Summary
Background
Androgen abuse is an increasing problem amongst professional and amateur athletes. Moreover, testosterone, apart from its widely accepted indications, is used for a variety of other indications such as aging and ischemia. Its actions are mainly attributed to a specific genomic mechanism through the androgen receptor, but emerging evidence reveals non-genomic effects as well. The use of androgens has been linked with several adverse effects. The purpose of this study was to examine the effects of testosterone on the morphology and the ultrastructure of the myocardium and to investigate the possible role of apoptosis.
Material/Methods
We used 12 adult male Wistar rats, separated into 2 groups. Group A consisted of 6 rats that were administered high doses of testosterone enanthate, while group B consisted of 6 male Wistar rats that received placebo (normal saline) intramuscularly. After the last day of treatment, all rats were anesthetized and sacrificed, and the hearts were removed and processed for optical and electron microscopy and immunohistochemical detection of caspase-3, an apoptosis marker.
Results
We found significant myocardial hypertrophy along with abundant ultrastructural alterations. The immunohistochemical staining of the myocardial cells for caspase-3 was positive in group A (experimental group), which is interpreted as an activation of apoptosis by testosterone treatment.
Conclusions
Testosterone abuse has serious adverse effects, including myocardial hypertrophy, myocardial fibrosis and activation of apoptosis. These findings need to be taken into account whenever androgens are prescribed to improve performance or as hormone therapy.
Keywords: myocardial hypertrophy, mitochondrial edema, apoptosis, androgen abuse
Background
Testosterone is the main androgen hormone and exerts its physiological effects through a genomic mechanism that is mediated by the androgen receptor [1]. However, a non-genomic pathway has recently been suggested [2]. These non-genomic effects are considered to be rapid, in contrast to the genomic effects, and several possible pathways have been identified. The activation of myocardial inflammation signaling enzymes of the MAPK family, mediated by the androgen receptor, has been documented, but interestingly this activation is not related to the receptor’s transcriptional activity [3,4].
Testosterone and its related androgens has significant anabolic and androgenic effects. Since its molecular identification, testosterone has been used as a therapy for hormone replacement, infertility and libido dysfunction, as well as an anti-aging agent [5,6]. The abuse of androgens for improvement of performance has become a common, yet serious, condition that may have detrimental effects on the athletes. Testosterone abuse has been linked to an increase of cardiovascular adverse events such as significant cardiac hypertrophy and myocardial infarction, virilization of women, prostate hyperplasia in men [6,7], liver hypertrophy and hepatocellular adenomas or carcinomas [8–12], apoptotic death of myocardial [13,14] and neuronal cells [15]. On the other hand, recent investigations have shown that testosterone might have anti-ischemic effects [16,17] and possibly inhibits plaque development [18,19].
The aim of this study was to examine the effect of testosterone administration at the ultrastructural level of the rat myocardium, as well as to investigate whether testosterone abuse can induce apoptosis. We used high doses of testosterone enanthate for a long period of time mimicking androgen abuse. The hypothesis was that testosterone induces hypertrophy, fibrosis and apoptosis of cardiac cells.
Material and Methods
All procedures for animal use were in accordance with the guidelines of the Greek Government and the Bioethics Committee of the Medical School of the Aristotle University of Thessaloniki. Every possible measure was taken to minimize pain or discomfort of treated animals.
We used adult male Wistar rats (weighing between 350–400 g) separated into 2 groups. Group A (experimental) consisted of 6 rats that received 10 mg testosterone enanthate (which equals 7. 5mg testosterone) intramuscularly for 20 consecutive days, while group B (control group) included 6 rats that were given normal saline via the same route. Having completed the administration period, all rats were anaesthetized and sacrificed and their hearts were removed. Sections from the hearts were placed in 10% formalin for light microscopy and glutaraldehyde 2.5% for electron microscopy.
Myocardial tissue samples designated for light microscopy study were dehydrated through a series of increasing ethanol concentrations (25%, 50%, 70%, 80%, 96% and absolute) and finally cleared with xylene. Then, the samples were embedded in paraffin wax and sections of 5 μm were cut and stained with hematoxylin and eosin. We also used Masson trichrome staining on heart sections to evaluate cardiac fibrosis.
The myocardial tissue samples for electron microscopy were sectioned into small (<1 cm3) pieces and were placed into glutaraldehyde 2.5% for 2 hours and then into osmium tetraoxide 1% for 1 hour. This was followed by staining with uranyl acetate 1% for 16 hours, dehydration with advancing ethanol concentrations and intubation into Epon resin. Subsequently, ultra-thin sections (600–900 Å) were taken and stained with lead citrate (Reynolds’s stain) and studied using a JOEL transition electron microscope. We used both qualitative and quantitative analyses which were conducted by 3 of the authors, blinded to the experimental groups.
