Summary
Background
Obstructive sleep apnea (OSA) is a condition contributing to oxidative stress. The aim of this study was to ascertain if there is any connection between OSA and novel oxidative stress-related markers. Matrix metalloproteinases 2 and 9 (MMP-2, MMP-9), high sensitive C-reactive protein (hsCRP), pregnancy-associated plasma protein-A (PAPP-A), soluble receptors for advanced glycation end-products (sRAGE), zinc (Zn) and copper (Cu) were measured. Further biochemical markers were evaluated.
Material/Methods
Fifty-one men suspected for OSA indicated for night polygraphy were included. Apnea/hypopnea index (AHI), oxygen desaturation index (ODI), mean blood hemoglobin oxygen saturation (SpO2) and time of blood hemoglobin oxygen saturation below 90% (SpO2 <90%) were measured. Morning venous blood samples were taken.
Results
For body mass index (BMI) we found strong positive correlation with levels of Cu, MMP-9, hsCRP and fibrinogen, and negative correlation with sRAGE. Concerning ventilation parameters, we found positive correlation of ODI and SpO2 <90% with markers MMP-9 and hsCRP. sRAGE level correlated with AHI and ODI negatively. SpO2 correlated negatively with Cu, MMP-9, hsCRP and fibrinogen. There was no correlation between ventilation parameters and markers MMP-2, PAPP-A and Zn. Compared to severity of OSA, there was significant difference in levels of hsCRP and Cu between patients with AHI ≤5 and AHI ≥30 independent of BMI.
Conclusions
MMP-9, hsCRP, sRAGE and Cu seem to be strong predictors of oxidative stress in OSA patients. The strong correlation between oxidative stress-related markers and OSA is elucidated by connection of these to BMI, which is probably a primary condition of oxidative stress, but OSA is an additive condition.
Keywords: obstructive sleep apnea, oxidative stress, MMP-9, sRAGE, hsCRP, Cu
Background
Obstructive sleep apnea (OSA) has been classified as a sleep breathing disorder. OSA is thought to be an important risk factor for cardiovascular disease. Associations have been reported between sleep apnea and systemic hypertension, pulmonary hypertension, ischemic heart disease and stroke.
OSA is characterized by recurrent obstructions of the upper airway during sleep, which are followed by lowering of blood hemoglobin oxygen saturation. This repeated hypoxia and reoxygenation cycle is similar to hypoxia–reperfusion injury, which initiates oxidative stress. Oxidative stress is characterized by an imbalance between oxidant-producing systems and anti-oxidant defence mechanisms, which results in excessive formation of reactive oxygen species (ROS). ROS can damage various biomolecules and cellular components, but they also act as important signalling molecules which play roles in induction and activation of multiple genes [1]. ROS can oxidize lipids, proteins, carbohydrates and DNA, and thus can alter their functions. Several studies have suggested that OSA is associated with increased levels of oxidative stress markers [2–5] or decreased antioxidant defence [6]. Mainly the phase of reoxygenation is thought to be the period during which increased production of ROS occurs.
It has been established that treating OSA patients by continuous positive airway pressure (CPAP) decreases levels of oxidative stress-related markers [7].
Previous studies found correlation of levels of some molecules (which are possible markers related to oxidative stress) and various diseases including chronic renal failure and hemodialysis patients, diabetes mellitus, diabetic kidney disease, arterial hypertension, myocardial ischemic syndromes and atherosclerosis [8–19]. This study was conducted to determine whether these molecules are also altered in OSA patients.
We measured levels of soluble receptor for advanced glycation end-products (sRAGE), pregnancy-associated plasma protein-A (PAPP-A), matrix metalloproteinases 2 and 9 (MMP-2 and MMP-9) and high sensitive C-reactive protein (hsCRP).
hsCRP is an acute phase protein and serves as a marker of inflammation. High CRP levels are linked to risk of atherosclerosis and it appears to be a marker for cardiovascular risk [20]. Oxidative stress is associated with higher CRP levels [21]. Previous works showed an association between CRP and OSA [22].
PAPP-A (or pappalysin 1) is a metzincin superfamily metalloproteinase. PAPP-A has been used in prenatal genetic screening. It was also used as an oxidative stress marker [11] and a marker of risk of atherosclerosis [12,13].
RAGE is a multiligand transmembrane receptor. Deleterious effects mediated by AGEs are prevented in part by this receptor lacking the transmembrane domain- soluble RAGE (sRAGE), which occurs in vivo. Higher sRAGE was found in patients with diminished renal function [14]. Low sRAGE level was described in patients with arterial hypertension [15] and diabetes mellitus complications [17]. Association between AGEs and OSA was also previously published [23].
