Summary
Background
Oral graft-versus-host disease (GVHD) is a significant complication after allogeneic stem cell transplantation (SCT) and there is no consistent information about its characteristics in patients after reduced-intensity conditioning regimen FLU/MEL (fludarabine 120 mg/m2 and melphalan 140mg/m2).
Material/Methods
This was a single-centre prospective observational study of patients after allogeneic SCT with FLU/MEL conditioning performed during the period 1/2005-12/2007. Characteristics of oral GVHD were observed in 71 patients. The observation was discontinued due to death, donor lymphocyte infusion (DLI) or new chemotherapy administration.
Results
In 10/2010, the median duration of the observation of the cohort of the patients was 13 (0.2–69) months, and 42 (35–69) months in the still-ongoing 20/71 (28%) patients. Oral acute GVHD had sporadic 7% incidence, whereas oral chronic GVHD was observed in 33% of patients and persisted with median duration of 188 (11–665) days. Clinical and histopathological features were similar in both acute and chronic oral GVHD and included mucosal lichenoid changes, erythema, ulcerations and pseudomembranes, satellite necrosis, apoptotic bodies and lichenoid interface inflammation.
Conclusions
It is necessary to consider complex clinical symptomatology and pathological correlations when classifying the oral GVHD, because local oral symptoms and histopathological features in both acute and chronic oral GVHD forms can be similar. Even though the oral chronic GVHD was mild in the majority of patients, it can be considered as clinically significant due to its incidence, duration and symptomatology. The FLU/MEL conditioning regimen should not be considered as low-risk protocol in this context.
Keywords: graft-versus-host disease, melphalan, allogeneic transplantation, oral
Background
Allogeneic stem cell transplantation (SCT) with the FLU/MEL conditioning regimen (fludarabine total dose 125–150 mg/m2, melphalan total dose 140–180 mg/m2) is an effective and well-established treatment modality in patients with hematological malignancies [1–5]. Graft-versus-host disease (GVHD) has an incidence between 10–80% and is one of the most important complications of allogeneic SCT [6]. The definition of acute or chronic GVHD is based on the specificity of signs and symptoms rather than the criterion of time of onset [6,11]. The oral cavity can be affected with chronic GVHD (Figure 1) in 38–46% of transplanted patients and in 54–80% of patients with ongoing chronic GVHD [7–10]. Oral acute GVHD is less common [14]. As clinical and histopathological characteristics of oral GVHD in the FLU/MEL conditioning regimen have not yet been published in detail, and the literature on oral acute GVHD is scant, we conducted this prospective observational study to obtain more information.
Figure 1.
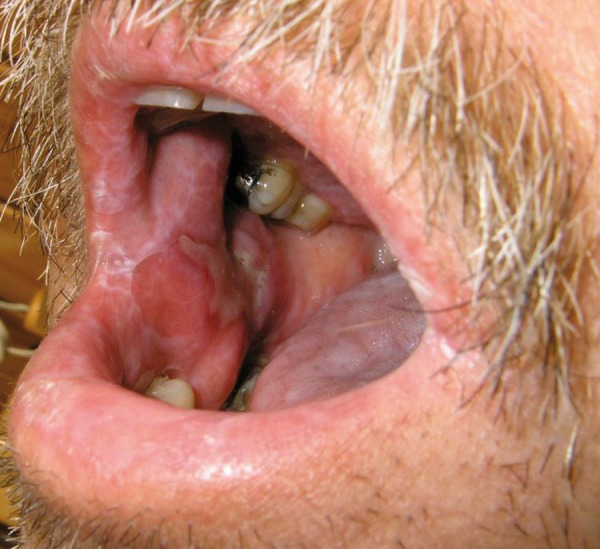
Oral GVHD – diffuse lichenoid changes, atrophy and buccal mucosa defect (ulceration) covered with pseudomembrane in a 54-year-old man with clinically chronic GVHD of the skin on day +330 post-transplant, reduced-intensity conditioning regimen FLU/MEL.