In order to examine the possible activation of apoptosis, we used the high temperature antigen unmasking immunohistochemical technique. Mouse monoclonal antibodies (NCL-CPP32, Novocastra) against caspase-3 (CCP32) were incubated with sections of myocardial tissue after appropriate processing. The paraffin sections were dewaxed, rehydrated and placed in 0.5% hydrogen peroxide/methanol. After washing with tap water, the sections were placed into high temperature and high pressure unmasking solution (0.01 M citrate buffer, pH 6.0) for 1 minute. The sections subsequently were placed into a bath of tap water, washed with TBS buffer and placed in diluted normal serum. Then, the sections were incubated with primary (mouse anti-rat-antibody) and secondary biotinylated antibody. The final steps included incubation in ABC reagent and in DAB, counter-staining with hematoxylin and dehydration.
Statistical analysis
The quantitative evaluation was made from photomicrographs of the myocardial tissue samples from each group by 1 of the authors (V.P.) blinded to the experimental groups. The area of fibrosis was quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA), and the fibrosis ratio was calculated by dividing the area of fibrosis by the total myocardial area. The dimensions and areas of mitochondria were measured with the use of ImageJ and were expressed as mean ± SE for each animal group. The Mann-Whitney U-test was used for comparisons between the 2 groups. Data was analyzed using PASW Statistics 18, Release Version 18.0.0, 2009 (SPSS, Inc., Chicago, IL). A value of p<0.05 was considered statistically significant. Finally, a quantitative measurement of the immunohistochemical staining for caspase-3 was performed using the ImageJ software.
Results
Optical study
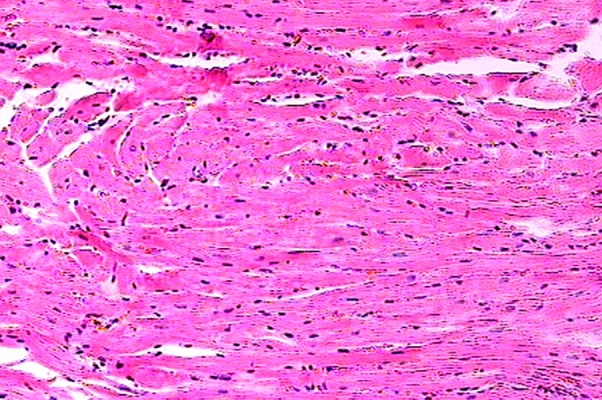
We observed significant myocardial hypertrophy of rats that received testosterone (Figure 1), whereas the myocardium of control rats appeared normal, without signs of hypertrophy (Figure 2).
Figure 1.

Significant myocardial hypertrophy (experimental Group A). Hematoxylin-Eosin ×25.
Figure 2.
The myocardial section from group B (control group) appears normal. Hematoxylin-eosin ×25.
Electron microscopy
Significant alterations were observed in the myocardial cells and the endothelial cells of the heart capillaries. We found edematous mitochondria in the myocardial cells of the experimental group along with some degree of intracellular edema (Figure 3). On the other hand, the mitochondria of the control group appeared to be normal (Figure 4). In order to evaluate any differences quantitatively, we measured and analyzed the shape and size characteristics of mitochondria of length exceeding 1.0 μm. The maximum length found was 7.96 μm.
Figure 3.
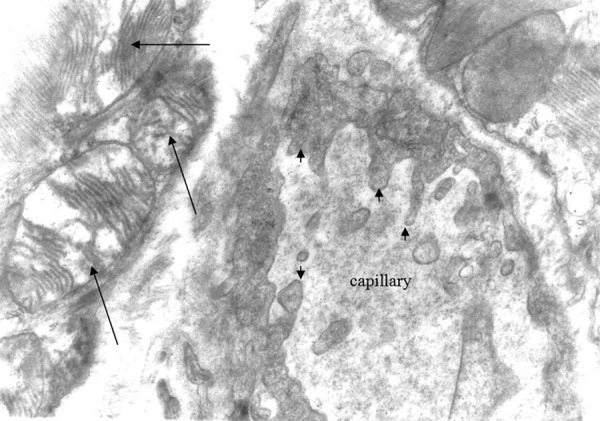
Mitochondria of oedematous morphology (large arrows) and endothelial cytoplasmic foot processes Group A ×12000.
Figure 4.

The appearance of the myofibrils and the mitochondria is normal. Group B (control rats) ×15000.
We measured and compared the area (A), the length (L), the width (W) of the equivalent (same area) ellipse W=A/(πL) and the elongation (L/W) of 219 mitochondria in the experimental group and 23 mitochondria in the control group. The results presented in Table 1, show that the area and the width of the mitochondria were larger for the experimental group, whereas the length and the elongation were larger for the control group. This shows that the mitochondria in the experimental group are larger and more rounded. It is indicative that the top 5 values of the area of the mitochondria in the experimental group (9.68–30.39 μm2) were all higher than the highest value of the corresponding area in the control group (7.67 μm2); however, the difference was non-significant (p=0.93), as were the differences in length (p=0.30) and width (p=0.42). On the other hand, the difference in elongation between the 2 groups was statistically significant (p=0.04).
