. 3). These data suggest that OspB binds to a protein or protein complex in the I. scapularis gut.
FIG. 3.
OspB binding to TGE is abolished by trypsin treatment. The TGE was treated with heat-denatured glycosidases (−Gly), active glycosidases (+Gly), heat-denatured lipase (−Lip), active lipase (+Lip), soybean trypsin inhibitor and trypsin (−Try), or trypsin (+Try). The treated TGEs were then used to coat microtiter wells and probed with labeled OspB. The OD of the binding of labeled OspB to BSA is also shown as a control (B). Bars represent the OD450 values at 15 min (means ± standard deviations) from three experiments. Statistically nonsignificant differences were obtained in the cases of the glycosidase (−Gly versus +Gly)- and lipase (−Lip versus +Lip)-treated group; the difference for the protease group (−Try versus +Try) was significant (P < 0.01) by Student's t test.
OspB F(ab)2 fragments lack in vitro bactericidal activity but inhibit the association of spirochetes with the tick gut in vivo.
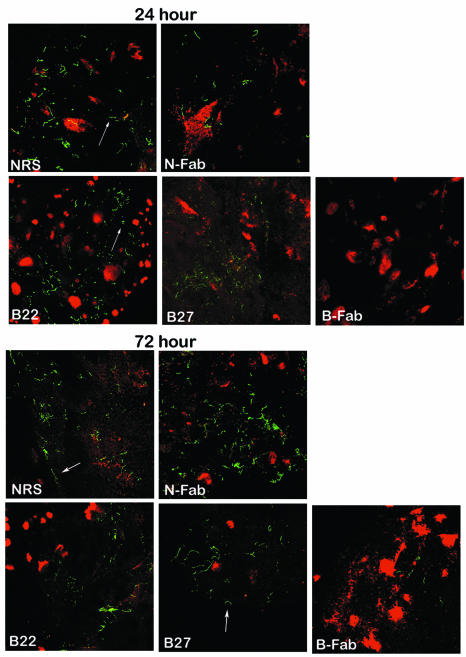
Previous studies have indicated that B. burgdorferi OspA plays an important role in the attachment of spirochetes to the tick gut (19). Since, like OspA, OspB also binds to the tick gut, we were interested in determining whether B. burgdorferi N40 exposed to OspB antibody could effectively colonize I. scapularis. Certain OspB antibodies or F(ab)2 fragments have been shown to exert in vitro bactericidal activity, so we first characterized whether selected OspB antibodies or F(ab)2 fragments can kill the spirochete in vitro. We prepared F(ab)2 fragments from a polyclonal OspB antiserum and used MAb B22 or MAb B27 as whole IgG, as these MAbs were previously characterized as nonbactericidal and bind to defined N-terminal epitopes of OspB as well as to the surface of unfixed B. burgdorferi N40 (7). MAb B22 binds an OspB epitope within amino acids 34 to 91, and MAb B27 binds a region within amino acids 134 to 167 (7). Both MAbs only react with OspB in an immunoblot with B. burgdorferi lysates (7). As expected, neither MAb B22 or MAb B27 or OspB F(ab)2 fragments were found to exert significant bactericidal activity against B. burgdorferi N40 grown in vitro (Table 1). For in vivo adherence studies, SCID mice were used because the animals cannot mount their own humoral response against B. burgdorferi during infection. The effects of the administered antibodies or F(ab)2 fragments can therefore be evaluated without the contribution of any acquired host response to the spirochete. Groups of three SCID mice were challenged with B. burgdorferi N40, and within 3 weeks all the mice had developed visible swelling of the tibiotarsal joints due to disseminated infection with the spirochete. Next, either normal rabbit serum, F(ab)2 fragments prepared from a polyclonal OspB antiserum or normal rabbit serum, or an OspB MAb (B22 or B27) was administered to groups of animals. Twenty-four hours after the antibody transfer, 10 uninfected ticks were allowed to feed to repletion on each mouse and the engorged ticks were collected. Guts from ticks that fed on different groups of mice were dissected and aliquots of the luminal contents were examined for B. burgdorferi by dark-field microscopy to confirm that the F(ab)2 fragments or antibodies did not interfere with the migration of spirochetes from mice into the tick gut (data not shown). To determine whether specific OspB fragments or antibodies could interfere with the ability of B. burgdorferi to adhere to the tick gut, we examined Borrelia-tick tissue associations 24, 48, and 72 h after tick engorgement. The tick gut diverticula were dissected, washed to remove unbound luminal contents, and examined by double-labeled confocal microscopy. Twenty-four to 72 h after repletion, B. burgdorferi cells in the control groups with IgG F(ab)2 or normal rabbit sera as well as OspB MAb B22 or B27 were found in close proximity to the lumen of the nymphal tick gut (Fig. 4 and Table 1). In contrast, OspB F(ab)2-treated samples had fewer spirochetes associated with the gut (Fig. 4 and Table 2). These data suggest that OspB is important for spirochete-tick interactions in vivo.