Material and Methods
This was a single-centre prospective observational study of patients after allogeneic SCT with the FLU/MEL conditioning regimen performed during the period 1/2005–12/2007. The FLU/MEL regimen consisted of fludarabine 30 mg/m2 IV once daily for 4 days (total dose 120 mg/m2) and melphalan 140 mg/m2 IV once daily 1 day prior to transplantation. Monitoring of the patients started on the day of transplantation (day 0) and ceased when a patient died or began new chemotherapy, transplantation or donor lymphocyte infusion (DLI) administration. The patients signed informed consent. For more characteristics of the patients, see Table 1.
Table 1.
Characteristics of the patients.
| Characteristics | FLU/MEL |
|---|---|
| n= | 71 |
|
| |
| Age (years), median | 56 (23–68) |
|
| |
| Gender – males | 51% |
|
| |
| Diagnosis: | |
| AML | 30/71 (42%) |
| ALL | 5 |
| NHL | 8 |
| CLL | 5 |
| MDS | 6 |
| MM | 7 |
| CML | 3 |
| HL | 5 |
| OMF | 2 |
|
| |
| Donor HLA identical: | 57/71 (80%) |
| matched unrelated | 25/71 |
| matched related | 32/71 |
| Donor HLA mismatched (unrelated): | 14/71 (20%) |
|
| |
| Peripheral stem cells graft | 71/71 (100%) |
|
| |
| CD34+ cells in graft (×106/kg), median | 4.95 (1.6–15.2) |
|
| |
| CD3+ cells in graft (×108/kg), median | 2.8 (1.3–6.3) |
|
| |
| Granulocytes ≥1×109/l on day post-transplant, median | 13 (0–26) |
The GVHD prophylaxis consisted of cyclosporine A (CyA) from day –1 and methotrexate 10 mg/m2 IV on day +1, +3 and +6 with rescue leucovorin. The median duration of CyA prophylaxis was 4.5 (2.7–24) months. The duration of the CyA administration was with respect to the individual kinetics of the donor-patient chimerism status, the underlying malignancy and GVHD occurrence post-transplantation. Dexamethasone 0.2–0.4% solutions and indifferent viscose gels were used for local therapy in patients with oral GVHD. Intensive immunosuppressive treatment with CyA and methylprednisolone 1–2 mg/kg/day was started in patients with acute GVHD grade 2–4 (Glucksberg’s), whereas patients with grade 1 continued with CyA monotherapy. In chronic GVHD, the patients with limited oral involvement had only local dexamethasone solution treatment, the patients with mild to moderate GVHD continued with CyA monotherapy, and patients with moderate to severe GVHD were given a combination of CyA and methylprednisolone.
GVHD was assessed with respect to the National Institutes of Health (NIH) [11] criteria accepted by the European Group for Blood and Marrow Transplantation (EBMT) [6]. The overall grading of acute GVHD was assessed by the Glucksberg’s system [13] with grades 0 to 4. Chronic GVHD was globally scored according to the NIH criteria: mild, moderate and severe. The intensity of oral GVHD was scored according the NIH criteria: 0 = no symptoms, 1= mild symptoms not limiting oral intake, 2= moderate symptoms with partial limitation of oral intake, 3= severe symptoms with major limitation of oral intake. Histopathological verification of GVHD in the skin, liver or gut was not routinely performed.
Buccal mucosa samples (4–11 mm in the largest diameter) were excised from lichenoid lesions in patients who developed clinically evident systemic GVHD and who agreed with the excision. Trimecain was used for local anesthesia. The biopsy samples were fixed in 10% formaldehyde. The 2 μm-thick tissue sections were hematoxylin-eosin stained.
