Summary
Background
The aim of this study was to evaluate the concentration of malondialdehyde (MDA) in erythrocytes and in blood plasma and the activity of blood paraoxonase (PON1) of patients with osteoarthrosis (OA) submitted to endoprosthesis implantation for evaluating oxidative stress.
Material/Methods
Study was conducted on 55 patients with OA and on 54 total movement-efficient volunteers. The material for the study was venous blood plasma, serum and erythrocytes.
Results
Increased concentration of MDAe before surgery was observed in the group of men and in patients with a degenerative process affecting hip joints. After an implantation of endoprosthesis, MDAe decreased to the level observed in the control groups. In the study group MDA concentration in plasma was slightly lower before surgery, and after an operation it reached the value of the parameter of the reference groups. Regardless of sex or age, paraoxonase activity was almost twice as high in almost all subgroups as in the reference group. A positive correlation between PON 1 activity and MDAe concentration was demonstrated both before and after surgery in the group of men.
Conclusions
The increase of PON1 activity in patients’ serum in relation to the control groups indicates a probable pathogenic role of the increased formation of reactive oxygen species in the course of OA and may suggest acute inflammation of the synovial joint. The high level of PON 1 activity after endoprosthesis implantation indicates that surgical treatment may additionally stimulate ROS generation. MDAe concentration indicate more intensive process of lipid peroxidation in the elderly.
Keywords: thiobarbituric acid, paraoxonase, osteoarthrosis, endoprosthesis
Background
Bone tissue is dynamic and subject to a continuous formation and resorption process [1]. During this process there is a balance in the activities of osteoblasts and osteoclasts. Many reports have shown that reactive oxygen species (ROS) may be involved in the pathogenesis of bone loss and can contribute to degradation of the cartilage matrix.
The presence of oxygen free radicals (OFR) inside and outside cells causes many pathological processes including inflammation, carcinogenesis, age-related diseases, atherosclerosis, ischemia, and neurological diseases (Parkinson’s, Alzheimer’s) [2–5]. They may easily react with deoxyribonucleic acid, lipids and proteins because of very high reactivity. Their mode of action changes the biological functions of basal cell components and thus probably leads to inappropriate enzymic activation or inactivation, irreversible transformation, mutations and even cell death [6–8]. OFR causes the damage of protein physico-chemical structure, lipid peroxidation, nucleic acids injury and hyaluronic acid depolymerization [9].
Lipid peroxidation involves the process of oxidative decomposition of polyunsaturated fatty acids (PUFA) of membrane phospholipids, leading to formation of mixtures of lipid hydroperoxide, and aldehydic end-products such as MDA [10–12]. Lipid peroxidation changes permeability and fluidity of cell membranes. Bonner et al. [13] demonstrated that lipids, especially polyunsaturated fatty acids, accumulate with normal aging of cartilage. In several models of degenerative arthritis, lipid accumulation generally precedes local tissue degeneration [14,15]. Age-related changes in the lipid composition of cartilage could push the normally contained lipid peroxidation process into a state of uncontrolled oxidative stress, leading to the oxidation of cartilage collagen. Oxidation of collagen could cause fragmentation which makes collagen fibrils more brittle and prone to mechanical fatigue failure [16]. Such failure could initiate osteoarthritis.
One of the best-known products of OFR reaction with lipids is malondialdehyde (MDA), formed in the lipid peroxidation process [6,17].
MDA is believed to be largely responsible for cytopathological effects observed during oxidative stress of lipid peroxidation [10,11]. MDA reacts with histidine and lysine residues of proteins to form stable adducts. MDA in some conditions may also induce changes in DNA by cross-linking formation [18,19].
Serum paraoxonase (PON1) is a calcium-dependent esterase known to catalyze hydrolysis of organophosphates, aryl esters, lactones and hydroperoxides and is widely distributed among tissues such as the liver, kidneys, intestine and plasma [20–22]. PON1 is exclusively bound to high-density lipoprotein (HDL) and is recognized as an antioxidant enzyme because it hydrolyses lipid peroxides in oxidized lipoproteins [23,24]. PON1 activity was suggested to be inversely associated with oxidative stress in serum and macrophages [25]. In several groups of patients with diabetes, hypercholesterolemia, and cardiovascular disease who are under increased oxidative stress, the reduced activity of PON1 has been reported [26–28].
MDA has not been searched for together with the antioxidant enzyme PON1 in OA patients thus far. We aimed to determine a possible correlation between serum PON1 activities as known antioxidant and MDA levels, end-products of lipid peroxidation, induced by ROS for evaluating oxidative stress in OA patients.
Material and Methods
Subjects
Subjects included 36 women aged 46–78 (average age: 66.47±9.94) and 19 men aged 40–77 (average age: 59.05±10.75) with clinically recognised degenerative disease of hip or knee joint who had been treated in the Department of Orthopedics and Traumatology at the Ludwik Rydygier Collegium Medicum in Bydgoszcz (part of Nicolaus Copernicus University in Toruń). Unilateral or bilateral hip osteoarthritis had been reported in 46 patients (29 women and 17 men); OA of unilateral or bilateral knee joint disease in 9 patients (7 women and 2 men). In some of the patients other concomitant diseases had been reported, among which the most frequent were myocardial ischaemia, diabetes and hypothyreosis.