MMP-2 (or gelatinase A) and MMP-9 (or gelatinase B) are Zn2+ and Cu2+ dependent endopeptidases. MMP-2 and MMP-9 are involved in degradation of collagen type IV, elastin and fibronectin. ROS have been shown to regulate the levels of MMPs in various cellular and in vivo systems [24]. MMPs, among others, play a role in many pathological processes in cancerogenesis and cardiovascular diseases [25]. Increase in MMPs activity was shown to be associated with the progression of atherosclerotic plaques to their rupture [26] and recently published elevation of MMP-9 in OSA [27].
Copper (Cu) and zinc (Zn) are necessary trace element for the body. Copper is a part of an antioxidant enzymes, as are superoxide dismutase (SOD), ceruloplasmin, and cytochrome c oxidase [28,29]. Copper, together with zinc, plays an important role in antioxidant defense mainly as a part of Cu/Zn SOD. Zn is necessary for correct function of MMPs and PAPP-A. It was shown that Cu-deficient rats had higher level of isoprostanes as marker of oxidative stress compared to Cu normal rats [30]. However, it was also found that high levels of Cu and other metal elements impair oxidative stress [31]. Low zinc levels are also thought to be associated with increased oxidative stress and reduced antioxidant defense [32,33].
Material and Methods
Study protocol
Adult men with suspicion of OSA indicated for nocturnal polygraphy in the sleep laboratory were enrolled in the study. A total of 51 men were examined. All subjects signed an informed consent, and the procedures were approved by the local ethics committee. Enrollment was preceded by examination with special respect to the subject’s medical history. Excluded from the study were patients with the following diseases: stroke or cerebral injury less than 6 month before the study, major cardiological diseases including cardiac insufficiency and manifest coronary artery disease, pulmonary hypertension, and patients with diabetes mellitus treated with insulin.
Sleep study
During the night (from 11 p.m. through 6 a.m.) patients were examined using the method of nocturnal polygraphy (monitored parameters were: nasal airflow, thoracoabdominal wall motion, heart rate, blood hemoglobin oxygen saturation, respiratory noises and body position). The nocturnal polygraphy was scored visually according to recommendations of the American Academy of Sleep Medicine (AASM) 2007 using 3% blood hemoglobin oxygen saturation decrease as the level of significance. The following parameters were scored: the apnea/hypopnea index (AHI – mean number of apneas and hypopneas per hour of subjective sleep duration), the oxygen desaturation index (ODI – mean number of oxygen hemoglobin saturation drops of 3% or more per hour of subjective sleep duration), mean blood hemoglobin oxygen saturation (SpO2) and duration of oxygen blood saturation dip below 90% (SpO2<90%).
Biochemical examination
In the morning after polygraphy, fasting venous blood samples were taken. Selected markers were measured: hsCRP, PAPP-A, MMP-2, MMP-9, sRAGE, Cu, Zn. Blood samples were centrifuged for 10 minutes at 1450 g and then serum was frozen and stored until analysis. Cu concentration in the diluted (1+9) serum samples were determined by inductively-coupled plasma mass spectrometry on a PE Elan 6000 instrument (Perkin Elmer, Norwalk, CT, USA). Calibration solutions were spiked by the important serum matrix compounds (NaCl, KCl, NaNO3, KBr). PAPP-A and hs-CRP were measured by TRACE (Time Resolved Amplified Cryptate Emission) on the KRYPTOR analyser using standard kits (Brahms, Henningsdorf, Germany). sRAGE, MMP-2, MMP-9 (all kits RD Systems, USA) were assessed with standard ELISA (enzyme-linked immunosorbent assay). We further used standard laboratory methods to measure blood count, minerals, renal function, liver enzymes, lipids, glycemia. Mean levels (± SD) of these parameters are shown in Table 1.
Table 1.
Biochemical characteristic of the group.
| Leukocytes ×109/l | 7.1±2.0 | Potassium mmol/l | 4.3±0.4 |
| Erytrocytes ×1012/l | 4.8±0.4 | Chlorides mmol/l | 103.7±3.1 |
| Hemoglobin g/l | 151.2±12.2 | Bilirubin umol/l | 13.7±5.6 |
| Hematocrite | 0,4±0 | ALT ukat/l | 0.6±0.3 |
| Trombocytes ×109/l | 243.8±47.2 | AST ukat/l | 0.5±0.1 |
| Cholesterol mmol/l | 4.9±0.9 | GGT ukat/l | 0.7±0.5 |
| Triglycerides mmol/l | 2±1.3 | ALP ukat/l | 1±0.3 |
| HDL cholesterol mmol/l | 0.2 | ApoA1 g/l | 0.2 |
| LDL cholesterol mmol/l | 2.8±0.7 | ApoB g/l | 1±0.2 |
| Athero Index | 3.7±1.2 | Alfa LP% | 25.5±7.5 |
| Urea mmol/l | 5.4±1.3 | LP (a)% | 1.9±3.0 |
| Creatinine umol/l | 81.2±10.8 | Prebeta Lp% | 28±10.4 |
| Uric acid umol/l | 378.9±70.3 | Beta Lp% | 44.2±6.3 |
| Total protein g/l | 69.6±4.5 | Chylomicrons% | 40±6.3 |
| Albumin g/l | 42.9±2.4 | TSH uIU/l | 1.6±1.0 |
| Natrium mmol/l | 142.2±2.2 | Blood glucose mmol/l | 4.9±0.7 |
Athero index – Atherogenic index; ALT – Alanine Aminotransferase; AST – Aspartate Aminotransferase; GGT – Gamma Glutamyl Transferase; ALP – Alkaline Phosphatase; ApoA1 – Apolipoprotein A-1; ApoB – Apolipoprotein B; alfa LP – alfa Lipoprotein in%; LP (a) – Lipoprotein in%; Prebeta Lp% – Prebetalipoprotien in%; Beta Lp – Betalipoprotein in%; TSH – Thyroid Stimulating Hormone.