Table 1.
Shape and size characteristics of mitochondria.
| Parameter | Group | Mean | SEM | Min | Max | Median | IQ range | Significance M-W test |
|---|---|---|---|---|---|---|---|---|
| Area (μm2) | Experimental | 2.35 | 0.21 | 0.23 | 30.39 | 1.87 | 0.91–2.83 | Z=−0.086 |
| Control | 2.17 | 0.34 | 0.89 | 7.67 | 1.61 | 1.16–2.24 | p=0.931 | |
| Length (μm) | Experimental | 2.12 | 0.06 | 1.00 | 7.96 | 1.99 | 1.42–2.53 | Z=0–1.035 |
| Control | 2.39 | 0.21 | 1.30 | 4.80 | 2.00 | 1.64–2.65 | p=0.301 | |
| Width (μm) | Experimental | 1.23 | 0.06 | 0.29 | 9.98 | 1.11 | 0.80–1.50 | Z=−0.803 |
| Control | 1.08 | 0.07 | 0.65 | 2.03 | 0.98 | 0.85–1.22 | p=0.422 | |
| Elongation | Experimental | 1.94 | 0.06 | 0.20 | 8.42 | 1.71 | 1.42–2.24 | Z=−2.034 |
| Control | 2.25 | 0.17 | 1.25 | 4.19 | 1.97 | 1.50–2.88 | p=0.042 |
SEM – Standard error of mean; IQ range – Inter-quartile range; M-W test – Mann-Whitney test. Experimental group n=8, control group n=4.
There was also disarrangement of the sarcomeres, with disorganization of the myofibrils and the Z discus (Figure 5). Between the capillaries and the myocardial cells we observed collagen fibers, which are an indication of myocardial fibrosis (Figures 6, 7). The nuclei in some endothelial cells had irregular shape (Figure 7). Endothelial cytoplasmic foot processes were abundant (Figure 3). A large number of pinocytoplasmic cysts inside the endothelial cytoplasm was observed (Figures 5,8). The appearance of the myofibrils and the mitochondria was normal in the control rats (Figure 4).
Figure 5.
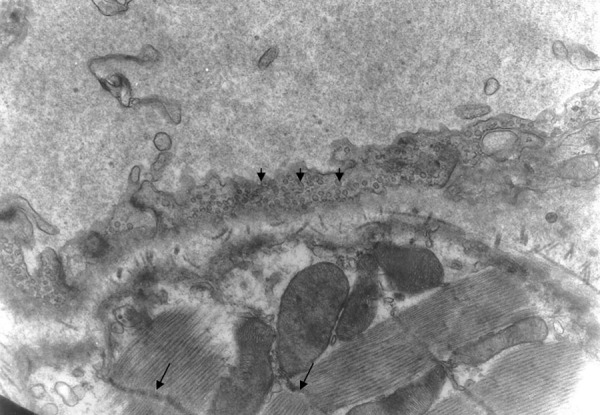
The Z band has curved shape (arrows). There are numerous pinocytoplasmic cysts (small arrows) inside the cytoplasm of the endothelial cell. Group A ×10000.
Figure 6.
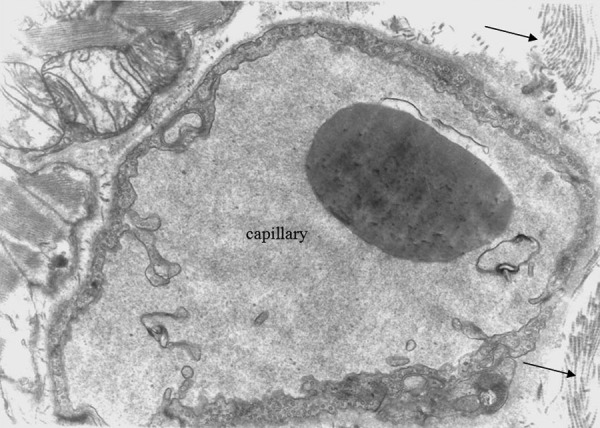
Oedematous mitochondria, collagen fibres (arrows). There is some degree of intracellular oedema. Group A ×8000.
Figure 7.