Results
A total of 71 patients were included in the observation. In 10/2010, the median duration of the observation within the whole cohort of the patients was 13 (0.2–69) months. The median duration of the observation of the still-ongoing 20/71 (28%) patients was 42 (35–69) months. In 51/71 (72%) patients, the observation was discontinued due to death, DLI or new chemotherapy administration. For more characteristics of the patients, see Table 1.
Acute GVHD developed with the median onset on day +25 (15–128) post-transplant in 28/71 (39%) patients and affected skin, gut and livers in 88%, 77% and 37% of the patients, respectively. The severity of acute GVHD by the Glucksberg’s global grading was: grade 1 – 4%, grade 2 – 66%, grade 3 – 26% and grade 4 – 4%.
Oral symptomatology was observed in 5/71 (7%) patients and in 5/27 (18%) of those with acute GVHD, and the concomitant gut GVHD was observed in 5/5 oral symptomatology patients, skin GVHD in 3/5 and liver GVHD in 2/5. Clinical characteristics are described in detail in Table 2.
Table 2.
Clinical characteristics of acute and chronic oral graft-versus-host disease (GVHD).
| Characteristics | Oral acute GvHD | Oral chronic GvHD |
|---|---|---|
| Incidenceian | 5/71 (7%) | 22/62 (33%) |
| Onset on day post-transplant, median: | 80 (40–125) | 237 (107–540) |
| Duration (days), median | 24 (7–54) | 188 (11–665) |
| Resolution on day post-transplant, med | 113 (93–150) | 422 (178–900) |
|
| ||
| Sites involved: | ||
| Buccal mucosa | 5/5 (100%) | 22/22 (100%) |
| Tongue | 2/5 (40%) | 10/22 (45%) |
| Lips | 1/5 (20%) | 7/22 (32%) |
| Palate | 1/5 (20%) | 7/22 (32%) |
| Cavity global | 0 | 4/22 (18%) |
|
| ||
| Clinical manifestation: | ||
| Lichenoid changes | 3/5 (60%) | 22/22 (100%) |
| Erythema | 3/5 (60%) | 8/22 (36%) |
| Defect-pseudomembrane | 1/5 (20%) | 12/22 (54%) |
| Atrophy | 0 | 3/22 (13%) |
| Hyperkeratosis | 0 | 1 (4.5%) |
|
| ||
| Symptoms: | ||
| No problems | 1/5 (20%) | 3/22 (13%) |
| Dryness | 3/5 (60%) | 15/22 (68%) |
| Discomfort | 0 | 7/22 (32%) |
| Pain | 2/5 (40%) | 11/22 (50%) |
|
| ||
| Oral GvHD scoring (NIH criteria) | ||
| Grade 1 | 5/5 (100%) | 16/22 (73%) |
| Grade 2 | 0 | 4/22 (18%) |
| Grade 3 | 0 | 2/22 (9%) |
Oral GvHD scoring (NIH criteria): 0 = no symptoms, 1= mild symptoms not limiting oral intake significantly, 2= moderate symptoms with partial limitation of oral intake, 3= severe symptoms with major limitation of oral intake.
Chronic GVHD developed with the median onset on day +234 (107–540) post-transplant in 31/71 (43%) patients and in 31/62 (50%) patients surviving ≥100 days post-transplant. The GVHD affected the skin (76%), oral cavity (73%), liver (26%), eyes (20%) and gut (16%). The severity by NIH global scoring was: mild 10/31 (32%), moderate 16/31 (52%), severe 5/31 (16%).
Oral chronic GVHD was observed in 22/62 (33%) patients surviving ≥100 days post-transplant and in 22/31 (71%) of these with chronic GVHD. The oral symptoms developed with the median onset on day +237 (107–540), persisted for 188 (11–665) days and resolved on day +420 (178–900) post-transplant. Oral GVHD reoccurrence was observed in 7/22 (32%) patients. Clinical characteristics are described in detail in Table 2.
