ABSTRACT
Fe(II)-oxidizing aerobic bacteria are poorly understood, due in part to the difficulties involved in laboratory cultivation. Specific challenges include (i) providing a steady supply of electrons as Fe(II) while (ii) managing rapid formation of insoluble Fe(III) oxide precipitates and (iii) maintaining oxygen concentrations in the micromolar range to minimize abiotic Fe(II) oxidation. Electrochemical approaches offer an opportunity to study bacteria that require problematic electron donors or acceptors in their respiration. In the case of Fe(II)-oxidizing bacteria, if the electron transport machinery is able to oxidize metals at the outer cell surface, electrodes poised at potentials near those of natural substrates could serve as electron donors, eliminating concentration issues, side reactions, and mineral end products associated with metal oxidation. To test this hypothesis, the marine isolate Mariprofundus ferrooxydans PV-1, a neutrophilic obligate Fe(II)-oxidizing autotroph, was cultured using a poised electrode as the sole energy source. When cells grown in Fe(II)-containing medium were transferred into a three-electrode electrochemical cell, a cathodic (negative) current representing electron uptake by bacteria was detected, and it increased over a period of weeks. Cultures scraped from a portion of the electrode and transferred into sterile reactors consumed electrons at a similar rate. After three transfers in the absence of Fe(II), electrode-grown biofilms were studied to determine the relationship between donor redox potential and respiration rate. Electron microscopy revealed that under these conditions, M. ferrooxydans PV-1 attaches to electrodes and does not produce characteristic iron oxide stalks but still appears to exhibit bifurcate cell division.
IMPORTANCE
Electrochemical cultivation, supporting growth of bacteria with a constant supply of electron donors or acceptors, is a promising tool for studying lithotrophic species in the laboratory. Major pitfalls present in standard cultivation methods used for metal-oxidizing microbes can be avoided by the use of an electrode as the sole electron donor. Electrochemical cultivation also offers a window into the poorly understood metabolism of microbes such as obligate Fe(II), Mn(II), or S0 oxidizers by replacing the electron source with the controlled surface of an electrode. The elucidation of redox-dependent behavior of these microbes could enhance industrial applications tuned to oxidation of specific metals, provide insight into how bacteria evolved to compete with oxygen for reactive metal species, and model geochemical impacts of their metabolism in the environment.
Observation
Neutrophilic obligate Fe(II)-oxidizing bacteria (FeOB) are prevalent throughout the world in both marine and freshwater environments, and these microbes play a direct role in the global iron cycle (1). Neutrophilic FeOB also contribute to corrosion of industrial steel pipelines and structures such as bridges, piers, and ships by greatly enhancing rates of Fe(II) oxidation (2). However, the molecular mechanism enabling oxidation of Fe(II) is among the most poorly understood lithotrophic metabolic strategies (1). Related work with bacteria capable of aerobic acidophilic (pH < 2) oxidation (3, 4) and anaerobic photosynthetic Fe(II) oxidation (5, 6) has identified very few proteins, many of which are c-type cytochromes predicted to be in the periplasm or outer membrane. However, no proteins required for Fe(II) oxidation in neutrophilic species have been identified. This lack of information is largely due to the difficulties inherent in cultivation of obligate Fe(II)-oxidizing lithotrophs.
Many challenges are present when studying bacteria catalyzing Fe(II) oxidation at circumneutral pH. Issues of gas and metal solubility and competition with the abiotic autocatalytic oxidation of Fe(II) by O2 (7), as well as the abiotic self-reactivity of Fe(II)/Fe(III) minerals, limit the amount of Fe(II) and O2 that can be supplied. In the case of Mariprofundus ferrooxydans strain PV-1 (8), growth is optimal at micromolar Fe(II) levels and microaerophilic O2 concentrations. To support growth, medium conditions are typically designed to create Fe(II) and O2 gradients that intersect and create a thin zone favorable to the organism. Once Fe(II) becomes oxidized, Fe(III) instantaneously forms insoluble iron oxyhydroxides (9). As formation of copious intracellular Fe(III) oxides could result in cell death, Fe(II) oxidation mechanisms are predicted to occur at the outer cell membrane (1), and different species appear to have evolved mechanisms for directing extracellular Fe(III) deposition. M. ferrooxydans PV-1 deposits a twisted stalk of polysaccharide and Fe(III) oxide on one side of the cell (10–12), while other species form sheaths (13), capsules (14), or amorphous polysaccharide precipitations (15), to avoid entombment by insoluble Fe(III) oxide.