Statistical analyses
Correlation analyses were performed with the parametric Pearson test. The group comparisons were performed with one-way parametric ANOVA, and the correction for BMI was performed using analysis of covariance (ANCOVA). Multiple regression was used to obtain and compare the effects of individual predictors of oxidative stress-related markers levels. All these test were performed using Statistica 8 software (StatSoft, Inc. STATISTICA for Windows Tulsa, OK, http://www.statsoft.com). Results are presented as mean ±SD.
Group characteristics
We examined 51 patients. Mean age was 51.1 years (SD ± 11.1), mean BMI was 30.8 (SD ± 5.3), mean AHI was 31.2 (SD ± 22.9), mean ODI was 32.2 (SD ± 26), mean SpO2 was 92.8% (SD ± 4), and mean SpO2 <90% was 15.1% (SD ± 25) of the night. The characteristic of each group concerning comorbidity is shown in Table 2. Concerning comorbidities, we present the number of patients with selected diseases in the whole group: 26 patients arterial hypertension, 2 hyperuricemia, 14 hyperlipidemia, 3 atrial fibrillation, 2 diabetes mellitus (excluded patients with insulin therapy), 4 chronic obstructive pulmonary disease, 4 ischemic cardiac disease and 1 myocardial infarction, 1 stroke. Fifteen patient were smokers.
Table 2.
Significant correlations.
| BMI | AHI | ODI | Mean SpO2 | SpO2 <90% | |
|---|---|---|---|---|---|
| Cu ng/ml | 0.3058 | 0.3333 | −0.3203 | ||
| p=.035 | p=.021 | p=.026 | |||
| sRAGE pg/ml | −0.3941 | −0.2922 | −0.3198 | ||
| p=.006 | p=.044 | p=.027 | |||
| MMP-9 ng/ml | 0.5363 | 0.3771 | −0.49 | 0.4692 | |
| p=.000 | p=.008 | p=.000 | p=.001 | ||
| hsCRP ug/ml | 0.5328 | 0.4793 | 0.4497 | −0.4394 | 0.4476 |
| p=.000 | p=.001 | p=.001 | p=.002 | p=.001 |
BMI – Body Mass Index; AHI – Apnea/Hypopnea Index; ODI – Oxygen Desaturation Index; Mean SpO2 – mean night O2 saturation; SpO2 <90% – time of saturation below 90%; Cu – Copper; MMP-9 – Matrix-Metalloproteinase 9; hsCRP – high sensitive C-reactive Protein; sRAGE – soluble Receptors for Advanced Glycation End-products.
Results
Body mass index (BMI) and ventilation parameters were correlated to chosen oxidative stress-related markers for the whole group of patients independent of OSA intensity.
We found strong correlations between BMI and levels of MMP-9 (P≤0.001), Cu (P=0.035) (Figure 1) and hsCR (P≤0.001) (Figure 2). BMI was also positively correlated with fibrinogen (P=0.019) and negatively correlated with sRAGE (P=0.006) (Table 2).
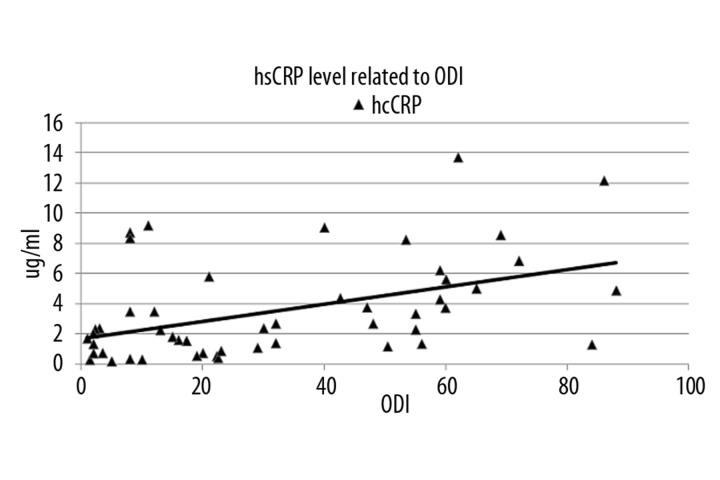
Figure 1.
hsCRP level related to ODI. ODI – Oxygen Desaturation Index, hs CRP – high sensitive C-reactive Protein.