There were 12 representative samples of buccal mucosa excised in 12 patients with clinically evident systemic GVHD and oral lichenoid changes. The samples were excised on median day +165 (78–420) post-transplant; 3 patients had systemic acute and 9 had chronic GVHD. The observed histopathological features were in full concordance with the diagnosis of GVHD and included apoptotic bodies, satellite necrosis and lichenoid interface inflammation in 100% of the samples (Table 3).
Table 3.
Histopathological features of oral graft-versus-host disease (GVHD).
| Characteristics | Oral acute GVHD | Oral chronic GVHD |
|---|---|---|
| n= | 3 | 9 |
| Interface inflammation | 3 (100%) | 9 (100%) |
| Apoptotic bodies | 3 (100%) | 9 (100%) |
| Lymphocytic infiltration at junction of the epithelium and subepithelial connective tissue | 3 (100%) | 9 (100%) |
| Hydropic degeneration of the basal layer of the epithelium | 3 (100%) | 7 (77%) |
| Acanthosis | 2 (66%) | 5 (55%) |
| Eosinophil infiltration | 2 (66%) | 4 (44%) |
| Epithelial atrophy | 1 (30%) | 2 (22%) |
| Parakeratosis | 0 | 3 (33%) |
| Spongiosis | 0 | 1 (12.5%) |
Discussion
This observational study was primarily designed to characterize clinical and histopathological characteristics of oral GVHD in patients after allogeneic stem cell transplantation with the fludarabine- and melphalan-containing preparative regimen FLU/MEL. We considered this topic to be of interest because no concise data on oral GVHD within this cohort of patients have been published to date.
This study included and evaluated 71 patients treated with protocolized FLU/MEL conditioning regimen. To reduce any possible impact of DLI infusions, chemotherapy or retransplantation on the GVHD occurrence in the cohort, the observation and monitoring of a patient was interrupted in case of such conditions. This restriction policy and post-transplant mortality contributed to the 72% (51/71) rate of drop-out from observation; however, it helped to keep the cohort more homogenous and the data and results more reliable.
Without any clinical information and awareness of the systemic GVHD characteristics, it could be rather problematic to clinically distinguish acute and chronic oral GVHD. The basic functional problems including oral discomfort, pain, dryness of mucosa, and morphological features, were similar in both forms of GVHD. The oral chronic GVHD, however, had later onset (median on day +237), longer persistence (median 188 days) and more marked lichenoid changes and mucosal defects. In addition, it is also important to be aware of other complications that can mimic oral GVHD, including local toxic or allergic reactions, viral infection, lichen ruber planus and dental-prosthesis lichenoid reaction. There were no specific tests performed in our patients to specifically rule out local oral viral infection. We assume, however, based on clinical observations, symptomatology and characteristics, that none of our patients who were considered as acute or chronic oral GVHD had viral oral infection.
From the histopathologist’s point of view, it can be also very difficult to distinguish the acute or chronic oral GVHD in the absence of any other clinical information describing the systemic and oral GVHD involvement and the time since the transplantation. No specific markers were found to conclusively differentiate acute and chronic GVHD in the histology samples. Interface inflammation (mostly lymphocytic infiltration at the interface between the epithelium and the subepithelial connective tissue), apoptotic bodies and satellite necrosis were typical findings in all samples, which was in full concordance with the diagnosis of GVHD [12]. The cleavage of the epithelium from the connective tissue (which may also be caused by mechanical damage to the tissue samples) or significant fibrosis was not observed. Even though hyperkeratosis is acknowledged an important marker of oral chronic GVHD, there was only 1 case with this feature in our cohort. The reason might be that the early recognition and treatment of the GVHD prevented the oral damage from progression into more developed morphological changes, including marked hyperkeratosis and fibrosis.