The combination of low reactant concentrations, abiotic side reactions, metal precipitate formation, and variations across intersecting gradients makes it difficult to grow and recover significant numbers of these bacteria under controlled conditions. Estimates of cell yields, Fe(II) oxidation rates, or calculations of thermodynamic driving forces required by these bacteria are also confounded by these culture variables. Further, the presence of mixed Fe(II)/Fe(III) species on and around cells results in biomass contaminated with metal oxides that can limit downstream analytical methods. An alternative cultivation method eliminating Fe(II) as the electron donor could provide insight into how neutrophilic Fe(II)-oxidizing bacteria obtain electrons from their environment and produce cells without contaminating oxides.
Here we report the use of electrochemical techniques to create a surface which is poised at a constant redox potential designed to mimic that of Fe(II) in the environment and supply cells with a stream of electrons at a known potential. Under these conditions, M. ferrooxydans PV-1 was able to attach to and accept electrons from an electrode and could be propagated autotrophically via serial dilution onto new electrodes, removing Fe(II) to the point where the electrode was the sole available donor. Data collected using electrode surfaces as electron donors support the hypothesis that the mechanism of electron entry into these cells occurs at the outer membrane (16). Moreover, it allows accurate determination of the thermodynamics of M. ferrooxydans PV-1 respiration, calculation of per-cell electron transfer rates, and imaging of cells in the absence of Fe(III) oxide contaminants.
M. ferrooxydans strain PV-1 was obtained from the Provasoli-Guillard National Center for Marine Algae and Microbiota (NCMA), located at the Bigelow Laboratory (East Boothbay, ME), and grown on zero-valent iron (ZVI) plates with an overlay of NCMA-1 minimal medium (as described by Bigelow Laboratory protocols [http://ncma.bigelow.org]). Growth in this medium produces a characteristic insoluble Fe(III) oxide precipitate, and cells connected to bifurcating twisted stalks (12) are easily observed via phase microscopy. Electrode-based growth experiments were conducted in 80-ml three-electrode reactors containing polished graphite electrodes poised at −0.076 V (versus standard hydrogen electrode [SHE]) and flushed continuously with a mix of O2-CO2-N2 (8:10:82). The three-electrode cells contained a platinum counter electrode and a calomel reference electrode connected via a salt bridge and were autoclaved with all electrodes in place before each use. Similar microbial electrolysis cells have been used routinely to cultivate microbes capable of anode respiration where the electrode was poised at a potential to accept electrons from cells (17, 18).
While electron flow from electrodes into cells has been observed in anaerobes such as Shewanella and Geobacter spp. (19, 20) as well as in environmental enrichments (21), these bacteria require low redox potentials, typically less than −0.3 V versus SHE, to drive electron uptake. For growth of M. ferrooxydans PV-1 (Fig. 1A), a much higher potential (−0.076 V) was chosen, based on the estimated redox potential of the Fe(OH)3/Fe(II) redox couple in neutral environments [Fe2+ + 0.25O2 + 2.5H2O → Fe(OH)3 + 2H+] (1). In addition, abiotic O2 reduction by graphitic electrodes was much slower (less than 20% of maximum) at this potential, ensuring that O2 remained available for microbial respiration at the electrode surface (Fig. 1B). Finally, the potential at which the electrode was poised was well above the formal potential of the 2H+/H2 couple (−0.41 V versus SHE). Even though M. ferrooxydans strain PV-1 has never been shown to utilize H2 gas, poising the electrode at this higher potential prevents the risk of H2 gas serving as a significant electron donor.
FIG 1 .