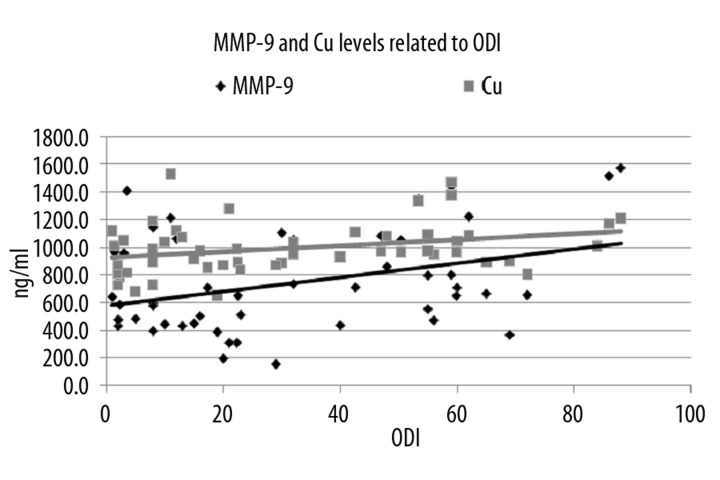
Figure 2.
MMP-9 and Cu levels related to ODI. ODI – Oxygen Desaturation Index, MMP-9 – Matrix-Metalloproteinase-9, Cu – Copper.
Concerning ventilation parameters, we found significant positive correlations for hsCRP and ODI (R=0.450, P=0.001) (Figure 1), AHI (R=0.479, P=0.001) and SpO2<90 (R=0.480, P=0.001).
MMP-9 also correlated positively with ODI (R=0.380, P=0.008) and SpO2<90 (R=0.469, P=0.001). Positive correlation for Cu was found only with ODI (R=0,330, P=0.020) (Figure 2).
Significant negative correlations were found for SpO2 and markers Cu (R=−0.320, P=0.026), MMP-9 (R=−0.490, P<0.001), hsCRP (R=−0,440, P=0.002) and fibrinogen (R=−0.420, P=0.020). sRAGE correlated negatively with AHI (R=−0.290, P=0.044) and ODI (R=−0.320, P=0.027). There were no correlations between OSA parameters and markers MMP-2 and PAPP-A. There were no correlations between oxidative stress markers and lipid spectrum parameters and other biochemical parameters. BMI correlated significantly with all the parameters of ventilation. Multiple regression showed that strong correlation between oxidative stress-related parameters and OSA is explained by connection of these parameters to BMI (R=0.322, beta 0.397, P=0.011).
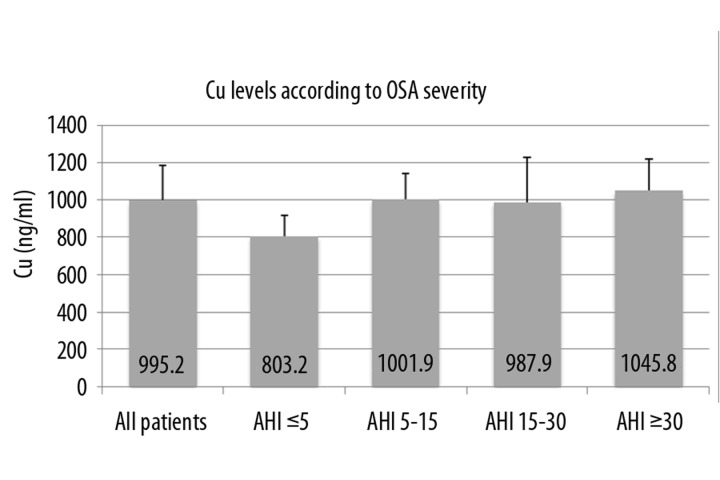
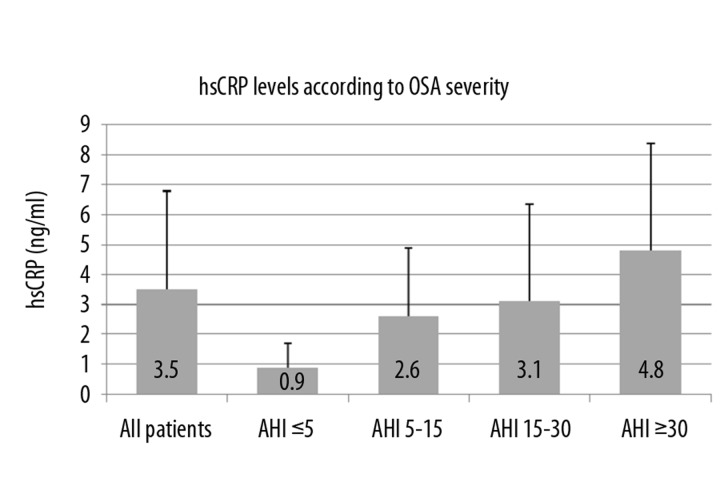
Further patients’ results were divided into 4 groups according to OSA severity, because we wanted to ascertain if markers’ levels are dependent on ventilation parameters. Group 1 was patients with AHI ≤5 (considered as controls without OSA), group 2 was AHI between 5 and 15, group 3 was AHI between 15 and 30, and group 4 was AHI ≥30. Values of oxidative stress-related markers according to OSA severity are shown in Table 3. By ANOVA test we found significant difference in serum level of Cu and hsCRP between groups 1 and 4 (Figure 3 and Figure 4). Post-hoc Tukey HSD testing verified the difference in level of Cu between group 1 (803.2 ng/ml ± 113.4) and group 4 (1045.8 ng/ml ± 170.1), p=0.023. There was also significant difference between serum levels of hsCRP between groups 1 and 4 (0.9 μg/ml ± 0.8 vs. 4.8 μg/ml ± 3.6), p=0.039. These 2 differences are significant even after correction for BMI (ANCOVA).
Table 3.
Oxidative stress related markers levels according to OSA intensity.
| Cu (ng/ml) | Zn (ng/ml) | MMP-2 (ng/ml) | MMP-9 (ng/ml) | hsCRP (ug/ml) | PAPP-A (mU/l) | sRAGE (pg/ml) | |
|---|---|---|---|---|---|---|---|
| All | 995.2±187.6 | 1117.8±262.8 | 168.8±37 | 743.5±360.3 | 3.5±3.3 | 8.1±3.1 | 1247.9±503.5 |
| AHI ≤5 | 803.2±113.4 | 935.4±121.4 | 165.4±20.2 | 722.1±388.4 | 0.9±0.8 | 8.6±2.5 | 1522.2±586.2 |
