Summary
Background
The aim of this paper was to evaluate functional and anatomical results of intravitreal ranibizumab injections and the course of exudative age-related macular degeneration (AMD) treatment over a 12-month observation period.
Material/Methods
In 25 patients with active dominantly classic exudative AMD, treatment was performed according to the following schedule: 3 intravitreal injections of 0.5 mg ranibizumab at monthly intervals (saturation phase); further injections were based on activity of the neovascular process. Changes in VA and central retinal thickness (CRT) during treatment were evaluated with ANOVA testing.
Results
Mean pre-treatment best corrected visual acuity was 0.73±0.27 logMAR. After the third ranibizumab injection the best results, 0.54±0.27 logMAR, were seen; 12-month results were 0.58±0.26 logMAR. Patients had a mean improvement of 10.6 letters at 12 months. In 92% of patients stabilization or improvement of vision was observed. The mean number of injections in the 12-month period was 6.
Baseline mean CRT was 351.12±74.15 μm. After the first ranibizumab injection it decreased significantly to 221.96±60.85 μm, after the third injection it was 200.80±47.63 μm, and after 12 months it was 213.16±44.37 μm. Mean correlations between baseline average CRT and baseline average VA measured in ETDRS letters (p=0.017) and in logMAR scale (p=0.033) and between average CRT after the third injection and average VA in logMAR scale after the third injection (p=0.047) were noted.
Conclusions
Treatment with intravitreal ranibizumab injections according to the presented scheme provides AMD patients with a chance of stabilization and improvement of the topical state, with a lower number of injections and preserved topical and general safety. Our results suggest that regular monthly controls are necessary to be able react rapidly to the smallest signs of deterioration, not only in visual acuity, but also in OCT images.
Keywords: exudative AMD, ranibizumab, central retinal thickness
Background
Vascular endothelial growth factor (VEGF) is currently considered the most important mediator of angiogenesis in physiological and pathological processes, including various eye diseases such as exudative age-related macular degeneration (AMD) [1,2]. Ranibizumab (Lucentis®, Novartis, Basel, Switzerland) is a monoclonal anti-VEGF antibody fragment, designed and registered for intravitreal injections in treatment of neovascular age-related macular degeneration (AMD). It acts selectively in locations where choroidal neovascularisation (CNV) forms, blocking and inactivating all known biologically active VEGF isoforms. At the same time it tightens and stabilizes vessels. Because of these effects, retraction of subretinal fluid and intraretinal edema, which are signs of the degeneration process, is observed during ranibizumab treatment [3–6].
The aim of this paper is evaluation of functional and anatomical results of intravitreal ranibizumab injections and of exudative AMD treatment course in our 12-month observational study.
Material and Methods
After approval by the Bioethics Commission, 25 patients from the Retinal Clinic at the Department Of Ophthalmology, Military Medical Institute in Warsaw were included in the study. Qualification criteria were as follows: (1) age of over 50 years; (2) active subfoveal dominantly classic neovascular AMD, confirmed on fluorescein angiography (FA) (Heidelberg Engineering HRA 2) and optical coherence tomography (SLO OCT OTI), not treated previously; (3) overall lesion size not exceeding 12 optic disc diameters; and (4) baseline VA of 22–76 letters (Early Treatment of Diabetic Retinopathy Study – ETDRS chart). Patients with the following conditions and past treatments were not qualified for ranibizumab treatment: (1) permanent structural foveal damage (subretinal scar or geographic atrophy); (2) macular hemorrhage exceeding 50% of lesion size; (3) retinal detachment; (4) vitreoretinal or filtration surgery, or transplantation of the cornea; (5) peripheral retinal photocoagulations within the last month; (6) macular photocoagulations; (7) cataract surgery within the last 2 months; (8) unstabilized glaucoma; (9) active infection of the eyeball or its protective apparatus; (10) past or active uveitis; and (11) significant degeneration of peripheral retina. The most important general exclusion criteria were cerebral stroke or myocardial infarction within the last 6 months, severe unstabilized hypertension, and unstable coronary heart disease.