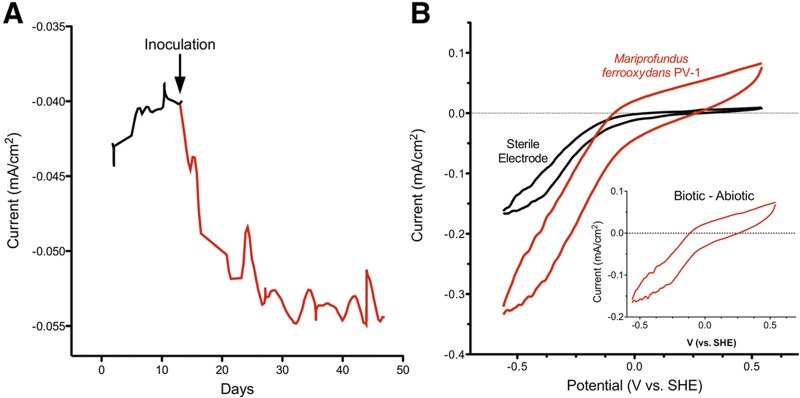
(A) Representative chronoamperometry of M. ferrooxydans PV-1 on a graphite electrode, after the third serial transfer from an electrode poised at −0.076 V as the sole electron donor. Negative (cathodic) current represents electron flow from the electrode surface to cells and/or oxygen. In total, three reactors were inoculated from iron-containing medium, and six reactors representing the third transfer away from iron-containing medium were inoculated to verify growth of M. ferrooxydans PV-1. Raw data are shown from a representative reactor; fluctuations in current reflect periodic gas accumulation on electrodes. (B) Representative cyclic voltammetry of M. ferrooxydans PV-1 after 30 days of growth on a graphite electrode, 5 mV/sec. (Inset) Biotic cyclic voltammogram minus abiotic cyclic voltammogram.
Upon introduction of approximately 106 cells grown in ZVI medium into a reactor containing electrodes poised at −0.076 V versus SHE, electrical current representing uptake of electrons increased steadily and reached a plateau within 4 weeks. After this initial growth, removal of the medium and replacement with sterile medium did not decrease the rate at which electrons were drawn from the electrode, suggesting that the source of increased current was an attached catalyst, and the reaction was not influenced by soluble mediators. A portion of these electrodes were scraped under sterile conditions and used to inoculate a second series of electrochemical cells. Following transfer into the second chamber, the rate of electrical current flowing to the electrode again increased steadily to a similar value after a 3-week period. At all time points, current was dependent upon the presence of oxygen, as flushing of oxygen from reactors eliminated electron flow.
When subsamples from these “second-transfer” electrodes were transferred to a third reactor, similar increases in current resulted. A representative trace showing the increase in electron flow from background levels (representing abiotic oxygen reduction) after a third transfer is shown (Fig. 1A). All voltammetry data (Fig. 1) and images (Fig. 2) are from “third-transfer” reactors, where the only iron present is from the 0.3 µM FeSO4 added with trace minerals for nutritional purposes, which is 6 orders of magnitude less than what is used to support growth by Fe(II) oxidation (1). Observations are the results of three separate inoculation and serial propagation experiments, and each reactor contained at least three independent electrodes for subsequent imaging and testing.
FIG 2 .
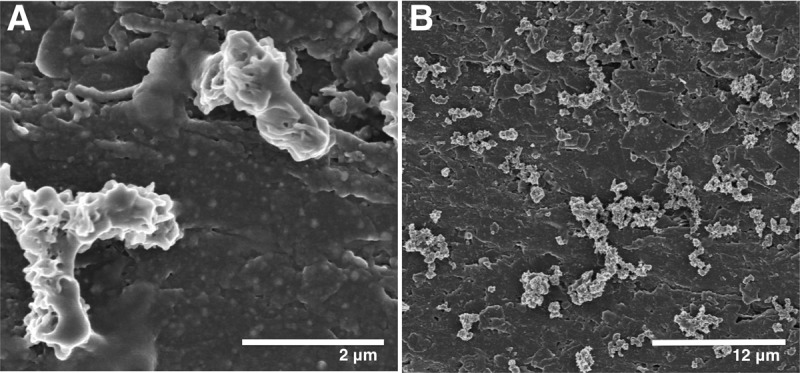
Scanning electron micrographs of M. ferrooxydans PV-1 attached to graphite electrodes. (A) M. ferrooxydans PV-1 cells undergoing longitudinal bifurcate cell division. (B) M. ferrooxydans PV-1 cells attached to graphite electrode.
When electrodes from third-transfer reactors were placed in sterile ZVI medium, a characteristic zone of Fe(III) precipitation again formed, and Y-shaped twisted stalks could be easily visualized in samples from this zone via light microscopy (results from two separate reactors). In contrast, sterile poised electrodes incubated over 8 weeks did not demonstrate any current increase, and no M. ferrooxydans-like growth was observed when these electrodes were placed in ZVI medium. Scanning electron micrographs of colonized electrodes from all stages of propagation consistently revealed cell densities between 1 × 106 and 5 × 106 cells/cm2. The fact that reactors typically contained 18 cm2 of electrodes, combined with the small amount of electrode (typically ~3 cm2) scraped for each transfer, required that the initial inoculum of ~106 cells undergo at least three doublings per transfer to produce the observed final cell density. This indicates that M. ferrooxydans PV-1 could accept electrons at this redox potential and use these electrons to generate ATP, fix CO2, and produce biomass. The fact that the electron donor is a solid surface is consistent with the hypothesis that electron-harvesting proteins are exposed on the outer membrane.