TABLE 1.
Borreliacidal activity of OspB antibodies against B. burgdorferi N40a
| Antibody | No. of viable B. burgdorferi cells
|
|
|---|---|---|
| 24 h | 48 h | |
| Control (no addition) | 100 | 100 |
| NRS | 92 ± 4.5 | 104 ± 6 |
| NRS-F(ab2) | 93 ± 10 | 97 ± 13 |
| OspB-F(ab2) | 102 ± 8.5 | 99 ± 26 |
| MAb B22 | 99 ± 19 | 96 ± 18 |
| MAb B27 | 98 ± 6.7 | 90 ± 5.5 |
| Human B. burgdorferi antiserum | 10 ± 9 | 0 |
Spirochetes were incubated in BSK medium in the absence (control) or presence of normal rabbit serum (NRS), F(ab2) fragments prepared from normal rabbit serum [NRS-F(ab2)], polyclonal anti-OspB serum [OspB-F(ab2)], OspB MAb B22 or B27 or a borreliacidal serum from a patient with Lyme disease. The numbers of spirochetes were assessed by dark-field microscopy after 24 and 48 h and are expressed relative to controls without antibody or serum treatment. Data represent the mean numbers of spirochetes remaining viable after treatment (means ± standard errors from three independent experiments). Differences between the control and normal rabbit serum as well as OspB antibodies or F(ab2)-treated samples were not statistically significant (Student's t test).
FIG. 4.
OspB F(ab)2 fragments interfered with the attachment and colonization of B. burgdorferi within I. scapularis. The distribution of B. burgdorferi within the I. scapularis gut 24 and 72 h after feeding is shown. Nymphal ticks fed on B. burgdorferi-infected mice that had been treated with either normal rabbit serum (NRS), F(ab)2 fragments from normal rabbit serum (N-Fab), or OspB MAb B22 or B27 or F(ab)2 fragments prepared from polyclonal anti-OspB sera (B-Fab). The spirochetes (arrows) were stained with a FITC-labeled goat anti-B. burgdorferi antibody (green), and the nuclei of the gut epithelial cells were stained with propidium iodide (red). Images were recorded at ×400 magnification and are presented as a merged image for clarity.
TABLE 2.
Effect of OspB antibodies on attachment of B. burgdorferi to the tick guta
| Time (h) | No. of spirochetes attached to the tick gut per field with indicated antibody
|
||||
|---|---|---|---|---|---|
| NRS | NRS-Fab2 | Mab-B22 | MabB27 | OspB-Fab2 | |
| 24 | 121 ± 28 | 159 ± 57 | 265 ± 88 | 221 ± 142 | 7 ± 1.6 |
| 48 | 199 ± 45 | 102 ± 51 | 135 ± 37 | 47 ± 11 | 10 ± 3 |
| 72 | 227 ± 34 | 117 ± 29 | 297 ± 74 | 205 ± 55 | 5 ± 2.3 |
NCr SCID mice were infected with B. burgdorferi before the administration of antibodies 24 h before tick placement. Engorged ticks were examined from 24 to 72 h after detachment by confocal microscopy to enumerate B. burgdorferi cells in direct contact with tick gut tissue. In each experiment, the entire gut diverticulum was examined, and counts were made from at least three fields from two separate gut diverticula. The average number of microscopic fields per diverticulum was 6; the range was 2 to 7 fields/diverticulum. Data shown are the numbers of spirochetes per field from three independent experiments (means ± standard errors). Differences between the OspB-F(ab2)-treated group and all of the other individual groups at each time period (24, 48, and 72 h) were significant (P values were <0.05). Statistical differences were determined by analysis of variance and Fisher's protected least significant difference test.