The mean age of patients was 73.23±8.55 years (range: 58–88 years of age); 14 women and 11 men were treated. Treatment was performed according to the following schedule: each patient received 3 intravitreal injections of 0.5 mg ranibizumab at monthly intervals (saturation phase); further treatment was based on activity of the exudative degeneration process. After the end of the saturation phase, each month the patient’s VA was tested with the ETDRS chart and OCT was performed. The following re-injection criteria were employed: (1) loss of 5 or more ETDRS letters compared to best result during the initial phase of treatment; (2) persistence or appearance of fluid under the retina or pigment epithelium, or intraretinal edema, on OCT; (3) increase in central retinal thickness (CRT) of at least 100 μm compared to lowest CRT during the saturation phase; (4) new macular hemorrhage; and (5) new CNV focus on FA.
After 12 months the results of this therapeutic approach were summarized. Each ranibizumab injection was performed in an operation theatre with aseptic protocol maintained during preparation of instruments, procedure field, anesthesia, the drug and its administration. Before and after the injection, a broad-spectrum antibiotic was administered for 3 days. During treatment the patients were monitored for topical and general adverse events, and mean number of ranibizumab injections was counted.
Mean VA (ETDRS letters, decimal scale, logMAR) and mean CRT were evaluated and compared at baseline, after the 1st, 2nd and 3rd injection, and at 12 months. Existence of correlations between changes in VA and retinal thickness in succeeding periods was tested.
Results
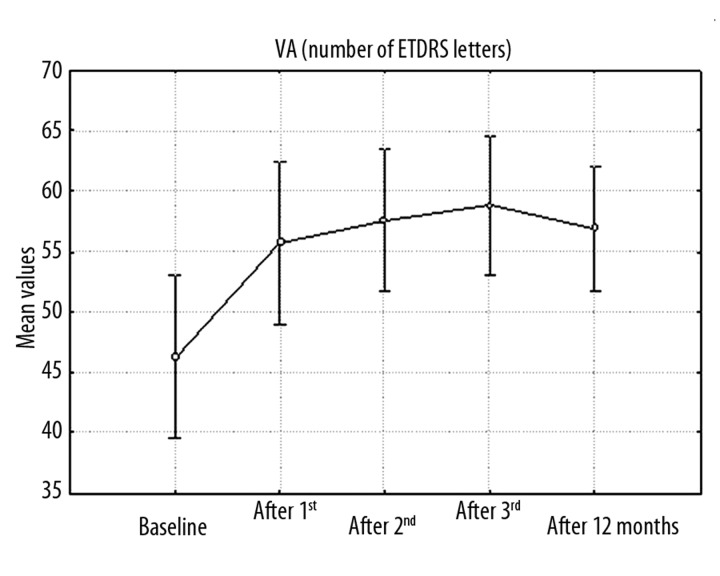
ANOVA testing was used for statistical analysis of changes in VA and CRT. Variation analysis with p<0.05 showed that mean VA (ETDRS letters, decimal scale, logMAR) was significantly different at successive intervals. Mean best-corrected visual acuity (BCVA) at baseline was 46.28±16.38 ETDRS letters (0.23±0.15, 0.73±0.27 logMAR). After the first injection a significant improvement to 55.72±16.24 letters (0.32±0.21, 0.58±0.29 logMAR) was observed, and after the third injection the best VA of 58.80±13.79 letters (0.34±0.21, 0.54±0.27 logMAR) was observed. At 12 months mean VA was 56.88±12.52 letters (0.31±0.19, 0.58±0.26 logMAR) (Figure 1). Patients had a mean improvement of 10.6 letters at 12 months. Visual stabilization or improvement was observed in 92% of patients. In 8% a deterioration of up to 5 ETDRS letters was seen, and 32% achieved a significant improvement of 15 letters or more.
Figure 1.
Graph of changes in mean VA (in number of ETDRS letters) in consecutive periods (baseline, after 1st, 2nd, 3rd injection, after 12 months) during ranibizumab therapy.