There were 26 women aged 24–90 (average age 64.03±14.57) and 28 men aged 32–89 (average age 61.86±14.68) with normal motor fitness in the control group.
The biochemical tests were conducted in the Department of Medical Biology at Ludwik Rydygier Collegium Medicum in Bydgoszcz. The research was approved by the Bioethics Committee at L. Rydygier Medical University in Bydgoszcz (KB/470/2004).
The material for the study was venous blood plasma, serum and erythrocytes. Blood was taken after fasting, from the cubital vein, during a routine exam and kept in dry and sterile test-tubes (Grainer Bio-one, Austria). In the group of patients blood was taken twice – once before the arthroplasty and once on the tenth day after the surgery.
The whole blood with addition of 3.2% sodium citrate was centrifuged at 4°C to obtain erythrocytes and plasma. The plasma was collected for assessing MDA level. After removing plasma, the cells were washed using 3 volumes of phosphate buffered saline (PBS). The haemoglobin concentration in the hemolysates was assayed with the colorimetric standard method using Drabkin’s reagent and expressed as g/dl.
The blood samples without anticoagulant, after complete clotting, were centrifuged for 10 min. at 3000 rotations per min., at 4°C. Serum from the samples was placed into Eppendorf test-tubes and kept frozen at −20°C until the marking of lysosomal enzymes was performed. Serum was thawed about an hour before the study.
Chemicals
Thiobarbituric acid (TBA), trichloroacetic acid (TCA), adrenaline and hydrogen peroxide were purchased from Fluka (Sigma-Alrich Sp. z o.o, Poland), butylated hyroxytoluene (BHT) and paraoxon was from Aldrich (Sigma-Aldrich sp. z o.o, Poland). Folin phenol reagent, sodium carbonate, sodium potassium tartrate ×4 H20, copper sulfate, Folin-Ciocalteau solution, fibrinogen, and calcium chloride were purchased from POCH (Gliwice, Poland). Other reagents were of analytical grade.
Concentration of thiobarbituric acid reactive substances (TBARS) – malondialdehyde (MDA) level in erythrocytes and blood plasma
Determination of MDA level in erythrocytes
The erythrocyte malondialdehyde (MDAe) level was determined according to the method of Buege and Aust [29] in Esterbauer and Cheeseman’s later modification [30]. This method is based on the reaction with thiobarbituric acid (TBA) in an acidic pH at 90–100°C. In the TBA test reaction, MDA or MDA-like substances (produced during lipid peroxidation) and TBA react with production of a pink pigment with a 532 nm absorption maximum. The specimens were joined with butylated hydroxytoluene (BHT) and TCA, then incubated at room temperature for 10 min and centrifuged (4000 g, 10 min.). The supernatants reacted with a mixture of TBA and TCA in a boiling water bath for 20 min. After cooling, the absorbance was read at 532 nm.
Determination MDA level in blood plasma
The samples were mixed with mixture of TBA and trichloroacetic acid (TCA) in HCl to precipitate protein. The reaction was performed at pH 2–3 at 90°C for 20 min. The precipitate was pelleted by centrifugation at 4000 g at room temperature for 15 min. Absorption of supernatants was read at a wavelength of 532 nm.
The majority of TBARS are malondialdehydes, thus the concentration of MDA in blood plasma was expressed as nmol MDA/ml. The results were calculated using an index of absorption for MDA of 1.56×105/M/cm. The concentration of MDA in erythrocytes was expressed as nmol MDA/g Hb.
Determination of paraoxonase activity
Paraoxonase activities were measured according to the method of Playfer et al. [31] with later modification of Sogorb et al. [32]. The assay buffer contained 0.1M TRIS-HCl (pH 9.0), 5 mM CaCl2. The reaction was initiated by adding blood serum to the freshly prepared paraoxon (diethyl-p-nitrophenyl phosphate), and the increase in absorbance was monitored at 412nm with the use of a continuously recording spectrophotometer. The rate of generation of p-nitrophenol was determined at 37°C. Enzyme activity was calculated from the rate of p-nitrophenol production (molar extinction coefficient – 18029 M/cm) and was expressed as units per l of blood serum (1U PON activity was defined as 1 μM paraoxon hydrolyzed min-1 under the above assay conditions).
Statistical analysis
The Statistical Package for Social Sciences (SPSS Inc., Chicago, USA, version 12.0) was used for the analyses. Kolmogorov-Smirnov and Shapiro-Wilks tests were applied for the compatibility estimation of the studied parameters’ distribution with normal distribution. All results are expressed as mean ±SD; differences between groups were assessed using non-parametric Mann-Whitney U-test. Spearman rank correlation (for categorical variables) and Pearson correlation coefficients were used to assess the relationship between parameters. A two-tailed P value of <0.05 was considered statistically significant.
Results
Characteristics of the study group are presented in Table 1. The results obtained in the studied group are presented together (Figure 1, Table 2) and separately with division into men and women (Table 3), and also into groups of patients under 69 and over 70 year of life (Table 4).
Table 1.