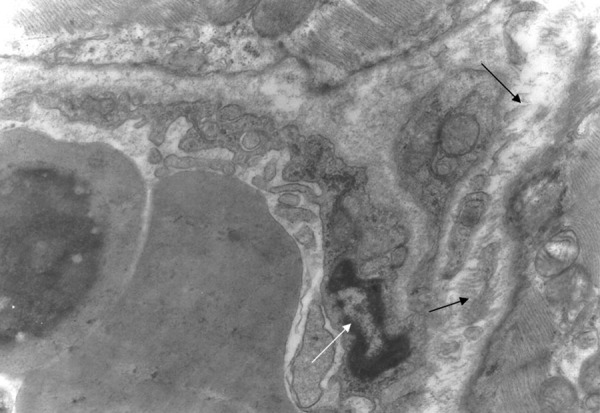
Between the capillary wall and myocardial cell there are collagen fibres (arrows). The nucleus of the endothelial cell is irregularly shaped (white arrow). Group A ×10000.
Figure 8.
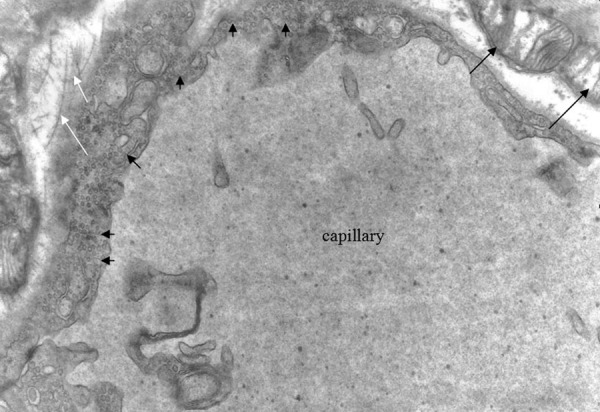
Oedematous mitochondria (black arrows), collagen fibres (white arrows). The abundance of pinocytoplasmic cysts is easily noticeable (small arrows). Group A ×10000.
Immunohistochemical study
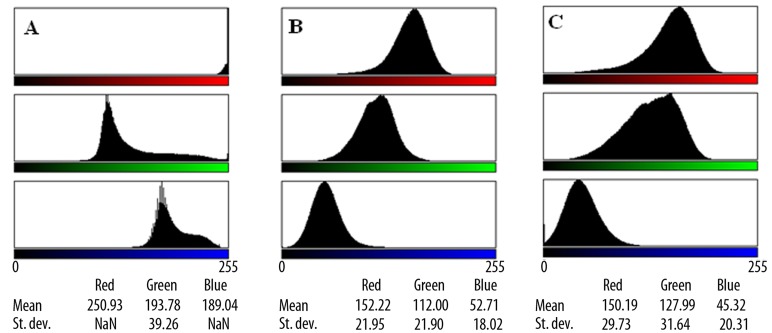
Myocardial immunohistochemical staining for caspase-3 was negative for the rats in group B (control group) (Figure 9). On the other hand, the myocardial tissue of the rats which received testosterone (group A) exhibited significantly positive staining for caspase-3, which indicates the detection of apoptosis (Figures 10, 11). The differences are presented in Figure 12, in the form of RGB color histograms, determined by the use of ImageJ software. To quantify the differences, we calculated the Delta-E (ΔE76), as defined by the International Commission on Illumination (CIE), after conversion of the mean RGB values of each image into L*, a* and b* values in the L*a*b* color space [20]. The values of ΔE76 between the control and experimental groups were found to be 63.0 and 74.3, for Figures 9, 10 and 9–11, respectively, and only 13.7 between Figures 10, 11, which correspond to experimental group images. A value of ΔE76 approximately equal to 2.3 corresponds to a JND (just noticeable difference).
Figure 9.
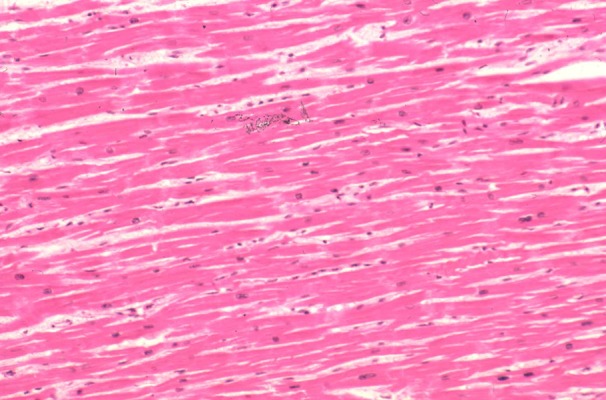
Negative staining of rat myocardial cells of group B. No apoptosis detected in the control rats. ×125.
Figure 10.