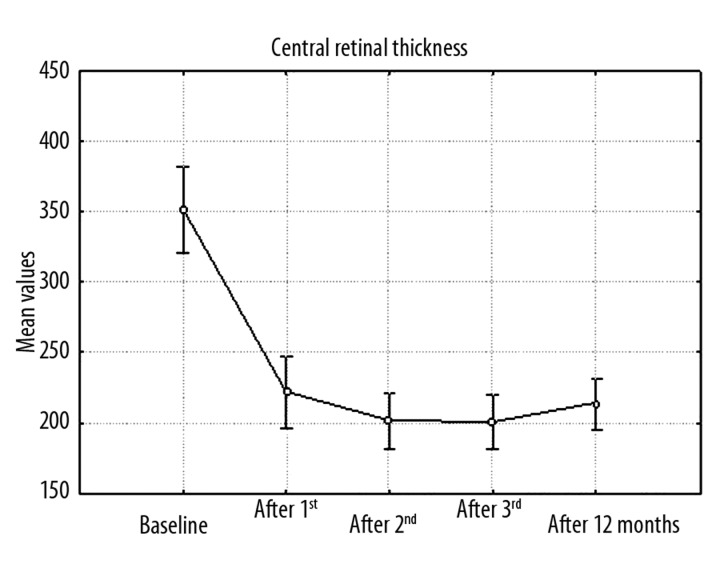
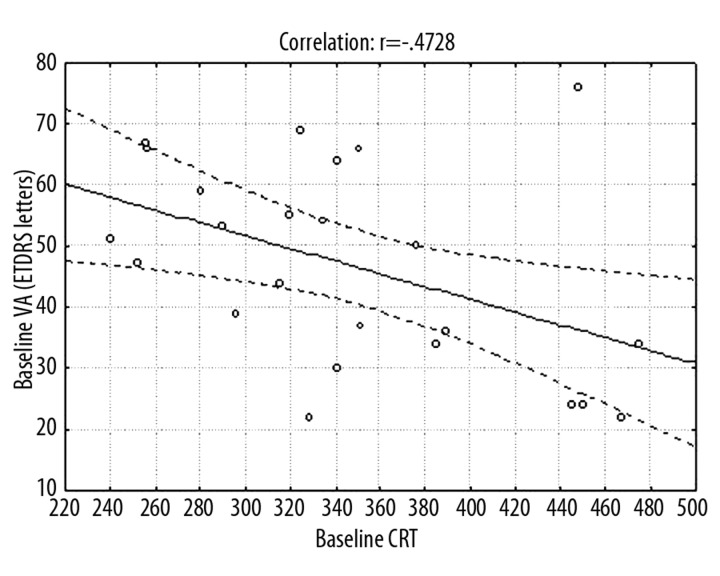
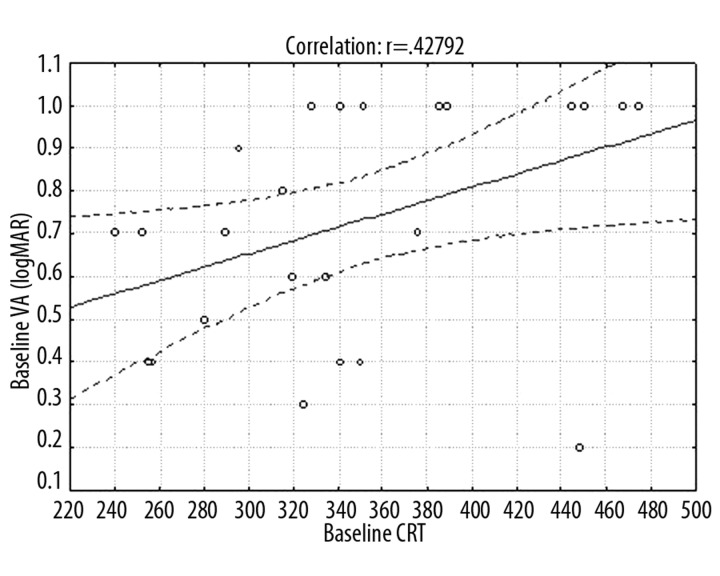
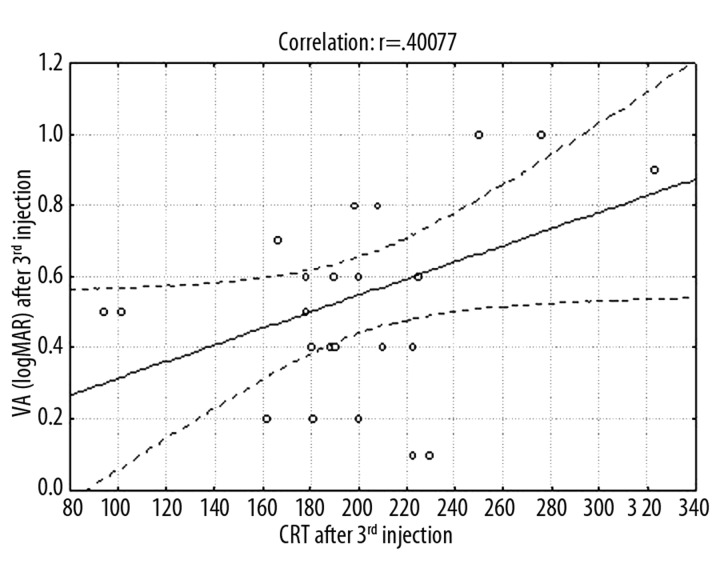
Variation analysis at p<0.05 showed that mean CRT differed at successive periods (Figure 2). Mean CRT at baseline was 351.12±74.15 μm. After the first ranibizumab injection it decreased significantly to 221.96±60.85 μm, after the third injection it was 200.80±47.63 μm, and after 12 months it was 213.16±44.37 μm. Frequencies of eyes with CRT >200 μm were: baseline 100%, 1 month 48%, 2 months 40%, 3 months 40%, and 12 months 56%. Mean correlations between baseline average CRT and baseline average VA measured in ETDRS letters (p=0.017) and in logMAR scale (p=0.033) (Figures 3,4) and between average CRT after the third injection and average VA in logMAR scale after the third injection (p=0.047) (Figure 5) were noted.