After 30 days, all electrodes (n = 15) containing M. ferrooxydans PV-1 increased their rate of current consumption by at least 10 µA/cm2 above background abiotic rates. At the relatively high potential (−0.076 V) used to colonize electrodes, abiotic O2 reduction by sterile electrodes accounted for approximately 80% of the current consumed. However, as electron transfer rates could be measured across a range of redox potentials and compared to sterile electrodes, it was possible to determine if M. ferrooxydans PV-1 was able to compete for oxygen differently at stronger driving forces (low redox potentials). Rates obtained from sterile electrodes were subtracted from rates obtained from colonized electrodes to infer the contribution of M. ferrooxydans to oxygen reduction at each redox potential (Fig. 1B). These studies revealed that at low redox potentials (−0.3 V) the presence of cells more than doubled the maximal rate of electron transfer from the electrode surface. Also, biotic electron transfer increased to account for over 50% of total electron flux (Fig. 1B).
The bacteria appeared to require an electron donor of at least 0 V versus SHE to drive respiration and reached 50% of maximum electron uptake rates near −0.2 V versus SHE (Fig. 1B). Based on these data, in the presence of 10% O2, M. ferrooxydans PV-1 was capable of respiration at a minimum ∆G′ approaching −77 kJ/mol e− and accelerated to half-maximum rates when the energy available approached −97 kJ/mol e−. While the actual ∆G′ available from environmental Fe(II) oxidation is affected by Fe(II) and O2 concentrations, as well as the form of Fe(III) precipitated, these electrode-derived values agree with published estimates of −90 kJ/mol Fe(II) for the energy available to Fe(II) oxidizers in microaerophilic zones (1).
Based on the number of attached cells and rate of electron consumption, it was also possible to estimate the rate of electrode oxidation by M. ferrooxydans PV-1. The only known cell-normalized rates of Fe(II) oxidation corrected for abiotic reactions are from planktonic incubations with Sideroxidans sp., which oxidizes pulses of Fe(II) at rates of 0.005 to 0.042 pmol electrons cell−1 h−1 (22, 23). In our experiments with an electrode continuously poised at −0.076 V versus SHE, cell-normalized electrode oxidation rates corrected for abiotic oxidation fell near the top of this range, equivalent to 0.075 pmol electrons cell−1 h−1 (calculated using cell densities of 5 × 106/cm2, 10 µA/cm2 of current consumed, 1 ampere = 1 coulomb/second, 1 mol electrons = 96,485 C).
To determine cell abundance and cell morphology during growth on the electrode, scanning electron microscopy of electrodes was performed. Electrodes were immersed for 20 h at 4°C in a mixture of 2% paraformaldehyde-2% glutaraldehyde-0.14 M (pH 7.4) sodium cacodylate buffer, containing 0.15% alcian blue (24). After primary fixation, electrodes were washed three times in sodium cacodylate buffer and postfixed for 90 min in 1% OsO4-0.14 M cacodylate buffer-1.5% potassium ferrocyanide. Electrodes were rinsed in cacodylate buffer and dehydrated in an ascending ethanol series (50, 70, 80, 90, 95, 100%) and then immediately critical point dried with CO2. Samples mounted on carbon tape were coated with approximately 1 nm of Pt using an Ion Tech argon ion beam coater and examined in a low-voltage Hitachi S-900 field emission scanning electron microscope (SEM) at 3 keV.
All cells were closely associated with the graphite electrode, typically attached as single cells and never as layered films or microcolonies (Fig. 2). Cell division of PV-1, resulting in Y-shaped dividing cells connected by a Fe(III)/polysaccharide matrix, has been documented by video (12). Similar to what has been observed in Fe(II)-containing medium, cells attached to the electrode typically were joined as if undergoing longitudinal bifurcation, appearing as Y or V shapes (Fig. 2A). This cell phenotype suggests that the phenotype observed in laboratory and environmental samples is not an artifact of Fe(III) precipitate formation but is fundamentally linked to growth of M. ferrooxydans PV-1. The genome of M. ferrooxydans PV-1 (16) does not appear to contain homologs to traditional cell division genes found in Escherichia coli, for example, minC, minD, minE, or zipA, all of which are genes for well-characterized proteins that play a role in standard E. coli cell division (25). Based on the lack of traditional cell division genes, further investigation is warranted into the possibility of a unique mechanism of cell division being used by M. ferrooxydans PV-1.