Characteristics of study groups.
| Number | Percent | Age [years]
|
|||
|---|---|---|---|---|---|
| Mean | SD | Min-Max | |||
| Osteoarthrosis | 55 | 100.0 | 63.91 | 10.73 | 40–78 |
| Gender | |||||
| Women | 36 | 65.5 | 66.47 | 9.94 | 46–78 |
| Men | 19 | 34.5 | 59.05 | 10.75 | 40–77 |
| Age group | |||||
| <69 | 31 | 56.4 | 56.39 | 8.164 | 40–69 |
| >70 | 24 | 43.6 | 73.63 | 2.96 | 70–78 |
| Controls | 57 | 100.0 | 62.96 | 14.57 | 24–90 |
| Gender | |||||
| Women | 29 | 50.9 | 64.03 | 14.63 | 24–90 |
| Men | 28 | 49.1 | 61.86 | 14.68 | 32–89 |
| Age group | |||||
| <69 | 36 | 63.2 | 54.47 | 10.89 | 24–69 |
| >70 | 21 | 36.8 | 77.52 | 5.84 | 70–90 |
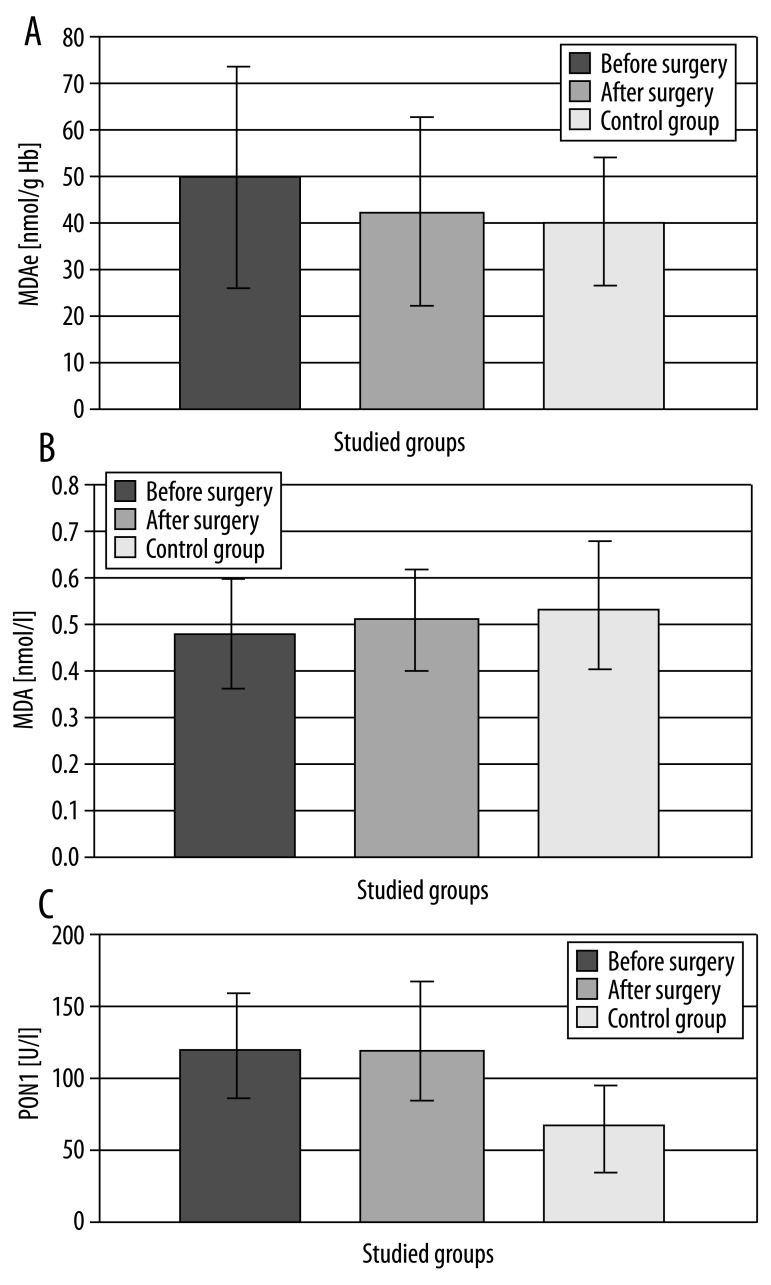
Figure 1.
Level of malondialdehyde in erythrocytes (MDAe), malondialdehyde in blood plasma (MDA) and activity of paraoxonase 1 (PON1) in blood serum of patients with osteoarthrosis (studied group) and in the control group.
Table 2.