| AHI 5–15 | 1001.9±139.3 | 1233.2±340.1 | 183.9±60.6 | 711.7±253.5 | 2.6±2.3 | 7.2±3 | 1290±468.1 |
| AHI 15–30 | 987.9±238.2 | 1069.4±110.5 | 146.6±17.8 | 544.4±330.2 | 3.1±3.2 | 8.5±2.1 | 1263.4±567.1 |
| AHI ≥30 | 1045.8±170.1 | 1138.4±284 | 173.7±30.7 | 858±380.6 | 4.8±3.6 | 8.3±3.8 | 1150.5±468.4 |
All – All patients independently to AHI; AHI – Apnea/Hypopnea Index; Cu – Copper; Zn – Zinc; MMP-2 – Matrix-Metalloproteinase 2; MMP-9 – Matrix-Metalloproteinase 9; hsCRP – high sensitive C-reactive Protein; PAPP-A – Pregnancy Associated Plasma Protein-A; sRAGE – soluble Receptors for Advanced Glycation End-products.
Figure 3.
Cu levels according to OSA severity. OSA – Obstructive Sleep Apnea, Cu – Copper, AHI – Apnea Hypopnea Index.
Figure 4.
hsCRP levels according to OSA severity. OSA – Obstructive Sleep Apnea, hs CRP – high sensitive C-reactive Protein, AHI – Apnea Hypopnea Index.
Discussion
Oxidative stress is a condition resulting from an imbalance between the prooxidative state and the antioxidant defence. Oxidative stress results in production of reactive oxygen species (ROS). ROS affect other biomolecules and change their characters. It is difficult to measure levels of ROS, mainly because of their extreme instability and biochemical reactivity, so it is easier to measure oxidative stress indirectly by ROS effects on other molecules (changes in structure, function and quantity of these molecules). General standardization tests for quantification of oxidative stress are lacking. This study was designed to assess the impact of OSA on novel parameters related to oxidative stress.
In our study we again statistically confirmed that higher BMI leads to higher OSA intensity. Higher BMI is associated with increased oxidative stress, independently of other parameters such as OSA [34].
We found strong positive correlation for hsCRP, MMP-9 and ODI and SpO2 <90%. AHI correlated positively only with hsCRP. Together with another study [2] we suggest that ODI is a stronger predictor of oxidative stress level than is AHI. Markers hsCRP, MMP-9, Cu and fibrinogen correlated well with BMI. The strongest correlation was demonstrated for MMP-9, hsCRP and Cu and parameters of OSA (especially ODI and SpO2). MMP-9, hsCRP and Cu levels are higher in severe OSA patients (according to ODI) and this correlation is further explained by association of ventilation parameters to BMI. These markers could be used as general biochemical molecules associated with oxidative stress in obese patients. Obesity seems to be primary risk factor of the increased oxidative stress state.