Conclusions
It is necessary to consider complex clinical symptomatology and clinical and pathological correlations when classifying oral GVHD because local oral symptoms, morphological and histotopathological features in both forms of GVHD can be similar. Even though the oral chronic GVHD was mild in the majority of the FLU/MEL patients, this mucosal condition can be considered as clinically significant due to its incidence (33%), prolonged duration (median 188 days) and feeling of local discomfort and even pain. The FLU/MEL conditioning regimen should not be considered as a low-risk protocol in this situation.
Acknowledgements
The authors would like to thank Skardova Jana, Cervena Jarmila, Dolejsova Lucie, Kabatova-Maxova Klara, Karasova Lenka, Kibitzova Petra, Krivankova Irena, Lastovkova Alena, Novotna Romana, Rerichova Michaela, Ruttnerova Marta, Schroderova Ruzena, Vohrnova Dana, Zikova Jindra – nurses, University Hospital in Plzen (Pilsen), Haemato-Oncological dept., Transplant unit – for the care of the patients. We would also like to thank the medical and nursing team of the Department of Dentistry and the Department of Pathology, University Hospital in Plzen (Pilsen) for their cooperation.
Footnotes
Disclosures
The authors declare no conflict of interest.
Source of support: Departmental sources
References
- 1.Giralt S, Thall PF, Issa K, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–37. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 2.Kröger N, Sayer H, Schwerdtfeger R, et al. Unrelated stem cell transplantation in multiple myeloma after a reduced-intensity conditioning with pretransplantation antithymocyte globulin is highly effective with low transplantation-related mortality. Blood. 2002;100:3919–24. doi: 10.1182/blood-2002-04-1150. [DOI] [PubMed] [Google Scholar]
- 3.Inamoto Y, Oba T, Miyamura K, et al. Stable engraftment after a conditioning regimen with fludarabine and melphalan for bone marrow transplantation from an unrelated donor. Int J Hematol. 2006;83:356–62. doi: 10.1532/IJH97.05168. [DOI] [PubMed] [Google Scholar]
- 4.Anderlini P, Saliba R, Acholonu S, et al. Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin’s lymphoma: the updated M.D. Anderson Cancer Center experience. Haematologica. 2008;93:257–64. doi: 10.3324/haematol.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aisa Y, Mori T, Kudo M, et al. Oral cryotherapy for the prevention of high-dose melphalan-induced stomatitis in allogeneic hematopoietic stem cell transplant recipients. Support Care Cancer. 2005;13:266–69. doi: 10.1007/s00520-004-0726-y. [DOI] [PubMed] [Google Scholar]
- 6.Devergie A. Graft versus host disease. The 2008 revised edition of the ESH-EBMT Handbook on Haemopoietic Stem Cell Transplantation 2008. In: Apperley J, Carreras E, Gluckman E, et al., editors. Forum Service Editore. 2008. pp. 219–34. [Google Scholar]
- 7.Treister NS, Woo SB, O’Holleran EW, et al. Oral chronic graft-versus-host disease in pediatric patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:721–31. doi: 10.1016/j.bbmt.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Busca A, Locatelli F, Vai S, et al. Clinical grading of oral chronic graft-versus-host disease in 104 consecutive adult patients. Haematologica. 2005;90:567–69. [PubMed] [Google Scholar]
- 9.Lee S, Klein JP, Barrett AJ, Ringden O, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–14. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 10.Flowers M, Parker P, Johnston L, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100:415–19. doi: 10.1182/blood-2002-01-0011. [DOI] [PubMed] [Google Scholar]
- 11.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Soares AB, Faria PR, Magna LA, et al. Chronic GVHD in minor salivary glands and oral mucosa: histopathological and immunohistochemical evaluation of 25 patients. J Oral Pathol Med. 2005;34:368–73. doi: 10.1111/j.1600-0714.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- 13.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Schubert MM, Correa ME. Oral graft-versus-host disease. Dent Clin North Am. 2008;52:79–109. doi: 10.1016/j.cden.2007.10.004. [DOI] [PubMed] [Google Scholar]