DISCUSSION
B. burgdorferi selectively produces distinct sets of proteins to enhance infectivity and to survive in mammalian or arthropod hosts (3). OspA and OspB are examples of spirochete proteins which are abundantly synthesized in ticks (10, 17, 20). B. burgdorferi also produces proteins such as OspC or BBK32 in feeding ticks during the process of transmission to new hosts. Their expression profile, however, is quite distinct from that of OspA or OspB. For example, OspA or OspB is produced by B. burgdorferi organisms during their entrance into the vector and during their residence for the long intermolt period within the tick gut, when little or no OspC or BBK32 is synthesized by the spirochetes (4, 6, 10, 20, 21). This selective and temporal expression of OspA and OspB within ticks suggests that these proteins might have a function in the vector during early spirochete colonization and persistence within the arthropod gut. Indeed, we have shown that OspA is involved in the colonization of spirochetes within the tick gut (18). OspA-mediated adherence was also highlighted in another recent study in which B. burgdorferi and Borrelia afzelii expressing OspA were shown to adhere to tick cells more readily than spirochetes not producing this protein (8). Since OspA and OspB are transcribed on a bicistronic operon, we speculated that OspB might also have a specific function for B. burgdorferi within ticks. Here we demonstrated that B. burgdorferi N40 OspB binds to a protein or protein complex in the gut of I. scapularis. It was previously shown that OspA also interacts with a protein in the tick gut (18). Epitope mapping and mutagenesis studies demonstrated that the binding domains reside in the central portion and carboxy terminus of OspA (18). Although the protein sequences of OspA and OspB share 50% homology (9), the amino acid sequences of the OspA peptides at positions 85 to 103 and 229 to 247, which demonstrated the highest levels of binding to the tick gut (18), are 90 to 95% homologous to the OspB amino acid sequences from positions 110 to 128 and 289 to 305, respectively. OspA and OspB colocalize on the surface of B. burgdorferi N40 (5), and both are coexpressed in unfed tick guts (17). Therefore, it is possible that B. burgdorferi can use both lipoproteins to bind a promiscuous tick receptor or, alternatively, that different tick ligands are responsible for binding to OspA and OspB.
OspB antibodies, either as whole IgGs or Fab fragments, have been reported to be either bactericidal or nonbactericidal in vitro (7, 13, 15). The lack of an in vitro bactericidal effect of the MAb B22 or B27 in our studies supports an earlier observation that such antibodies failed to provide protective immunity in vivo (7). Interestingly, F(ab)2 fragments prepared from a polyclonal OspB antiserum also failed to show significant killing of B. burgdorferi in our in vitro assay. Although MAbs B22 and B27 as well as OspB F(ab)2 fragments were found to be nonborreliacidal, these antibodies can bind to the surface of B. burgdorferi N40 (7). We therefore used them for further in vivo adherence studies with the murine model of tick-transmitted Lyme borreliosis.
Our in vivo studies examined the capacity of nonbactericidal OspB F(ab)2 fragments or antibodies to prevent effective tick colonization by B. burgdorferi. We administered the F(ab)2 fragments or antibodies to B. burgdorferi-infected mice, allowed ticks to acquire the antibodies together with the spirochetes, and assessed whether gut tissue colonization by spirochetes is affected in the presence or absence of OspB antibodies. Our in vivo studies demonstrated that OspB F(ab)2 fragments, even those that did not kill spirochetes in vitro, effectively prevented B. burgdorferi from associating with the I. scapularis gut. Such OspB antibodies or F(ab)2 fragments may bind directly to the tick gut binding region of OspB. It is likely that the tick gut binding site of OspB is recognized by antibodies that do not bind the epitopes identified by either MAb B22 or B27. Alternatively, these polyclonal antibody fragments may bind to several epitopes of OspB on the B. burgdorferi surface, and steric hindrance might then interfere with OspB binding to the tick gut. Since OspA is also involved in the attachment of spirochetes to the tick gut and since we have found that polyclonal OspB antibodies prevented the binding of spirochetes, it is possible that steric hindrance by OspB antibodies also affected OspA-mediated binding of spirochetes to the tick gut. This is not surprising, as both lipoproteins have been shown to be colocalized on the spirochete surface (5). Overall, these results, together with our in vitro studies on OspB binding to the tick gut, indicate that OspB serves as a functional ligand for B. burgdorferi-tick gut interactions. The mechanism by which B. burgdorferi interacts with ticks and survives therein remains to be explored. The characterization of B. burgdorferi ligands that play significant roles in bacterial colonization and survival at the arthropod-pathogen interface will enhance our knowledge of vector-spirochete interactions and highlight the contribution of differentially expressed genes in the enzootic life cycle of B. burgdorferi.
Acknowledgments
The human experimentation guidelines of Yale University were followed in the conduct of clinical research, and animal experimentation guidelines were followed for animal studies.
This work was supported by grants from the NIH. E. Fikrig is the recipient of a Burroughs Wellcome Clinical Scientist Award in Translational Research. R. A. Flavell is an investigator of the Howard Hughes Medical Institute.
We sincerely thank Debby Beck, Syed A. Morshed, and Denise Lusitani for their help in the study and Fran Manzo for help with manuscript preparation.