Implications.
The results support the model that neutrophilic Fe(II)-oxidizing bacteria, specifically M. ferrooxydans PV-1, are capable of accepting electrons from an external source, which suggests that the Fe(II) oxidation mechanism consists of at least one or more extracellular components. Further investigation of the proteins and pathways involved in this process may be more easily accomplished in an iron-free system, where an electrode can be substituted for Fe(II) and cells can be maintained at consistent rates of respiration.
The existence of cellular machinery able to link Fe(II) oxidation to internal metabolism has long suggested that cells could use electrical resources to power the synthesis of organic compounds. Bacteria active at this bioelectrical interface are capable of multistep enzymatic redox reactions that would be difficult to achieve with precious metals. In particular, cathodes have emerged as possible tools in biofuel synthesis, where they are proposed to serve as electron sources for microbially driven reductive metabolism (26). In addition, cathodes can serve as electron donors in microbially mediated reductive bioremediation (27). This work with M. ferrooxydans PV-1 shows that neutrophilic Fe(II)-oxidizing bacteria are capable of directing a stream of external electrons into internal respiratory and metabolic reactions, where they can be used for simultaneous biological energy generation and reductive capture of CO2.
ACKNOWLEDGMENTS
We thank David Emerson (Bigelow Laboratory) for helpful discussion and encouragement. We thank Caleb Levar for assistance with electrochemical cultivation.
This work was supported by a grant from the Minnesota Environment and Natural Resources Trust Fund.
Footnotes
Citation Summers ZM, Gralnick JA, Bond DR. 2013. Cultivation of an obligate Fe(II)-oxidizing lithoautotrophic bacterium using electrodes. mBio 4(1):e00420-12. doi:10.1128/mBio.00420-12.
REFERENCES
- 1. Emerson D, Fleming EJ, McBeth JM. 2010. Iron-oxidizing bacteria: an environmental and genomic perspective. Annu. Rev. Microbiol. 64:561–583 [DOI] [PubMed] [Google Scholar]
- 2. Little BJ, Mansfeld FB, Arps PJ, Earthman JC. 2007. Microbiologically influenced corrosion, p 662–685. In Bard AJ, Stratmann M, Frankel GS, Encyclopedia of electrochemistry, vol 4. Corrosion and oxide films. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany [Google Scholar]
- 3. Valdés J, Pedroso I, Quatrini R, Dodson RJ, Tettelin H, Blake R, Eisen JA, Holmes DS. 2008. Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications. BMC Genomics 9:597 http://dx.doi.org/10.1186/1471-2164-9-597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singer SW, Chan CS, Zemla A, VerBerkmoes NC, Hwang M, Hettich RL, Banfield JF, Thelen MP. 2008. Characterization of cytochrome 579, an unusual cytochrome isolated from an iron-oxidizing microbial community. Appl. Environ. Microbiol. 74:4454–4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Croal LR, Jiao Y, Newman DK. 2007. The fox operon from Rhodobacter strain SW2 promotes phototrophic Fe(II) oxidation in Rhodobacter capsulatus SB1003. J. Bacteriol. 189:1774–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiao Y, Newman DK. 2007. The pio operon is essential for phototrophic Fe(II) oxidation in Rhodopseudomonas palustris tie-1. J. Bacteriol. 189:1765–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sung W, Morgan JJ. 1980. Kinetics and product of ferrous iron oxygenation in aqueous systems. Environ. Sci. Technol. 14:561–568 [Google Scholar]
- 8. Emerson D, Rentz JA, Lilburn TG, Davis RE, Aldrich H, Chan C, Moyer CL. 2007. A novel lineage of Proteobacteria involved in formation of marine Fe-oxidizing microbial mat communities. PLoS One 2:e667 http://dx.doi.org/10.1371/journal.pone.0000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. King DW, Lounsbury HA, Millero FJ. 1995. Rates and mechanism of Fe(II) oxidation at nanomolar total iron concentrations. Environ. Sci. Technol. 29:818–824 [DOI] [PubMed] [Google Scholar]