Figure 2.
Graph of changes in mean CRT in consecutive periods (baseline, after 1st, 2nd, 3rd injection, after 12 months) during ranibizumab therapy.
Figure 3.
Graph showing average correlations between CRT and mean VA at baseline (in number of ETDRS letters).
Figure 4.
Graph showing average correlations between CRT and mean VA at baseline (in logMAR scale results).
Figure 5.
Graph showing average correlations between CRT and mean VA (in logMAR scale results) after 3rd injection.
No topical or general adverse events were observed. The average number of injections in 12 months was 6 (4–8 injections in each patient).
Discussion
One of the most important clinical observations on ranibizumab, which should be referred to when discussing study results, is the ANCHOR trial, in which patients with dominantly classic AMD were qualified. The control group consisted of patients treated with photodynamic therapy (PDT) at 3-month intervals [7,8]. In 90% of eyes, monthly intravitreal injections led to stabilization of vision at 24-month follow-up, while in the PDT group the result was 66%. In 41% of those patients improvement of 3 lines or more was observed, in 12% the improvement was 30 letters or more, and in the PDT group vision improved in 6% of patients. Mean VA improvement in this group at 24 months was 11.2 letters. In the MARINA trial, which included patients with minimally classic or occult wet AMD, mean improvement was 7.2 letters [9]. Baseline VA and lesion size were not shown to have a statistical influence on final functional results. Endophthalmitis or inflammation was seen in 0.7% of eyes. There was no difference in mortality rate between the Lucentis® group and the control group. No statistical difference in rates of myocardial infarction or thromboembolic events was observed [7,8].
Ranibizumab therapy is indicated in all cases of exudative AMD with involvement of the fovea, including perifoveal, provided the degeneration is active. In our observation presented here, only patients with classic form CNV were qualified, because this form of neovascular AMD is uncommon, and in most studies patients with classic subretinal membranes constitute less than 50% of the study group.
Baseline visual acuity is not a factor that should limit qualification for treatment in cases without irreversible damage of foveal function. Clinical trials have shown that even in cases of very low baseline VA below 20/200, improvement of about 8 letters can be expected. Patients with active exudative sub- or perifoveal AMD and good baseline VA above 20/40 should be qualified for ranibizumab treatment due to a high potential for maintaining normal everyday functioning including reading and driving [7–10]. Both patients with poor BCVA (22 ETDRS letters) and with good VA (76 letters) were qualified for our study. According to the MARINA and ANCHOR studies, duration of degeneration is not decisive for treatment effects [7,9]. In our study we additionally isolated a subgroup of patients with baseline VA 22–24 ETDRS letters (4 cases). In this subgroup a significant mean improvement of 18.75 letters at 12 months was observed.
Treatment of neovascular AMD with ranibizumab usually begins with a saturation phase that consists of 3 monthly injections of 0.5 mg; our study proceeded according to this schedule. In this phase, effects are monitored through examination of VA, fundus examination, and OCT. Retraction of subretinal fluid, retinal edema and detachment of pigment epithelium correlates with VA improvement. Fluorescein angiography is performed in individual cases of visual deterioration or new ophthalmoscopic lesions in the macula [7,9,11].