Level of malondialdehyde in erythrocytes (MDAe), malondialdehyde in blood plasma (MDA) and activity of paraoxonase I (PON1) in blood serum of patients with osteoarthrosis (studied group) and in the control group.
| Osteoarthrosis group (n=55) | Control group (n=57) | |||||
|---|---|---|---|---|---|---|
| Before surgery | 10 days after surgery | Mean ±SD | Min–Max | |||
| Mean ±SD | Min–Max | Mean ±SD | Min–Max | |||
| MDAe [nmol/g Hb] | 49.75±23.98 | 19.51–206.99 | 42.37±20.33 | 9.23–161.00 | 40.24±13.81 | 22.86–86.99 |
| MDA [nmol/ml] | 0.48±0.11 | 0.19–0.74 | 0.52±0.13 | 0.31–0.84 | 0.54±0.14 | 0.26–0.90 |
| PON1 [U/l] | 116.62±41.58* | 33.99–269.71 | 113.30±51.59*,** | 22.85–369.14 | 61.83±30.61 | 12.52–200.59 |
Statistically significant as compared to a control group
p<0.01. Statistically significant as compared to a studied group before surgery
p< 0.01.
Table 3.
Level of malondialdehyde in erythrocytes (MDAe), malondialdehyde in blood plasma (MDA) and activity of paraoxonase (PON1) in blood serum of men and women with osteoarthrosis (studied groups) and in the control groups.
| Osteoarthrosis men group (n=19) | Control men group (n=28) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before surgery | 10 days after surgery | Mean | SD | Min–Max | |||||
| Mean | SD | Min–Max | Mean | SD | Min–Max | ||||
| MDAe [nmol/g Hb] | 64.42* | 29.53 | 23.98–206.99 | 45.38 | 17.74 | 23.45–92.46 | 42.58 | 17.08 | 22.86–86.99 |
| MDA [nmol/ml] | 0.48 | 0.12 | 0.19–0.69 | 0.53 | 0.14 | 0.31–0.84 | 0.55 | 0.15 | 0.26–0.88 |
| PON1 [U/l] | 119.54* | 40.19 | 40.53–193.70 | 97.60**,# | 40.11 | 22.85–178.20 | 64.60 | 30.72 | 12.52–200.59 |
| Osteoarthrosis women group (n=36) | Control women group (n=29) | ||||||||
| Before surgery | 10 days after surgery | Mean | SD | Min–Max | |||||
| Mean | SD | Min–Max | Mean | SD | Min–Max | ||||
| MDAe [nmol/g Hb] | 41.44** | 16.73 | 19.51–89.64 | 40.50 | 17.79 | 9.23–161.00 | 37.98 | 9.45 | 23.08–54.89 |
| MDA [nmol/ml] | 0.49 | 0.11 | 0.25–0.74 | 0.51 | 0.11 | 0.32–0.79 | 0.53 | 0.14 | 0.33–0.90 |
| PON1 [U/l] | 115.12** | 42.77 | 33.99–269.71 | 123.39*,## | 60.99 | 38.23–369.14 | 59.05 | 23.93 | 26.41–126.76 |
Statistically significant as compared to a control group
p< 0.05;
p< 0.01. Statistically significant as compared to a studied group before surgery
p<0.01;
p<0.05.
Table 4.
Level of malondialdehyde in erythrocytes (MDAe), malondialdehyde in blood plasma (MDA) and activity of paraoxonase (PON1) in blood serum of OA patients under 69 years and over 70 year (studied groups) and in the control groups.
| Studied group of <69 years (n=31) | Control group of <69 year olds (n=36) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before surgery | 10 days after surgery | Mean | SD | Min–Max | |||||
| Mean | SD | Min–Max | Mean | SD | Min–Max | ||||
| MDAe [nmol/g Hb] | 47.86** | 19.85 | 19.51–171.36 | 41.31 | 19.02 | 9.23–93.46 | 39.82 | 13.29 | 23.08–86.99 |
| MDA [nmol/ml] | 0.48* | 0.12 | 0.19–0.72 | 0.51 | 0.14 | 0.31–0.84 | 0.55 | 0.16 | 0.26–0.90 |
| PON1 [U/l] | 117.20* | 43.65 | 33.99–269.71 | 105.83*,# | 42.76 | 53.04–247.89 | 66.76 | 32.58 | 12.52–200.59 |
| Studied group >70 years (n=24) | Control group of >70 year olds (n=24) | ||||||||
| Before surgery | 10 days after surgery | Mean | SD | Min–Max | |||||
| Mean | SD | Min–Max | Mean | SD | Min–Max | ||||
| MDAe [nmol/g Hb] | 52.30* | 19.54 | 23.98–206.99 | 43.93**,# | 21.08 | 9.93–161.00 | 40.97 | 14.98 | 22.86–79.69 |
| MDA [nmol/ml] | 0.49 | 0.11 | 0.32–0.74 | 0.53 | 0.10 | 0.32–0.71 | 0.53 | 0.12 | 0.33–0.81 |
| PON1 [U/l] | 115.86* | 39.66 | 40.53–193.70 | 124.91*,## | 53.09 | 22.85–369.14 | 52.95 | 22.00 | 24.00–112.98 |
Concentration of malondialdehyde in all studied patients’ erythrocytes was higher than in the reference group; however, the stated difference was not statistically significant. Increased concentration of MDAe before surgery was noticed in the group of men with OA (64.42 nmol/g Hb, p<0.01). After implantation of the endoprosthesis, MDAe concentration in plasma decreased to the level found in the control groups. Only in the group of patients older than 70 did the postoperative concentration of MDAe differ significantly from the value of the parameter in the control group: accordingly (43.93 vs. 40.97 nmol/g Hb; p<0.05). Statistically significant differences in the level of malondialdehyde in erythrocytes before and after surgery were found in the group of the oldest patients. At the same time, there was a statistically significant difference in MDA concentration in erythrocytes before implantation of the endoprosthesis between men and women in both studied and control groups (higher in men, p<0.05). In case of determining MDA concentration in blood plasma, in the studied group it was slightly lower before surgery, and after surgical treatment it reached a level close to the value of this parameter in the reference group. Statistically significant differences between the level of malondialdehyde in blood plasma of patients before the endoprosthesis-plasty and those in the control group were noticed only in patients younger than 69 (p<0.01).