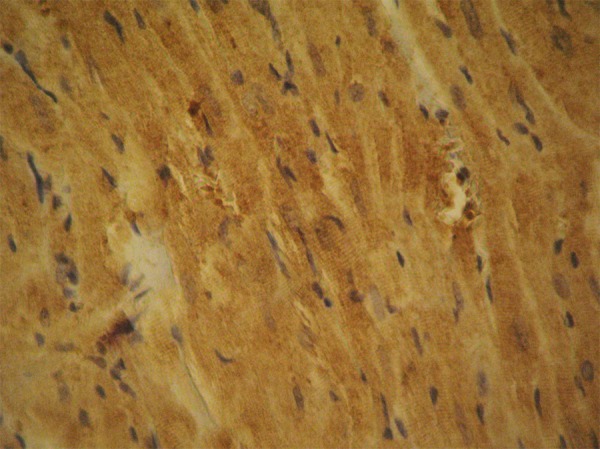
Positive myocardial staining for caspase-3. This is an indication of the activation of the apoptosis cascade after the administration of testosterone in rats of group A. ×480.
Figure 11.

Positive immunostaining of myocardial cells for caspase-3. Group A. ×1200.
Figure 12.
RGB colour histograms of microphotographs showing myocardial immunohistochemical staining for caspase-3, determined by ImageJ. (A) Rat myocardial cell in control rats, corresponding to Figures 9. (B, C) Rat myocardial cells in the experimental group, after the administration of testosterone, corresponding to Figures 10, 11.
Masson’s trichrome staining for collagen fibers
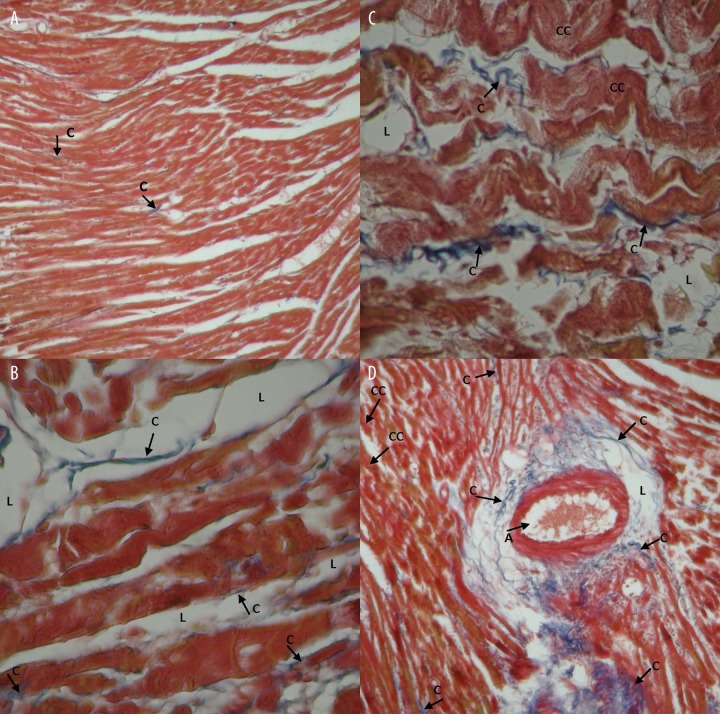
Masson trichrome staining showed traces of local collagen fibrils (connective tissue) among the cardiac cells in the control group. Figure 13A shows Masson’s trichrome staining of a representative heart tissue section from a rat of the control group. The amount of collagen fibrils in the experimental group that received testosterone was higher compared to the control group.
Figure 13.
Myocardial fibrosis induced by testosterone and evaluated by Masson trichrome staining. (A) Indicative heart section from rat of the control group (4×10), fibrosis ratio 1.11±0.15%. (B, C) Heart sections from the experimental group received testosterone (4×40), fibrosis ratio 3.92±0.05% (p<0.01 between the two groups). (D) section of cardiac vein from the experimental group (4×10), fibrosis ratio 4.22±0.32% (p<0.01 between the two groups). Each group n=6. C: Colagen, CC: Cardiac cells, L: lipids, A: Artery.
In sections of cardiac veins, thickening of the muscular tunic with many foamy cells was observed as well as many collagen fibrils in the outer vascular tunica, surrounded by thick adipose tissue (Figure 13D). The presence of adipose tissue was evident around the arterioles wall as well. Within the adipose tissue a number of collagen fibrils were observed. The control group did not show similar characteristics.
Discussion
Androgen abuse has been linked to several serious adverse cardiovascular events. Cardiac arrhythmias, QT dispersion, atrial fibrillation, myocardial infarction, heart failure and atherogenesis have all been linked to androgen abuse by athletes [14]. Melchert and Welder proposed 4 hypothetical models of anabolic-induced adverse cardiovascular effects [21,22]:
an ‘atherogenic’ model involving the effects of androgen-anabolic steroids (AASs) on lipoprotein concentrations;
a ‘thrombogenic’ model involving the effects of AASs on clotting factors and platelets;
a ‘vasospasm’ model involving the effects of AASs on the vascular nitric oxide system;
a ‘direct myocardial injury’ model involving the effects of AASs on myocardial cells.