- 10. Hanert HH. 2006. The genus Gallionella, p 990–995 In Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E, The prokaryotes, vol 7, 3rd ed. Springer Verlag, New York, NY. [Google Scholar]
- 11. Chan CS, Fakra SC, Edwards DC, Emerson D, Banfield JF. 2009. Iron oxyhydroxide mineralization on microbial extracellular polysaccharides. Geochim. Cosmochim. Acta 73:3807–3818 [Google Scholar]
- 12. Chan CS, Fakra SC, Emerson D, Fleming EJ, Edwards KJ. 2011. Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: implications for biosignature formation. ISME J. 5:717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghiorse WC, Ehrlich HL. 1992. Microbial biomineralization of iron and manganese. Catena 21:75–99 [Google Scholar]
- 14. Hanert HH. 2006. The genus Siderocapsa, p 1005–1015 In Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E, The prokaryotes, vol 7, 3rd ed. Springer Verlag, New York, NY. [Google Scholar]
- 15. Miot J, Benzerara K, Obst M, Kappler A, Hegler F, Schaedler S, Bouchez C, Guyot F, Morin G. 2009. Extracellular iron biomineralization by photoautotrophic iron-oxidizing bacteria. Appl. Environ. Microbiol. 75:5586–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singer E, Emerson D, Webb EA, Barco RA, Kuenen JG, Nelson WC, Chan CS, Comolli LR, Ferriera S, Johnson J, Heidelberg JF, Edwards KJ. 2011. Mariprofundus ferrooxydans PV-1 the first genome of a marine Fe(II) oxidizing Zetaproteobacterium. PLoS One 6:e25386 http://dx.doi.org/10.1371/journal.pone.0025386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marsili E, Rollefson JB, Baron DB, Hozalski RM, Bond DR. 2008. Microbial biofilm voltammetry: direct electrochemical characterization of catalytic electrode-attached biofilms. Appl. Environ. Microbiol. 74:7329–7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bond DR, Holmes DE, Tender LM, Lovley DR. 2002. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483–485 [DOI] [PubMed] [Google Scholar]
- 19. Strycharz SM, Glaven RH, Coppi MV, Gannon SM, Perpetua LA, Liu A, Nevin KP, Lovley DR. 2011. Gene expression and deletion analysis of mechanisms for electron transfer from electrodes to Geobacter sulfurreducens. Bioelectrochemistry 80:142–150 [DOI] [PubMed] [Google Scholar]
- 20. Ross DE, Flynn JM, Baron DB, Gralnick JA, Bond DR. 2011. Towards electrosynthesis in Shewanella: energetics of reversing the mtr pathway for reductive metabolism. PLoS One 6:e16649 http://dx.doi.org/10.1371/journal.pone.0016649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pisciotta JM, Zaybak Z, Call DF, Nam JY, Logan BE. 2012. Enrichment of microbial electrolysis cell biocathodes from sediment microbial fuel cell bioanodes. Appl. Environ. Microbiol. 78:5212–5219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neubauer SC, Emerson D, Megonigal JP. 2002. Life at the energetic edge: kinetics of circumneutral iron oxidation by lithotrophic iron-oxidizing bacteria isolated from the wetland-plant rhizosphere. Appl. Environ. Microbiol. 68:3988–3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Druschel G, Emerson D, Sutka R. 2008. Low-oxygen and chemical kinetic constraints on the geochemical niche of neutrophilic iron (II) oxidizing microorganisms. Geochim. Cosmochim. Acta 72:3358–3370 [Google Scholar]
- 24. Erlandsen SL, Kristich CJ, Dunny GM, Wells CL. 2004. High-resolution visualization of the microbial glycocalyx with low-voltage scanning electron microscopy: dependence on cationic dyes. J. Histochem. Cytochem. 52:1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Margolin W. 2000. Themes and variations in prokaryotic cell division. FEMS Microbiol. Rev. 24:531–548 [DOI] [PubMed] [Google Scholar]
- 26. Hallenbeck PC. 2011. The future of biofuels, biofuels of the future, p 261–268 In Hallenbeck PC, Microbial technologies in advanced biofuels production. Springer US, Boston, MA [Google Scholar]
- 27. Gregory KB, Lovley DR. 2005. Remediation and recovery of uranium from contaminated subsurface environments with electrodes. Environ. Sci. Technol. 39:8943–8947 [DOI] [PubMed] [Google Scholar]