Editor: D. L. Burns
REFERENCES
- 1.Barthold, S. W., M. S. deSouza, J. L. Janotka, A. L. Smith, and D. H. Persing. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:959-971. [PMC free article] [PubMed] [Google Scholar]
- 2.Brunet, L. R., A. Spielman, E. Fikrig, and S. R. Telford, 3rd. 1997. Heterogeneity of Lyme disease spirochaetes within individual vector ticks. Res. Microbiol. 148:437-445. [DOI] [PubMed] [Google Scholar]
- 3.de Silva, A. M., and E. Fikrig. 1997. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J. Clin. Investig. 99:377-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Silva, A. M., S. R. Telford, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escudero, R., M. L. Halluska, P. B. Backenson, J. L. Coleman, and J. L. Benach. 1997. Characterization of the physiological requirements for the bactericidal effects of a monoclonal antibody to OspB of Borrelia burgdorferi by confocal microscopy. Infect. Immun. 65:1908-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fikrig, E., S. W. Barthold, W. Sun, W. Feng, S. R. Telford, and R. A. Flavell. 1997. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity 6:531-539. [DOI] [PubMed] [Google Scholar]
- 7.Fikrig, E., H. Tao, F. S. Kantor, S. W. Barthold, and R. A. Flavell. 1993. Evasion of protective immunity by Borrelia burgdorferi by truncation of OspB. Proc. Natl. Acad. Sci. USA 90:4092-4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fingerle, V., H. Laux, U. G. Munderloh, U. Schulte-Spechtel, and B. Wilske. 2000. Differential expression of outer surface proteins A and C by individual Borrelia burgdorferi in different genospecies. Med. Microbiol. Immunol. (Berlin) 189:59-66. [DOI] [PubMed] [Google Scholar]
- 9.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 10.Gilmore, R. D., Jr., M. L. Mbow, and B. Stevenson. 2001. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microb. Infect. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 11.Guo, B. P., E. L. Brown, D. W. Dorward, L. C. Rosenberg, and M. Hook. 1998. Decorin-binding adhesins from Borrelia burgdorferi. Mol. Microbiol. 30:711-723. [DOI] [PubMed] [Google Scholar]
- 12.Howe, T. R., F. W. LaQuier, and A. G. Barbour. 1986. Organization of genes encoding two outer membrane proteins of the Lyme disease agent Borrelia burgdorferi within a single transcriptional unit. Infect. Immun. 54:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katona, L. I., S. Ayalew, J. L. Coleman, and J. L. Benach. 2000. A bactericidal monoclonal antibody elicits a change in its antigen, OspB of Borrelia burgdorferi, that can be detected by limited proteolysis. J. Immunol. 164:1425-1431. [DOI] [PubMed] [Google Scholar]
- 14.Ma, J., P. A. Bulger, D. R. Davis, B. Perilli-Palmer, D. A. Bedore, C. R. Kensil, E. M. Young, C. H. Hung, J. R. Seals, C. S. Pavia, et al. 1994. Impact of the saponin adjuvant QS-21 and aluminum hydroxide on the immunogenicity of recombinant OspA and OspB of Borrelia burgdorferi. Vaccine 12:925-932. [DOI] [PubMed] [Google Scholar]
- 15.Ma, J., C. Gingrich-Baker, P. M. Franchi, P. Bulger, and R. T. Coughlin. 1995. Molecular analysis of neutralizing epitopes on outer surface proteins A and B of Borrelia burgdorferi. Infect. Immun. 63:2221-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadelman, R. B., and G. P. Wormser. 1998. Lyme borreliosis. Lancet 352:557-565. [DOI] [PubMed] [Google Scholar]
- 17.Oliver, J. H., Jr., K. L. Clark, F. W. Chandler, Jr., L. Tao, A. M. James, C. W. Banks, L. O. Huey, A. R. Banks, D. C. Williams, and L. A. Durden. 2000. Isolation, cultivation, and characterization of Borrelia burgdorferi from rodents and ticks in the Charleston area of South Carolina. J. Clin. Microbiol. 38:120-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal, U., A. M. de Silva, R. R. Montgomery, D. Fish, J. Anguita, J. F. Anderson, Y. Lobet, and E. Fikrig. 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Investig. 106:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pal, U., R. R. Montgomery, D. Lusitani, P. Voet, V. Weynants, S. E. Malawista, Y. Lobet, and E. Fikrig. 2001. Inhibition of Borrelia burgdorferi-tick interactions in vivo by outer surface protein A antibody. J. Immunol. 166:7398-7403. [DOI] [PubMed] [Google Scholar]
- 20.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 23.Stepanova-Tresova, G., J. Kopecky, and M. Kuthejlova. 2000. Identification of Borrelia burgdorferi sensu stricto, Borrelia garinii and Borrelia afzelii in Ixodes ricinus ticks from southern Bohemia using monoclonal antibodies. Zentbl. Bakteriol. 289:797-806. [DOI] [PubMed] [Google Scholar]