Our study shows that the administration of testosterone in high doses exerts toxic effects on the myocardial cells. This probably correlates with the direct myocardial injury model of the effects of androgen-anabolic steroids on the myocardial cells. The mitochondria are particularly damaged and appeared edematous, with diminished cristae. The morphometric approach showed that the mitochondria of the experimental group were larger and more rounded.
The contractile apparatus showed signs of deterioration, with disorganization of the sarcomeres and the Z discus. These observations have been reported earlier and have been characterized as typical for early heart failure [23,24].
Another interesting finding of this study, using the Masson’s trichrome staining, was the presence of collagen fibrils inside the myocardium and particularly between the capillaries and the myocardium cells. This perivascular myocardial fibrosis, together with the hypertrophy that was also induced, may be the cause of myocardial ischemia [25,26] as well as a substrate for arrhythmias [27,28]. The presence of collagen was not noted in the hearts of the control rats. The collagen may increase the stiffness of the myocardium and reduce its compliance. Crisostomo et al. [3] reported that acute exposure of hearts to testosterone significantly reduces the −dP/dt (a measure of cardiac compliance).
The myocardial hypertrophy observed in the present study and also in our previous experiments [29], as well as by other researchers [30,31], has a significant role in the testosterone-induced reduction of myocardial compliance. The hypertrophy of the myocardium correlates with the enhanced expression of the androgen receptor after testosterone administration [29]. Myocardial hypertrophy is also associated with myocardial dysfunction due to abnormal intracellular calcium cycling [32]. Abnormalities of the circulating levels of other hormones may induce adverse cardiovascular effects. Increased levels of the protein hormone leptin have been found to induce cardiac hypertrophy although diastolic dysfunction was not associated with leptin levels [33]. Another set of hormones, the thyroid hormones, do have an impact on the myocardial diastolic properties among other various cardiovascular effects [34].
An unusually large number of micropinocytic vesicles was observed in the cytoplasm of the endothelial cells. Although it is common for these vesicles to occur in capillary endothelial cells, especially in the striated muscles, the abundance of such vesicles is a sign of heightened pinocytic activity that permits the cell to receive substances through the cell membrane [35].
Apoptosis is the programmed cell death and is mediated by 2 pathways: the extrinsic death receptor signaling pathway and the intrinsic mitochondrial control pathway [36,37]. Caspases exert significant action in both pathways. Caspase-3 (CPP 32) is a member of the interleukin-1 beta-converting enzyme (ICE) family of mammalian proteases that specifically cleaves substrates at the C-terminal side of aspartic residues. Members of this family have been implicated in apoptosis, and caspase-3 acts as a control mediator of programmed cell death in mammalian cells. Caspase-3 is synthesized as an inactive 32kD proenzyme and is processed during apoptosis to its active form, which is responsible for the cleavage of poly (ADP-ribose) polymerase (PARP), actin and sterol regulatory element binding protein (SREDP) [38–40]. In the present study, testosterone overdosing significantly activated apoptosis, as was clearly seen by immunohistochemistry. The staining for caspase-3 was negative in the control rats. Although it has been reported that apoptosis activated by testosterone enanthate is due to the ester [21], experimental exposure of myocardial cells to enanthate alone did not activate apoptosis [14]. Apoptosis causes the loss of myocardial cells and ultimately the depression of myocardial performance. The abuse of androgen anabolic substances has been causally linked with sudden cardiac death, myocardial infarction, ventricular remodeling and cardiomyopathy. These events are related to the activation of apoptosis due to AASs abuse. Myocardial death without coronary vessel disease or atherosclerosis has also been attributed to the activation of apoptosis by AASs [41,42].
Conclusions
This study showed that testosterone abuse produces significant myocardial hypertrophy and fibrosis as well as of the myofibrils, the mitochondria and the capillaries. The activation of apoptosis was a significant finding that indicates the direct myocardial injury caused by testosterone. As testosterone is now used for a variety of possible indications such as hormone replacement in the elderly or as an antianginal in cardiac patients, more research is required to clarify the exact biochemical route of action as well as the correct dose to prevent adverse effects.