Best effects have been achieved in the MARINA and ANCHOR trials with monthly injections of ranibizumab; however, modified treatment schedules with fewer injections are being sought because they are cheaper and safer for the patient. In the PIER study, after the saturation phase patients received quarterly injections of Lucentis® during an observation period of 24 months. The mean final VA was 2.2 letters worse than baseline, while in the untreated group VA deteriorated by 21.4 letters. These results formed the basis for the idea that the scheme of monthly injections can be modified with benefit for the patient [11].
In the PrONTO trial, after the saturation phase, further injections were administered according to strict monitoring of topical state and changes in VA, CRT, OCT and ophthalmoscopic macula image, and in some cases FA [12]. These reinjection criteria were also used in our study. Results of the PrONTO study (mean improvement in BCVA 9.2 letters, 35% patients gained >15 letters, mean 5.6 injections), comparable with MARINA or ANCHOR, have made this kind of approach, which assumes stabilization and improvement of visual function with reduced number of injections, more and more common in retinological practice [13]. In our study a mean of 6 injections, between 4–8 in each patient, were performed during a 12-month period and allowed an improvement 10.6 letters (32% of patients had improvement of 15 letters or more) and stabilization of VA and CRT parameters. In the PrONTO study the mean CRT was 394 μm at baseline and 216 μm at 12 months [12]. In our study, mean CRT declined from 351.12±74.15 μm to 213.16±44.37 μm at 12 months, thus the results are comparable, but in the PrONTO study only 17.5% (7 eyes) had predominantly classic lesions. The SUSTAIN study also utilized the scheme of 3 saturation injections and further injections according to activity of the disease [14]. In the SAILOR Cohort 1 study, after the saturation phase, patients were monitored quarterly and reinjected if vision deteriorated by at least 5 letters compared to best result or if CRT increased by at least 100 μm compared to the smallest value achieved. After the saturation phase, significant improvement was observed, but final VA deteriorated by 2.3 letters compared to baseline. These results prove that quarterly visits and reinjections are not sufficient for stabilization and maintenance of functional improvement [15].
In relation to our observations, a clinical study by Kumar et al reported the mean number of intravitreal ranibizumab treatment 5.6±2.3 in 81 patients with neovascular AMD with similar treatment protocol to the PrONTO study [16]. Frequency of predominantly classic lesions in this study was only 22%; 17.1% of all patients gained 15 letters or more (in our study 32% of patients achieved a significant VA improvement). Mean BCVA change at 12 months was +3.7±11.1 letters. The subgroup analysis by Kumar et al. showed a mean change in VA at 12 months of +6.9 letters for classic no occult CNV, +5.6 letters for predominantly classic with no occult lesions (in our study it was +10.6 letters). Best results were noted in the RAP subgroup, with mean VA improvement of 8.9 letters. On our good results influenced a significant VA improvement in the subgroup of patients with poor baseline VA 22–24 letters (16% of the group, at 12 months +18.75 letters) and systematic control visits every month during 1-year observation which allowed us to notice each sign of the neovascular activity process. Bandukwala et al reported the effectiveness of intravitreal ranibizumab in a 94-patient group with neovascular AMD [17]. The mean number of injections was 5.1, with a mean of 9.4 visits in the 12-month period. There was a gain of 2.88 letters in all eyes, and a loss of 2.5 letters in the subgroup of patients who met the visual acuity inclusion criteria for the clinical trials. In this subgroup 75% lost fewer than 15 letters and 11% gained more than 15 letters. Visual outcomes in the Bandukwala et al. study compared poorly with our study and with other clinical trials. One of the reasons may be a low frequency of follow-up visits. Cohen et al confirmed this conclusion by results of their 1-year study in 124 eyes treated on a pro re nata basis after 1 or 3 initial intravitreal ranibizumab injections [18]. The mean number of injections was 3.79 (range, 1–7), and the mean number of follow-up visits was 8.07 (range, 4–12) over a mean ±SD period of 52±6 weeks. Mean VA ±standard deviation changed from 56.15±14 to 56.89±17 letters (VA gain, +0.7 letters). CNV cases were of the classic type in 31 eyes (25%) and of the occult type in 93 eyes (75%). The results presented by Cohen et al. once again suggest that long-term regular follow-up