According to our results, the strongest biochemical markers of oxidative stress in OSA patients are Cu and hsCRP, independently of BMI as verified by analysis of OSA subgroups and using BMI as a covariate.
C-reactive protein (CRP) is an inflammatory marker; it is thought to be an acute phase reactant. Studies suggest that oxidative stress may have pro-inflammatory effects, but data on the relationship between oxidative stress and CRP in healthy persons is sparse. Higher levels of CRP and fibrinogen were found in OSA patients [21,35]. hsCRP was proved to be an oxidative stress marker [20,36]. Another study confirmed a positive correlation between CRP and AHI and a negative correlation between CRP and mean nocturnal saturation [37]. Difference in serum level of hsCRP and Cu in patients without OSA (AHI ≤5) and patients with severe OSA (AHI ≥30) remains significant even after correction for BMI. Therefore these 2 markers could be used as parameters of oxidative stress in OSA, independently of BMI. Increased Cu level in severe OSA patients and obese patients seems to be a strongly associated factor, but its significance is not well explained. Cu and Zn are trace elements important for functioning of the antioxidase system, especially as part of SOD.
MMPs have a primary function in degradation of the extracellular matrix proteins, and play a role in normal tissue remodeling. They also perform essential functions at the cell surface involved in signaling, cell survival, and cell death. MMP-9 is prepacked in neutrophils and released under neuroinflammatory conditions [38]. MMP-9 level is associated with oxidative stress in patients with acute coronary syndrome [39]. MMP-9 was found higher not only in the brain after reperfusion injury but also after liver hypoxia [38,40]. In our study MMP-9 level was associated with severity of OSA according to ODI and mean SpO2 and SpO2 <90%.
RAGE is a transmembrane receptor of the immunoglobulin superfamily. The interaction between RAGE and its ligands can cause activation of pro-inflammatory genes by activation of nuclear factor NF-κB. Isoforms of the RAGE protein, which lack the transmembrane and the signaling domain, are commonly referred to as soluble RAGE (sRAGE), a naturally occurring inhibitor of AGE-RAGE action. There was shown to be a higher level of AGEs in OSA patients, dependently on OSA severity [23]. We found that sRAGE level is associated with AHI, ODI and also negatively associated with BMI. Thus we can propose the hypothesis that sRAGE protects against oxidative stress and its level in OSA and obese patients is decreased.
OSA is an independent risk factor in obesity for increased oxidative stress state as shown by diminishing of some oxidative stress-related markers after CPAP therapy [3,7,41].
It is apparent that our group of patients is not homogenous as concerned the comorbidities. Oxidative stress is thought to be associated with many factors including obesity, smoking, age, hypertension, diabetes, and dyslipidemia. Therefore assessment of the relationship between OSA and oxidative stress intensity may be influenced by such confounding variables.
Conclusions
OSA, unlike obesity, is a treatable risk factor of systemic inflammation and oxidative stress. Our study provides evidence of increased oxidative stress in OSA patients, predominantly in obese ones. In patients with severe OSA (AHI over 30), this effect is additive to the influence of obesity.
Acknowledgements
Authors would like to thank all the staff in the sleep and biochemical laboratory.