Regardless of the sex or age, paraoxonase activity was twice as high in all studied groups as in the reference group, and the differences were statistically significant.
PON1 activity after surgical treatment increased in serum of studied women (by 7%, p<0.05) and also the group of patients older than 70 (about 8%, p<0.05). In the other groups, endoprosthesis implantation resulted in a decrease of PON1 activity: male patients (19%, p<0.01) and younger patients (10%, p<0.01). A positive correlation between PON1 activity and MDAe concentration was demonstrated, both before and after surgery in the group of male patients (r=0.318; p<0.01).
Discussion
The assumption that extremely labile reactive oxygen species (ROS) may play a role in the regulation of tissue oxidation-antioxidant processes in articular cartilage seems to be problematic due to a lack of vascularization and oxygenation. In vitro studies have shown that the concentration of oxygen on the surface of the cartilage was 5%, and in its deeper layer 1% [33]. Collagen is the element of tissue that is particularly prone to oxidative damages, because its mechanical properties are weakened by the damages [34]. The damage of the matrix proteoglycans and extracellular fluids caused by ROS exposes the joint cartilage to risk of mechanical damage, even at loads just under physiological conditions, and changes the tissue’s ability to maintain water [35]. Kempson et al. [36] showed that degenerative changes of articular cartilage are accompanied by the resistance to deformity forces together with damage to collagen fibrils. In most cases, patients with disease-involved oxidative stress have lower PON1 activity [37]. A logical explanation for the upregulation of PON1 activity patient toward control is the influence of PON1 genetic polymorphisms on serum PON1 activity. The PON1 gene has 2 common polymorphisms in the coding region, which lead to a glutamine – arginine substitution at position 192 and leucine – methionine substitution at position 55 [38]. There were 3 different phenotypes that corresponded to high, intermediate, and low serum PON1 activities. Also, the frequency for the low-activity allele varied considerably among populations of different geographical/ethnic origin, a higher frequency being observed in the Caucasian population. Considerable differences were observed in relation to the PON155 genotype, with individuals carrying a leucine at position 55 (L isoform) having higher serum PON1 concentrations than those with a methionine (M isoform) at this position. A practical and important consequence of the influence of PON1 genetic polymorphisms on serum PON1 activity is that it becomes necessary that, when performing case-control studies, the observed differences in enzyme activities are matched by genotype [39].
The decrease of PON1 activity after endoprosthesis implantation in examined men and younger persons confirms the results of experiments by Regan et al. [40], which proved a correlation between OA and the activity of ROS main scavengers in the articular cartilage, suggesting that damages caused by ROS play a key role at both initial and progressive stages of disease. Regan et al. also reported that with increasing body weight, excessive shear forces rooted in instability or improper setting of a joint in relation to its axis, as well as an excessive load of joint while performing daily activities, explain the increased activity of reactive particles in the articular cartilage. The ability of scavenging ROS within a joint may depend on genetic factors – it is possible that remaining under the influence of hormones, age at which first OA symptoms were observed, and other interindividual differences play a role. Buffering capacities of ROS can also be weakened by other diseases, in the course of which ROS are generated [40].
The other described joint element playing a vital role in the pathogenesis of OA is a synovial compartment [41]. Ayral et al. found that in the course of acute synovial inflammation, changes are observed in the nature of its proliferation and inflammation. Activated synovium may release proteases and cytokines that accelerate the deterioration of pathological changes adjacent to the synovial cartilage. Among some of our patients (especially those with post-traumatic and rheumatoid OA) high activity of PON1 before surgery may indicate acute inflammation of synovial joint. In the advanced stages of OA, “synovitis” may even resemble the image of synovial membrane inflammatory hyperplasia (pannus) observed in rheumatoid arthritis (RA) [42]. MDA, very harmful for an organism, being the final product of rapid oxidation of unsaturated fatty acids, is currently the most commonly assessed marker of lipid peroxidation. In numerous scientific studies, statistically higher levels of MDA were found in blood, synovial fluid and urine of patients with rheumatoid arthritis compared to the control group [43,44]. Our study also shows higher MDA concentration in erythrocytes of patients (aetiology of OA in 7 cases was rheumatoid) compared to the control group.