is necessary for patients treated with ranibizumab to obtain and preserve significant visual gain, and not only to achieve visual stabilization. One of the aims of current clinical studies in patients with wet AMD is to adapt the treatment to each individual to reduce the number of injections preformed. The results show great inter-patient variability in the number of injections needed, ranging from 1 to 23 over the course of 2 years [19–21]. In a study by Rothenbuehler et al. initial treatment consisted of 1 ranibizumab injection [22]; thereafter, all patients had follow-up examinations at monthly intervals as suggested by the MARINA and ANCHOR trials. Retreatment was performed monthly if indicated based of CNV activity in OCT, FA and ophthalmology examination with VA evaluation. In spite of using only 1 initial dose of ranibizumab, but with systematic control visits each month, after 24 months 30% of 129 treated eyes gained 15 or more letters. The mean change in BCVA at 24 months was +6.3±14.5 letters. Mean injection number per patient was 5.6±2.9 from baseline to month 12 and 4.3±3.8 from month 12 to month 24. Arias et al reported a case series study of 90 eyes that were initially treated with 3 consecutive monthly intravitreal injections of ranibizumab, and thereafter follow-up visits were progressively spread out to a maximum of 8 weeks apart [23]. Median VA improved from 56 letters at baseline to 60 letters at 12 months, with significant reduction in foveal thickness. The mean number of injections was 4.4 and the number of visits was 8.0; 40% of patients received 3 injections and 60% received more than 3 injections. In this study no significant association was observed between VA improvement and the number of injections (the same as in the PrONTO study). Like our study, Arias at al confirmed that a flexible regimen with ranibizumab therapy is efficacious and safe in patients with neovascular AMD, but reducing the burden of injections correlates here with reducing follow-up visits (fewer injections, control visits, and less effective in improving VA than in our study). In a short 6-month study Kloos et al reported no significant improvement in VA for classic CNV (42/195 eyes) +0.87 Snellen chart lines in patients treated with repeated intravitreal injections of ranibizumab as needed[24]. Better results were obtained in the occult or minimally classic lesions subgroup; 45% of eyes with predominantly classic CNV had received photodynamic therapies prior to the injections, and MR (SZB reading chart) remained stable in 80% over 6 months. Rotsos et al described a retrospective analysis of the 50 patients with neovascular AMD (classic form 10%) treated with ranibizumab [25]. The mean number of injections was 4.7 per 12 month period, with 26% gaining 15 letters or more, and mean change in VA was +4.6±2.2 letters. It is difficult to compare those results with ours because of the small subgroup with classic CNV and heterogenous qualifications criterions (some eyes were treated previously).
In our study 3D retinal topography full field 29.2 with high quality and high resolution frames was done in each patient at baseline and every month. We analyzed and used for statistical analysis parameters from macular volume and thickness maps. We also performed radial scans and reviewed them for presence of subretinal fluid, intraretinal edema, fluid under the retinal pigment epithelium. We noted mean correlations between baseline average CRT and baseline average VA measured in ETDRS letters (p=0.017) and in logMAR scale (p=0.033) and between average CRT after the third injection and average VA in logMAR scale after the third injection (p=0.047). Kumar et al did not observe statistically significant correlations between CRT and VA, finding only a slight increase in the number of patients with CRT over 200 μm at the 9-month visit corresponding to the drop in vision [16]. In the PrONTO study, statistically significant correlations were found between the OCT-CRT measurements and VA at months 2, 3 and 12 [12]. Another strategy in the PrONTO study was to examine the association between OCT changes at month 1 with VA changes thereafter to determine if OCT improvements could serve as a predictor of future VA improvements. Statistically significant correlations were detected when the OCT-CRT measurements at month 1 were correlated with the VA changes at month 2 [12]. Fung et al described significant correlations between the decrease in CRT at 1 month and subsequent improvement in VA seen at months 2 and 3 [13]. In addition, significant correlations were identified between the change in thickness at months 2 and 3 and VA at months 2 and 3. Rothenbuehler et al. did not observe significant correlation of