Abbreviations
- OSA
obstructive sleep apnea
- MMP-2,9
matrix metalloproteinases 2,9
- hsCRP
high sensitive C-reactive protein
- PAPP-A
pregnancy-associated plasma protein-A
- sRAGE
soluble receptors for advanced glycation end-products
- AHI
apnea/hypopnea index
- ODI
oxygen desaturation index
- SpO2
mean blood hemoglobin oxygen saturation
- SpO2 <90%
time of blood hemoglobin oxygen saturation below 90%
- BMI
body mass index
Footnotes
Source of support: This study was supported by Ministry of Education grant VZ0021620816
References
- 1.Hensley K, Robinson KA, Gabbita SP, et al. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28(10):1456–62. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 2.Yamauchi M, Maekawa J, Okamoto Y, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127(5):1674–79. doi: 10.1378/chest.127.5.1674. [DOI] [PubMed] [Google Scholar]
- 3.Schulz R, Mahmoudi S, Hattar K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162(2 Pt 1):566–70. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 4.Barcelo A, Miralles C, Barbe F, et al. Abnormal lipid peroxidation in patients with sleep apnoea. Eur Respir J. 2000;16(4):644–47. doi: 10.1034/j.1399-3003.2000.16d13.x. [DOI] [PubMed] [Google Scholar]
- 5.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27(1):123–28. [PubMed] [Google Scholar]
- 6.Christou K, Moulas AN, Pastaka C, Gourgoulianis KI. Antioxidant capacity in obstructive sleep apnea patients. Sleep Med. 2003;4(3):225–28. doi: 10.1016/s1389-9457(02)00253-8. [DOI] [PubMed] [Google Scholar]
- 7.Christou K, Kostikas K, Pastaka C, et al. Nasal continuous positive airway pressure treatment reduces systemic oxidative stress in patients with severe obstructive sleep apnea syndrome. Sleep Med. 2009;10(1):87–94. doi: 10.1016/j.sleep.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Spittle MA, Hoenich NA, Handelman GJ, et al. Oxidative stress and inflammation in hemodialysis patients. Am J Kidney Dis. 2001;38(6):1408–13. doi: 10.1053/ajkd.2001.29280. [DOI] [PubMed] [Google Scholar]
- 9.Himmelfarb J. Oxidative stress in hemodialysis. Contrib Nephrol. 2008;161:132–37. doi: 10.1159/000130658. [DOI] [PubMed] [Google Scholar]
- 10.Kalousová M, Sulková S, Fialová L, et al. Glycoxidation and inflammation in chronic haemodialysis patients. Nephrol Dial Transplant. 2003;18(12):2577–81. doi: 10.1093/ndt/gfg404. [DOI] [PubMed] [Google Scholar]
- 11.Fialová L, Kalousová M, Soukupová J, et al. Relationship of pregnancy-associated plasma protein-A to renal function and dialysis modalities. Kidney Blood Press Res. 2004;27(2):88–95. doi: 10.1159/000076390. [DOI] [PubMed] [Google Scholar]
- 12.Heeschen C, Dimmeler S, Hamm CW, et al. Pregnancy-associated plasma protein-A levels in patients with acute coronary syndromes: comparison with markers of systemic inflammation, platelet activation, and myocardial necrosis. J Am Coll Cardiol. 2005;45(2):229–37. doi: 10.1016/j.jacc.2004.09.060. [DOI] [PubMed] [Google Scholar]
- 13.Beaudeux JL, Burc L, Imbert-Bismut F, et al. Serum plasma pregnancy-associated protein A: a potential marker of echogenic carotid atherosclerotic plaques in asymptomatic hyperlipidemic subjects at high cardiovascular risk. Arterioscler Thromb Vasc Biol. 2003;23(1):7–10. doi: 10.1161/01.atv.0000047448.76485.b8. [DOI] [PubMed] [Google Scholar]
- 14.Kalousová M, Jáchymová M, Mestek O, et al. Receptor for advanced glycation end products-soluble form and gene polymorphisms in chronic haemodialysis patients. Nephrol Dial Transplant. 2007;22(7):2020–26. doi: 10.1093/ndt/gfm050. [DOI] [PubMed] [Google Scholar]
- 15.Geroldi D, Falcone C, Emanuele E, et al. Decreased plasma levels of soluble receptor for advanced glycation end-products in patient with essential hypertension. J Hypertens. 2005;23(9):1725–29. doi: 10.1097/01.hjh.0000177535.45785.64. [DOI] [PubMed] [Google Scholar]
- 16.Witko-Sarsat V, Friedlander M, Capeillére-Blandin C, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49(5):1304–13. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 17.Katakami N, Matsuhisa M, Kaneto H, et al. Decreased endogenous secretory advanced glycation end product receptor in type 1 diabetic patients: its possible association with diabetic vascular complications. Diabetes Care. 2005;28(11):2716–21. doi: 10.2337/diacare.28.11.2716. [DOI] [PubMed] [Google Scholar]
- 18.Hakim FA, Pflueger A. Role of oxidative stress in diabetic kidney disease. Med Sci Monit. 2010;16(2):37–48. [PubMed] [Google Scholar]
- 19.Misra MK, Sarwat M, Bhakuni P, et al. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15(10):RA209–19. [PubMed] [Google Scholar]