Tiku et al. [16] conducting in vitro studies found that lipid peroxidation, and not the release of ROS, intervened in the degradation of matrix-dependent activation of chondrocyte. The detrimental effect of lipid peroxidation is initiated by a chain reaction that generates free radicals, which induce further peroxidation [45,46]. Paraoxonase 1 is a part of 1 of the 3 systems of the organism’s defence against ROS, called the third line of defence, and is formed to repair damages already caused by ROS activity. Properties of reducing the products of lipid peroxidation by this enzyme may be associated with the increased levels of its activity in certain motor system diseases. There are, however, some controversies concerning the behavior of PON1 in patients with rheumatoid arthritis. Tantimoto et al. [47] explained that the stated decrease of the enzyme activity in patients with rheumatoid arthritis (depending on the stage of the disease) is a result of its intensified inactivation due to the increased formation of ROS. At the same time, the increase of PON1 activity after endoprosthesis implantation in women, and those older than 70, shows that the surgery might have an inhibitory influence on ROS generation. The main components of the extracellular matrix, collagen type II and proteoglycans, are subject to quantitative changes relating to structural arrangement and organization during the aging process [48]. However, despite the fact that changes in the composition and structure of cartilage are inevitable, the development of OA while aging, in spite of being common, does not relate to the general population [49]. In our work we have found no significant differences between the 2 age groups evaluated in terms of MDA activity in plasma, although in the subgroup of younger people the value of this parameter differed significantly from the control group (P<0.01). MDAe concentration, higher in the elderly group, indicates an intensified process of lipid peroxidation in this group of patients. A lower value of the studied parameter in the control group younger than 69 in relation to the group older than 70 may also confirm the dependence of the MDA concentration in erythrocytes on patients’ age. Our observations may be confirmed by the findings of Bonner et al. [13] who concluded who, developing the lipid profile of human articular cartilage, proved that lipids, particularly polyunsaturated fatty acids, accumulate as physiological aging of cartilage advances. At the same time, research into many models of osteoarthritis showed that local tissue degeneration usually precedes the process of lipid accumulation [50].
Consumption of PON1 to prevent oxidation results in a decrease of serum PON1 activity [51,52]. The protection against lipid peroxidation is achieved by PON1, during which free sulfhydryl groups of PON1 interact with specific oxidized lipids, and thus PON1 is inactivated [51]. Baskol et al. [43] concluded that the increased ROS levels might cause increased lipid peroxidation, and thus result in decreased antioxidant PON1 activity and increased serum MDA levels, and also determined a significant negative correlation between serum MDA and PON1 activities in the patient group. In our study the surgery seems (decreased PON1 activity and increased serum MDA but no significant correlation MDA/PON1) to cause an increase of ROS production in males and older patients.
Red blood cells are frequently used as an in vitro model for evaluation the antioxidant properties of different factors (in our study, surgery in OA). Erythrocytes exhibit a considerable oxidative stress resistance, but unsaturated fatty acids present in erythrocyte membrane can undergo peroxidation under these conditions [53]. Apparently, red blood cells of the examined men and patients over 70 years are more susceptible to oxidative stress in comparison to other studied groups.
Conclusions
Our research and statistical analysis of the obtained results show both an increased ROS generation and intensified lipid peroxidation in the course of OA. It is the peroxidation of lipids, more severe in the elderly, which probably plays a key role in the pathogenesis of osteoarthritis.
Acknowledgements
The authors thank Nordea Bank (Bydgoszcz, Poland) for technical support during the preparation of this manuscript.
Abbreviations
- BHT
butylated hydroxytoluene
- GR
glutathione reductase
- H2O2
hydrogen peroxide
- MDA e
erythrocyte malondialdehyde
- NADPH
nicotinamide adenine dinucleotide phosphate
- OA
osteoarthritis
- OFR
oxygen free radicals
- PBS
phosphate buffered saline
- PON
paraoxonase
- ROS
reactive oxygen species
- TBA
thiobarbituric acid
- TBARS
lipid peroxidation product reactive with TBA
- TCA
trichloroacetic acid
Footnotes
Source of support: Departmental sources
References
- 1.Eriksen EF. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord. 2010;11(4):219–27. doi: 10.1007/s11154-010-9153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkner E, Zalejska-Fiolka J, Antoszewski Z. Antioxidant enzymes activity and role of antioxidant vitamins in Alzheimer disease. Post Hig Med Dosw. 2004;58:264–69. [PubMed] [Google Scholar]