Footnotes
Source of support: Departmental sources
References
- 1.Magee JA, Chang LW, Stormo GA, Milbrandt J. Direct Androgen Receptor-Mediated Regulation of the FKBP5 Gene via a Distal Enhancer Element. Endocrinology. 2006;147(1):590–98. doi: 10.1210/en.2005-1001. [DOI] [PubMed] [Google Scholar]
- 2.Mendelsohn ME, Karas RH. Molecular and Cellular Basis of Cardiovascular Gender Differences. Science. 2005;308(5728):1583–87. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 3.Crisostomo PR, Wang, Wairiuko GM, et al. Brief exposure to exogenous testosterone increases death signaling and adversely affects myocardial function after ischemia. Am J Physiol Regul Integr Comp Physiol. 2006;290:1168–74. doi: 10.1152/ajpregu.00833.2005. [DOI] [PubMed] [Google Scholar]
- 4.Kousteni S, Bellido T, Plotkin LI, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–30. [PubMed] [Google Scholar]
- 5.Heutling D, Lehnert H. Hormone therapy and anti-aging: is there an indication? Internist (Berl) 2008;49(5):570, 572–76, 578–79. doi: 10.1007/s00108-008-2110-3. [DOI] [PubMed] [Google Scholar]
- 6.Payne JR, Kotwinski, Montogomery HE. Cardiac effects of anabolic steroids. Heart. 2004;90473(5):475. doi: 10.1136/hrt.2003.025783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appel HJ, Heller-Umpfenbach B, Feraudi M, Weicker H. Ultrastructural and morphometric investigations on the effect of training and administration of anabolic steroids on the myocardium of guinea pigs. International J Sport Medicine. 1983;4(4):268–74. doi: 10.1055/s-2008-1026048. [DOI] [PubMed] [Google Scholar]
- 8.Lin MC, Wu CC, Cheng SB, et al. The Influence of High Serum Testosterone Levels on the Long-term Prognosis in Male Patients Undergoing Hepatectomy for Early Stage Hepatocellular Carcinoma without Vascular Invasion. World J Surg. 2007;31(7):1469–73. doi: 10.1007/s00268-007-9094-3. [DOI] [PubMed] [Google Scholar]
- 9.Gorayski P, Thompson CH, Subhash HS, Thomas AC. Hepatocellular carcinoma associated with recreational anabolic steroid use. Br J Sports Med. 2008;42:74–75. doi: 10.1136/bjsm.2007.03932. [DOI] [PubMed] [Google Scholar]
- 10.Socas L, Zumbado M, Pérez-Luzardo, et al. Hepatocellular adenomas associated with anabolic androgenic steroid abuse in bodybuilders: a report of two cases and a review of the literature. Br J Sports Med. 2005;39:27–31. doi: 10.1136/bjsm.2004.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe S, Kobayashi Y. Exogenous hormones and human cancer. Japan J Clin Oncol. 1993;23:1–3. [PubMed] [Google Scholar]
- 12.Nagashe N, Kohno H. Hepatocellular carcinoma and sex hormones. HPB Surg. 1992;6:1–6. doi: 10.1155/1992/72761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Ascenzo S, Millimaggi D, Di Massimo C, et al. Detrimental effects of anabolic steroids on human endothelial cells. Toxicol Lett. 2007;169:129–36. doi: 10.1016/j.toxlet.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Zaugg M, Jamali NZ, Lucchinetti E, et al. Anabolic – androgenic steroids induce apoptotic cell death in adult rat ventricular myocytes. J Cell Physiol. 2001;187:90–95. doi: 10.1002/1097-4652(2001)9999:9999<00::AID-JCP1057>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 15.Estradab M, Varshney A, Ehrlich BE. Elevated previous testosterone induces apoptosis in neuronal cells. J Biol Chem. 2006;281:25492–501. doi: 10.1074/jbc.M603193200. [DOI] [PubMed] [Google Scholar]
- 16.Cornoldia A, Caminitia G, Marazzia G, et al. Effects of chronic testosterone administration on myocardial ischemia, lipid metabolism and insulin resistance in elderly male diabetic patients with coronary artery disease. Int J Cardiol. 2010;142(1):50–55. doi: 10.1016/j.ijcard.2008.12.107. [DOI] [PubMed] [Google Scholar]
- 17.English KM, Steeds RP, Jones TH, et al. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation. 2000;102:1906–11. doi: 10.1161/01.cir.102.16.1906. [DOI] [PubMed] [Google Scholar]
- 18.Tharp DL, Masseau I, Ivey I, et al. Endogenous testosterone attenuates neointima formation after moderate coronary balloon injury in male swine. Cardiovascular Res. 2009;82(1):152–60. doi: 10.1093/cvr/cvp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanke H, Lenz C, Hess B, et al. Effect of Testosterone on Plaque Development and Androgen Receptor Expression in the Arterial Vessel Wall. Circulation. 2001;103:1382–85. doi: 10.1161/01.cir.103.10.1382. [DOI] [PubMed] [Google Scholar]
- 20.Color Difference. http://en.wikipedia.org/wiki/Color_difference(Retrieved May 2, 2011)
- 21.Melchert RB, Welder AA. Cardiovascular effects of androgenic-anabolic steroids. Med Sci Sports Exerc. 1995;27:1252–62. [PubMed] [Google Scholar]