- 20.Abramson JL, Hooper WC, Jones DP, et al. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis. 2005;178(1):115–21. doi: 10.1016/j.atherosclerosis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Yeun JY, Kaysen GA. C-reactive protein, oxidative stress, homocysteine, and troponin as inflammatory and metabolic predictors of atherosclerosis in ESRD. Curr Opin Nephrol Hypertens. 2000;9(6):621–30. doi: 10.1097/00041552-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Lui MM, Lam JC, Mak HK, et al. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009;135(4):950–56. doi: 10.1378/chest.08-1798. [DOI] [PubMed] [Google Scholar]
- 23.Tan KC, Chow WS, Lam JC, et al. Advanced glycation endproducts in nondiabetic patients with obstructive sleep apnea. Sleep. 2006;29(3):329–33. doi: 10.1093/sleep/29.3.329. [DOI] [PubMed] [Google Scholar]
- 24.Gasche Y, Copin JC, Sugawara T, et al. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21(12):1393–400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Parks WC, Mecham RP. Matrix metaloproteinasis. San Diego: Academic Press; 1998. [Google Scholar]
- 26.Beaudeux JL, Giral P, Bruckert E, Foglietti MJ, Chapman MJ. Matrix metalloproteinases, inflammation and atherosclerosis: therapeutic perspectives. Clin Chem Lab Med. 2004;42(2):121–31. doi: 10.1515/CCLM.2004.024. [DOI] [PubMed] [Google Scholar]
- 27.Ye J, Liu H, Li Y, et al. Increased serum levels of C-reactive protein and matrix metalloproteinase-9 in obstructive sleep apnea syndrome. Chin Med J. 2007;120(17):1482–86. [PubMed] [Google Scholar]
- 28.Lynch SM, Strain JJ. Effects of copper deficiency on hepatic and cardiac antioxidant enzyme activities in lactose- and sucrose-fed rats. Br J Nutr. 1989;61(2):345–54. doi: 10.1079/bjn19890122. [DOI] [PubMed] [Google Scholar]
- 29.Prohaska JR. Changes in Cu,Zn-superoxide dismutase, cytochrome c oxidase, glutathione peroxidase and glutathione transferase activities in copper-deficient mice and rats. J Nutr. 1991;121(3):355–63. doi: 10.1093/jn/121.3.355. [DOI] [PubMed] [Google Scholar]
- 30.Schuschke DA, Adeagbo AS, Patibandla PK, et al. Cyclooxygenase-2 is upregulated in copper-deficient rats. Inflammation. 2009;32(5):333–39. doi: 10.1007/s10753-009-9140-4. [DOI] [PubMed] [Google Scholar]
- 31.Strausak D, Mercer JF, Dieter HH, Stremmel W, Multhaup G. Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res Bull. 2001;55(2):175–85. doi: 10.1016/s0361-9230(01)00454-3. [DOI] [PubMed] [Google Scholar]
- 32.Song Y, Elias V, Loban A, et al. Marginal zinc deficiency increases oxidative DNA damage in the prostate after chronic exercise. Free Radic Biol Med. 2010;48(1):82–88. doi: 10.1016/j.freeradbiomed.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee BH, Ho BY, Wang CT, Pan TM. Red mold rice promoted antioxidase activity against oxidative injury and improved the memory ability of zinc-deficient rats. J Agric Food Chem. 2009;57(22):10600–7. doi: 10.1021/jf902046s. [DOI] [PubMed] [Google Scholar]
- 34.Lima AM, Franco CM, Castro CM, et al. Obstructive sleep apnea contribution to oxidative stress in obesity. Arq Bras Endocrinol Metabol. 2008;52(4):668–76. doi: 10.1590/s0004-27302008000400013. [DOI] [PubMed] [Google Scholar]
- 35.Kasasbeh E, Chi DS, Krishnaswamy G. Inflammatory aspects of sleep apnea and their cardiovascular consequences. South Med J. 2006;99(1):58–67. doi: 10.1097/01.smj.0000197705.99639.50. [DOI] [PubMed] [Google Scholar]
- 36.Cottone S, Mule G, Nardi E, et al. Relation of C-reactive protein to oxidative stress and to endothelial activation in essential hypertension. Am J Hypertens. 2006;19(3):313–18. doi: 10.1016/j.amjhyper.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Hua Y, Xue J, Sun F, et al. Aspirin inhibits MMP-2 and MMP-9 expressions and activities through upregulation of PPARalpha/gamma and TIMP gene expressions in ox-LDL-stimulated macrophages derived from human monocytes. Pharmacology. 2009;83(1):18–25. doi: 10.1159/000166183. [DOI] [PubMed] [Google Scholar]
- 38.Gidday JM, Gasche YG, Copin JC, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289(2):558–68. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 39.Bittner A, Alcaíno H, Castro PF, et al. Matrix metalloproteinase-9 activity is associated to oxidative stress in patients with acute coronary syndrome. Int J Cardiol. 2010;143(1):98–100. doi: 10.1016/j.ijcard.2008.11.188. [DOI] [PubMed] [Google Scholar]
- 40.Fokkelman K, Haase E, Stevens J, et al. Tissue-specific changes in glutathione content of hypoxic newborn pigs reoxygenated with 21% or 100% oxygen. Eur J Pharmacol. 2007;562(1–2):132–37. doi: 10.1016/j.ejphar.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 41.Murri M, García-Delgado R, Alcázar-Ramírez J, et al. Continuous Positive Airway Pressure Therapy Reduces Oxidative Stress Markers and Blood Pressure in Sleep Apnea-Hypopnea Syndrome Patients. Biol Trace Elem Res. 2011 doi: 10.1007/s12011-011-8969-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]