- 3.Cobbs CS, Samanta M, Harkins LE, et al. Evidence for peroxynitrate-mediated modifications to p53 in human gliomas: possible functional consequences. Arch Biochem Biophys. 2001;394:167–72. doi: 10.1006/abbi.2001.2540. [DOI] [PubMed] [Google Scholar]
- 4.Pazdernik TL. The osmotic/calcium stress theory of brain damage is free radicals involved. Neurochem Res. 1992;17:11–21. doi: 10.1007/BF00966860. [DOI] [PubMed] [Google Scholar]
- 5.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B, Gutteridge JM. Free radicals in biology and medicine. Clarendon Press; Oxford: 1993. pp. 86–187. [Google Scholar]
- 7.Hinshaw DB. A cellular model of antioxidant mediated neuronal injury. Brain Res. 1993;615:13–26. doi: 10.1016/0006-8993(93)91110-e. [DOI] [PubMed] [Google Scholar]
- 8.Tews DS. Cell death and oxidative stress in gliomas. Neuropathol Appl Neurobiol. 1999;25:272–84. doi: 10.1046/j.1365-2990.1999.00186.x. [DOI] [PubMed] [Google Scholar]
- 9.Prabha PS, Das UN, Koratkar R, et al. Free radical generation, lipid peroxidation and essential fatty acids in uncontrolled essential hypertension. Prostaglandis Leukot Essent Fatty Acids. 1990;41:27–33. doi: 10.1016/0952-3278(90)90127-7. [DOI] [PubMed] [Google Scholar]
- 10.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 11.Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22(1–2):287–305. doi: 10.1016/s0891-5849(96)00327-9. [DOI] [PubMed] [Google Scholar]
- 12.Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39(8):1529–42. [PubMed] [Google Scholar]
- 13.Bonner WM, Jonsson H, Malanos C, Bryant M. Changes in the lipids of human articular cartilage with age. Arthritis Rheum. 1975;18(5):461–73. doi: 10.1002/art.1780180505. [DOI] [PubMed] [Google Scholar]
- 14.Silberberg M, Silberberg R. Effects of a high fat diet on the joints of aging mice. AMA Arch Pathol. 1950;50(6):828–46. [PubMed] [Google Scholar]
- 15.Silberberg M, Silberberg R, Orcutt B. Modifying effect of linoleic acid on articular aging and osteoarthrosis in lard-fed mice. Gerontologia. 1965;11(3):179–87. doi: 10.1159/000211491. [DOI] [PubMed] [Google Scholar]
- 16.Tiku ML, Shah R, Allison GT. Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation. Possible role in cartilage aging and the pathogenesis of osteoarthritis. J Biol Chem. 2000;275(26):20069–76. doi: 10.1074/jbc.M907604199. [DOI] [PubMed] [Google Scholar]
- 17.Smith CV. Correlation and apparent contradictions in assessment of oxidant stress status in vivo. Free Radic Biol Med. 1991;10:217–24. doi: 10.1016/0891-5849(91)90079-i. [DOI] [PubMed] [Google Scholar]
- 18.Dooley PA, Tsarouhtsis D, Korbel GA, et al. Structural studies of an oligodeoxynucleotide containing a trimethylene interstrand cross-link in a 5′-(CpG) motif: model of a malondialdehyde cross-link. J Am Chem Soc. 2001;123(8):1730–39. doi: 10.1021/ja003163w. [DOI] [PubMed] [Google Scholar]
- 19.Oliński R, Jurgowiak M. Oxidative DNA base modifications and their role in ageing and degenerative disease. Post Biol Kom. 1999;(Supl 3):3–22. [Google Scholar]
- 20.Draganov DI, La Du BN. Naunyn Schmiedebergs Arch Pharmacol. 2004;369(1):78–88. doi: 10.1007/s00210-003-0833-1. [DOI] [PubMed] [Google Scholar]
- 21.La Du BN. Structural and functional diversity of paraoxonases. Nat Med. 1996;2(11):1186–87. doi: 10.1038/nm1196-1186. [DOI] [PubMed] [Google Scholar]
- 22.La Du BN, Adkins S, Kuo CL, Lipsig D. Studies on human serum paraoxonase/arylesterase. Chem Biol Interact. 1993;87(1–3):25–34. doi: 10.1016/0009-2797(93)90022-q. [DOI] [PubMed] [Google Scholar]
- 23.Aviram M, Rosenblat M, Bisgaier CL, et al. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest. 1998;101(8):1581–90. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackness MI, Mackness B, Durrington PN, et al. Paraoxonase: biochemistry, genetics and relationship to plasma lipoproteins. Curr Opin Lipidol. 1996;7(2):69–76. doi: 10.1097/00041433-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Rozenberg O, Rosenblat M, Coleman R, et al. Paraoxonase (PON1) deficiency is associated with increased macrophage oxidative stress: studies in PON1-knockout mice. Free Radic Biol Med. 2003;34(6):774–84. doi: 10.1016/s0891-5849(02)01429-6. [DOI] [PubMed] [Google Scholar]
- 26.Ayub A, Mackness MI, Arrol S, et al. Serum paraoxonase after myocardial infarction. Arterioscler Thromb Vasc Biol. 1999;19(2):330–35. doi: 10.1161/01.atv.19.2.330. [DOI] [PubMed] [Google Scholar]
- 27.Feretti G, Bacchetti T, Masciangelo S, Bicchiega V. HDL-paraoxonase and membrane lipid peroxidation: a comparison between healthy and obese subjects. Obesity (Silver Spring) 2010;18(6):1079–84. doi: 10.1038/oby.2009.338. [DOI] [PubMed] [Google Scholar]
- 28.Mackness MI, Harty D, Bhatnagar D, et al. Serum paraoxonase activity in familial hypercholesterolaemia and insulin-dependent diabetes mellitus. Atherosclerosis. 1991;86(2–3):193–99. doi: 10.1016/0021-9150(91)90215-o. [DOI] [PubMed] [Google Scholar]