- 22.Deligiannis A, Björnstad H, Francois C, et al. ESC Study Group of Sports Cardiology Position Paper on adverse cardiovascular effects of doping in athletes. Eur J Cardiovascular Prev Reh. 2006;13(5):667–94. doi: 10.1097/01.hjr.0000224482.95597.7a. [DOI] [PubMed] [Google Scholar]
- 23.Behrendt H, Boffin H. Myocardial cell lesions caused by an anabolic hormone. Cell Tissue Research. 1977;181:423–26. doi: 10.1007/BF00223116. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Das B, Sen S. Cardiac hypertrophy: Mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2007;9(6):623–52. doi: 10.1089/ars.2007.1474. [DOI] [PubMed] [Google Scholar]
- 25.Nascimento JH, Medei E. Cardiac Effects of Anabolic Steroids: Hypertrophy, Ischemia and Electrical Remodelling as Potential Triggers of Sudden Death. Mini Rev Med Chem. 2011;11(5):425–29. doi: 10.2174/138955711795445899. [DOI] [PubMed] [Google Scholar]
- 26.Willenheimer R. Left ventricular remodelling and dysfunction. Can the process be prevented? Int J Cardiol. 2000;72(2):143–50. doi: 10.1016/s0167-5273(99)00182-5. [DOI] [PubMed] [Google Scholar]
- 27.Weber KT. Brilla Factors associated with reactive and reparative fibrosis of the myocardium. CG Basic Res Cardiol. 1992;87(Suppl 1):291–301. doi: 10.1007/978-3-642-72474-9_25. [DOI] [PubMed] [Google Scholar]
- 28.Klug D, Robert V, Swynghedauw B. Role of mechanical and hormonal factors in cardiac remodeling and the biologic limits of myocardial adaptation. Am J Cardiol. 1993;71(3):46A–54A. doi: 10.1016/0002-9149(93)90245-8. Related Articles, Books, LinkOut. [DOI] [PubMed] [Google Scholar]
- 29.Papamitsou T, Dermentzopoulou-Theodoridou M, Barlagiannis D, et al. Morphological and immunohistochemical study of the rat myocardium after testosterone administration. J Sports Medicine Association of Greece. 2006;1(1):24–28. [Google Scholar]
- 30.Appel HJ, Heller-Umpfenbach B, et al. Ultrastructural and morphometric investigations on the effect of training and administration of anabolic steroids on the myocardium of guinea pigs. Int J Sports Med. 1983;4(4):268–74. doi: 10.1055/s-2008-1026048. [DOI] [PubMed] [Google Scholar]
- 31.Urhausen A, Albers T, Kindermann W. Are the cardiac effects of anabolic steroids abuse in strength athletes reversible? Heart. 2004;90:496–501. doi: 10.1136/hrt.2003.015719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Barros R, Zornoff L, Ribeiro H, et al. Hypertrophied myocardium is more dependent on extracellular calcium than the normal cardiac muscle. Med Sci Monit. 2010;16(8):BR278–84. [PubMed] [Google Scholar]
- 33.Tatli E, Aktoz M, Altun A. Do plasma leptin levels predict diastolic dysfunction in patients with hypertension? Arch Med Sci. 2009;5(3):342–46. [Google Scholar]
- 34.Abdel Moniem SR, Nagwa AMA, Rahman Negm HA, Yousrey TM. Assessment of myocardial function in newly diagnosed Egyptian patients with clinical and subclinical thyroid diseases. Arch Med Sci. 2009;5(2):177–81. [Google Scholar]
- 35.Stockem W, Wohlfarth-Bottermann KE. Pinocytosis (endocytosis) In: Lima-de-Faria, editor. Handbook of Molecular Cytology. Amsterdam and London: North Holland Publ; 1969. p. 1373. [Google Scholar]
- 36.Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death and Differentiation. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konopleva M, Zhao S, Xie Z, et al. Apoptosis: Molecules and mechanisms. Adv Exp Med Biol. 1999;457:217–36. [PubMed] [Google Scholar]
- 38.Krajewska M, Wang H-G, Krajewsji S, et al. Immunohistochemical Analysis of in Vivo Patterns of Expression of CPP32 (Caspase-3), a Cell Death Protease. Cancer Research. 1997;57:1605–13. [PubMed] [Google Scholar]
- 39.Nicholson DW, Ambereen A, Thornberry N, et al. Identification and inhibition of the ICE/CED3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 40.Jeruc J, Vizjak A, Rozman B, Ferluga D. Immunohistochemical Expression of Activated Caspase-3 as a Marker of Apoptosis in Glomeruli of Human Lupus Nephritis. Am J Kid Dis. 2006;48(3):410–18. doi: 10.1053/j.ajkd.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Fantona L, Belhanib D, Vaillant F, et al. Heart lesions associated with anabolic steroid abuse: Comparison of post-mortem findings in athletes and norethandrolone-induced lesions in rabbits. Exp and Toxicol Pathol. 2009;61(4):317–23. doi: 10.1016/j.etp.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy CM, Lawrence C. Anabolic steroid abuse and cardiac death. Med J Aust. 1993;158:346–48. doi: 10.5694/j.1326-5377.1993.tb121797.x. [DOI] [PubMed] [Google Scholar]