morphological changes like subretinal fluid, intraretinal edema or sub-RPE fluid on the crosshair OCT scans to the visual outcome at months 12 and 24 [22]. After treatment with anti-VEGF agents, the recurrence of subretinal fluid precedes the visual loss [26]. The immediate effect of intravitreal ranibizumab on retinal anatomy, as seen by standard OCT technology, was proven by Fung et al., who reported a resolution of sub- and intraretinal fluid [13]. Kiss et al. described that ranibizumab rapidly diminishes the leakage activity of the neovascular net, and slower pace has a direct impact on the morphology of the RPE lesion as the subretinal lesion progressively flattens and the abnormal RPE area decreased in size during the initial treatment phase when visual function is positively affected in 80% of treated eyes [8,27]. Dadgostar et al. reported that the resolution of fluid is not always correlated with improvement in vision [28]. Visual recovery after resolution of macular fluid in wet AMD likely depends on many variables, including chronicity of disease, viability of photoreceptors and the RPE, progression of the underlying dry abnormalities, and the presence of epiretinal membranes, RPE tears, and fibrosis [12,29]. Framme et al. used spectral domain-optical coherence tomography (SD-OCT) to image CNV structure before and after anti-VEGF treatment. CNV (classic 16/78, occult 54/78, minimal classic 8/78) was imaged before and 4 weeks after anti-VEGF upload in 3 intravitreal injections of ranibizumab [30]; 59% of all post-treatment OCTs reveled complete dry retinal structures, 27% showed reduced edema, and 14% showed edema remaining unchanged. Macular thickness reduction was significantly enhanced, especially in CNV with classic components. In this study ranibizumab monotherapy was able to morphologically stop further CNV growth but in most patients did not lead to major regression of CNV, especially of its occult components.
Conclusions
The issue of how to deal with the increasing number of intravitreal injections in everyday practice is very important. The main goal is to improve vision and maintain this improvement at a maximum over time, with a minimum of inconvenience for patients (mostly elderly) and the treating physician. At the moment, it seems the reduction of numbers of injections to 6–8 per year in the first year will maintain visual gain. Nevertheless, at the same time, monthly controls seem to be necessary to be able to react quickly to the smallest signs of deterioration – this does not only mean deterioration in visual acuity, but also fluid increase in OCT. It seems that if visual acuity is once lost, complete recovery is no longer possible. Treatment with intravitreal ranibizumab injections according to the scheme of saturation phase followed by re-treatment based on OCT image and VA, provides AMD patients with a chance of stabilization and even improvement of topical state with a lower number of injections and preserved topical and general safety.
Footnotes
Source of support: Departmental sources
References
- 1.Krejca M, Plewska A, Szmagala P, et al. Outside stenting of the vein graft decreases VEGF-A expression and induces significant down-regulation of VEGFR-1 in the intimal and medial layers after the re-endothelization period. Med Sci Monit. 2010;16(3):BR89–96. [PubMed] [Google Scholar]
- 2.Czarkowska-Paczek B, Zendzian-Piotrowska M, Bartlomiejczyk I, et al. Skeletal and heart muscle expression of PDGF-A and VEGF-A after an acute bout of exercise and endurance training in rats. Med Sci Monit. 2010;16(5):BR147–53. [PubMed] [Google Scholar]
- 3.Lowe J. RhuFav V2 inhibits VEGF-isoforms stimulated HUVEC proliferation. Ophthalmol Vis Sci. 2003;44:1828–29. [Google Scholar]
- 4.Keyt BA, Nguyen HV, Berleau LT, et al. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem. 1996;271:5638–46. doi: 10.1074/jbc.271.10.5638. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Wiesmann C, Fuh G, et al. Selection and analysis of an optimized anti-VEGF antibody: Crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293:865–81. doi: 10.1006/jmbi.1999.3192. [DOI] [PubMed] [Google Scholar]
- 6.Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal ranibizumab (Lucentis) Ophthalmology. 2007;114:2179–82. doi: 10.1016/j.ophtha.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Brown DM, Kaiser PK, Michels M, et al. for the Anchor Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 9.Boyer DS, Antoszyk AN, Awh CC. Subgroup analisis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:246–52. doi: 10.1016/j.ophtha.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 10.Raja MSA, Saldana M, Goldsmith C, et al. Ranibizumab treatment for neovascular age-related macular degeneration in patients with good baseline visual acuity (better than 6/12): 12-month outcomes. Br J Ophthalmol. 2010;94:1543–45. doi: 10.1136/bjo.2009.174763. [DOI] [PubMed] [Google Scholar]