- 29.Buege SC, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1987;51:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 30.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Method Enzymol. 1990;186:407–21. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 31.Playfer JR, Eze LC, Bullen MF, Evans DA. Genetic polymorphism and interethnic variability of plasma paraoxonase activity. J Med Genet. 1976;13:337–42. doi: 10.1136/jmg.13.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sogorb MA, Díaz-Alejo N, Escudero MA, Vilanova E. Phosphotriesterase activity identified in purified serum albumins. Arch Toxicol. 1998;72(4):219–26. doi: 10.1007/s002040050492. [DOI] [PubMed] [Google Scholar]
- 33.Silver IA. Measurement of pH and ionic composition of pericellular sites. Philos Trans R Soc Lond B Biol Sci. 1975;271(912):261–72. doi: 10.1098/rstb.1975.0050. [DOI] [PubMed] [Google Scholar]
- 34.Monboisse JC, Borel JP. Oxidative damage to collagen. EXS. 1992;62:323–27. doi: 10.1007/978-3-0348-7460-1_32. [DOI] [PubMed] [Google Scholar]
- 35.McCord JM. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974;185(150):529–31. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- 36.Kempson GE, Muir H, Pollard C, Tuke M. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim Biophys Acta. 1973;297(2):456–72. doi: 10.1016/0304-4165(73)90093-7. [DOI] [PubMed] [Google Scholar]
- 37.Zielaskowska J, Olszewska-Słonina D. The polymorphism of paraoxonase and its effects in physiological and pathological processes. Adv Clin Exp Med. 2006;15(6):1073–78. [Google Scholar]
- 38.Ozkök E, Aydin M, Babalik E, et al. Combined impact of matrix metalloproteinase-3 and paraoxonase 1 55/192 gene variants on coronary artery disease in Turkish patients. Med Sci Monit. 2008;14(10):CR536–42. [PubMed] [Google Scholar]
- 39.Camps J, Marsillach J, Joven J. The paraoxonases: role in human diseases and methodological difficulties in measurement. Crit Rev Clin Lab Sci. 2009;46(2):83–106. doi: 10.1080/10408360802610878. [DOI] [PubMed] [Google Scholar]
- 40.Regan E, Flannelly J, Bowler R, et al. Extracellular superoxide dismutase and oxidant damage in osteoarthritis. Arthritis Rheum. 2005;52(11):3479–91. doi: 10.1002/art.21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayral X, Pickering EH, Woodworth TG, et al. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13(5):361–67. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Shibakawa A, Aoki H, Masuko-Hongo K, et al. Presence of pannus-like tissue on osteoarthritic cartilage and its histological character. Osteoarthritis Cartilage. 2003;11(2):133–40. doi: 10.1053/joca.2002.0871. [DOI] [PubMed] [Google Scholar]
- 43.Baskol G, Demir H, Baskol M, et al. Assessment of paraoxonase 1 activity and malondialdehyde levels in patients with rheumatoid arthritis. Clin Biochem. 2005;38:951–55. doi: 10.1016/j.clinbiochem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Jaswal S, Mehta HC, Sood AK, Kaur J. Antioxidant status in rheumatoid arthritis and role of antioxidant therapy. Clin Chim Acta. 2003;338:123–29. doi: 10.1016/j.cccn.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 45.van Acker SA, Koymans LM, Bast A. Molecular pharmacology of vitamin E: structural aspects of antioxidant activity. Free Radic Biol Med. 1993;15(3):311–28. doi: 10.1016/0891-5849(93)90078-9. [DOI] [PubMed] [Google Scholar]
- 46.Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41(12 Pt 2):1819–28. [PubMed] [Google Scholar]
- 47.Tanimoto N, Kumon Y, Suehiro T, et al. Serum paraoxonase activity decreases in rheumatoid arthritis. Life Sci. 2003;72(25):2877–85. doi: 10.1016/s0024-3205(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 48.Verzijl N, Bank RA, TeKoppele JM, DeGroot J. Ageing and osteoarthritis: a different perspective. Curr Opin Rheumatol. 2003;15(5):616–22. doi: 10.1097/00002281-200309000-00016. [DOI] [PubMed] [Google Scholar]
- 49.Carrington JL. Aging bone and cartilage: cross-cutting issues. Biochem Biophys Res Commun. 2005;328(3):700–8. doi: 10.1016/j.bbrc.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 50.Murphy JM, Dixon K, Beck S, et al. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46(3):704–13. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 51.Göçmen YA, Gümüşlü S, Semiz E. Association between paraoxonase-1 activity and lipid peroxidation indicator levels in people living in the Antalya region with angiographically documented coronary artery disease. Clin Cardiol. 2004;27(7):426–30. doi: 10.1002/clc.4960270714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Göçmen YA, Semiz E, Gümüşlü S. Relationship between paraoxonase-1 (PON1) activity and lipoprotein (a) levels in Turkish coronary artery disease patients living in the Antalya region. Eur J Cardiovasc Prev Rehabil. 2005;12(2):185–86. doi: 10.1097/00149831-200504000-00016. [DOI] [PubMed] [Google Scholar]
- 53.Sawicka E, Długosz A, Roszkowska A. Synergistic effect of Scutellaria baicalensis extract and Q10 coenzyme. Bromat Chem Toksykol. 2009;XLII(1):59–64. [Google Scholar]