- 11.Regillo CD, Brown DM, Abraham P. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145:239–48. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO study. Am J Ophthalmol. 2009;148:43–53. doi: 10.1016/j.ajo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Fung AE, Lalwani GA, Rosenfeld PJ. An optical coherence tomography – guided, variable dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–83. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Meyer CH, Eter N, Holz FG. Ranibizumab in patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Interim results from the SUSTAIN trial (abstract) Invest Ophthalmol Vis Sci. 2008:49. [Google Scholar]
- 15.Schmidt-Erfurth UM, Richard G, Augustin A, et al. Guidance for the treatment of neovascular age-related macular degeneration. Acta Ophthalmol Scand. 2007;85:486–94. doi: 10.1111/j.1600-0420.2007.00979.x. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Sahni JN, Stangos AN, et al. Effectiveness of ranibizumab for neovascular age-related macular degeneration using clinical-determined retreatment strategy. Br J Ophthalmol. 2011;95:530–33. doi: 10.1136/bjo.2009.171868. [DOI] [PubMed] [Google Scholar]
- 17.Bandukwala T, Muni RH, Schwartz C, et al. Effectiveness of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration in a Canadian retina practice: a retrospective review. Can J Ophthalmol. 2010;45:590–95. doi: 10.3129/i10-082. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SY, Dubois L, Tadayoni R, et al. Results of one year’s treatment with ranibizumab for exudative age-related macular degeneration in a clinical setting. Am J Ophthalmol. 2009;148:409–13. doi: 10.1016/j.ajo.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell P, Korobelnik JF, Lanzetta P, et al. Ranibizumab (Lucentis) in neovascular age-related macular degeneration: evidence from clinical trials. Br J Ophthalmol. 2010;94:2–13. doi: 10.1136/bjo.2009.159160. [DOI] [PubMed] [Google Scholar]
- 20.Spaide RF. The as-needed treatment strategy for choroidal neovascularization: a feedback-based treatment system. Am J Ophthalmol. 2009;148:1–3. doi: 10.1016/j.ajo.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Spaide RF. Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol. 2007;143:679–80. doi: 10.1016/j.ajo.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Rothenbuehler SP, Waeber D, Brinkmann ChK, et al. Effect of ranibizumab in patients with subfoveal choroidal neovascularization attributable to age-related macular degeneration. Am J Ophthalmol. 2009;147:831–37. doi: 10.1016/j.ajo.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Arias L, Roman I, Masuet-Aumatell C, et al. One-year results of a flexible regimen with ranibizumab therapy in macular degeneration: relationship with the number of injections. Retina. 2011;X:1–7. doi: 10.1097/IAE.0b013e318207d152. [DOI] [PubMed] [Google Scholar]
- 24.Kloos P, Bernasconi P, Estermann S, et al. Visual acuity and magnification requirement after ranibizumab in patients with wet age-related macular degeneration. Klin Monbl Augenheilkd. 2008;225:385–91. doi: 10.1055/s-2008-1027252. [DOI] [PubMed] [Google Scholar]
- 25.Rotsos T, Patel PJ, Chen FK, Tufail A. Initial clinical experience of ranibizumab therapy for neovascular age-related macular degeneration. Clinical Ophthalmology. 2010;4:1271–75. doi: 10.2147/OPTH.S14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for noevascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–35. [PubMed] [Google Scholar]
- 27.Kiss ChG, Geitzenauer W, Simader Ch, et al. Evaluation of ranibizumab-induced changes in high-resolution opical coherence tomographic retinal morphology and their impact on visual function. Investigative Ophthalmology & Visual Science. 2009;50:2376–83. doi: 10.1167/iovs.08-2017. [DOI] [PubMed] [Google Scholar]
- 28.Dadgostar H, Ventura AA, Chung JY, et al. Evaluation of injection frequency and visual acuity outcomes for ranibizumab monotherapy in exudative age-related macular degeneration. Ophthalmology. 2009;116:1740–47. doi: 10.1016/j.ophtha.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 29.Holz FG, Bindewald-Wittich A, Fleckenstein M, et al. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143:463–72. doi: 10.1016/j.ajo.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 30.Framme C, Panagakis G, Birngruber R. Effects on choroidal neovascularization after anti-VEGF upload using intravitreal ranibizumab, as determined by spectral domain-optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51:1671–76. doi: 10.1167/iovs.09-4496. [DOI] [PubMed] [Google Scholar]