ABSTRACT
Candida albicans has developmental programs that govern transitions between yeast and filamentous morphologies and between unattached and biofilm lifestyles. Here, we report that filamentation, intercellular adherence, and biofilm development were inhibited during interactions between Candida albicans and Pseudomonas aeruginosa through the action of P. aeruginosa-produced phenazines. While phenazines are toxic to C. albicans at millimolar concentrations, we found that lower concentrations of any of three different phenazines (pyocyanin, phenazine methosulfate, and phenazine-1-carboxylate) allowed growth but affected the development of C. albicans wrinkled colony biofilms and inhibited the fungal yeast-to-filament transition. Phenazines impaired C. albicans growth on nonfermentable carbon sources and led to increased production of fermentation products (ethanol, glycerol, and acetate) in glucose-containing medium, leading us to propose that phenazines specifically inhibited respiration. Methylene blue, another inhibitor of respiration, also prevented the formation of structured colony biofilms. The inhibition of filamentation and colony wrinkling was not solely due to lowered extracellular pH induced by fermentation. Compared to smooth, unstructured colonies, wrinkled colony biofilms had higher oxygen concentrations within the colony, and wrinkled regions of these colonies had higher levels of respiration. Together, our data suggest that the structure of the fungal biofilm promotes access to oxygen and enhances respiratory metabolism and that the perturbation of respiration by bacterial molecules such as phenazines or compounds with similar activities disrupts these pathways. These findings may suggest new ways to limit fungal biofilms in the context of disease.
IMPORTANCE
Many of the infections caused by Candida albicans, a major human opportunistic fungal pathogen, involve both morphological transitions and the formation of surface-associated biofilms. Through the study of C. albicans interactions with the bacterium Pseudomonas aeruginosa, which often coinfects with C. albicans, we have found that P. aeruginosa-produced phenazines modulate C. albicans metabolism and, through these metabolic effects, impact cellular morphology, cell-cell interactions, and biofilm formation. We suggest that the structure of C. albicans biofilms promotes access to oxygen and enhances respiratory metabolism and that the perturbation of respiration by phenazines inhibits biofilm development. Our findings not only provide insight into interactions between these species but also provide valuable insights into novel pathways that could lead to the development of new therapies to treat C. albicans infections.
Introduction
Candida albicans, a commensal resident of mucosal surfaces, can overgrow under favorable conditions, leading to a wide range of diseases, referred to as candidiasis (1). C. albicans infections often involve more than one cellular morphology; yeast, pseudohyphal, and hyphal growth forms have all been associated with virulence (2). It has been proposed that filaments are responsible for tissue penetration, whereas yeasts play an important role in early dissemination and in less invasive disease infections (3). C. albicans infections are also associated with its formation of biofilms, which are dense single-species and mixed-species populations adhering to one another through the action of surface adhesins and extracellular polymers. Biofilms on implanted medical devices can serve as a source for infection, and dense C. albicans populations within the host may have properties in common with biofilms, such as high levels of drug resistance and resistance to host immune defenses (4, 5). Importantly, biofilm formation involves filamentation and adhesin-mediated aggregation of cells (6, 7).
Many environmental cues impact fungal morphology and biofilm formation. Many conditions, such as hypoxia, elevated extracellular pH, N-acetylglucosamine (GlcNAc), amino acids, low concentrations of glucose, body temperature, and elevated CO2 have been associated with C. albicans filamentation (8, 9). There have been a number of studies that also indicate that metabolic status can influence the decision to form hyphae (10–16). There are still many questions regarding how the fungus integrates these different signals and whether there are important links between known inducers of hyphal growth, biofilm formation, and metabolism.
C. albicans and bacteria can coexist within the host (17–19), where the nature of the interspecies interactions can determine the fate of the microbial populations (20) and thus probably the outcome of polymicrobial diseases. C. albicans and Pseudomonas aeruginosa, two species commonly found together in mixed-species, biofilm-related infections (21), interact in many different ways through physical association, killing by secreted factors and signaling events that modulate virulence properties (22–29). P. aeruginosa adheres to and forms biofilms on C. albicans filaments (23) and ultimately causes the death of the fungal hypha. A P. aeruginosa quorum-sensing molecule also induces a transition to yeast growth, and yeasts are more resistant to killing by P. aeruginosa (23). Redox-active phenazines produced by P. aeruginosa play a key role in controlling C. albicans when the two species are in close proximity (24, 29). We reported that C. albicans growth was antagonized by two P. aeruginosa phenazines, pyocyanin (PYO) and its biosynthetic precursor 5-methyl-phenazinium-1-carboxylate (5MPCA), as well as by a synthetic methylphenazinium analog phenazine methosulfate (PMS), which we have shown to be a surrogate for studying 5MPCA’s antifungal activity (24, 29) (see Fig. S1 in the supplemental material for chemical structures). Phenazines play important roles in bacterial interactions with fungi in which high concentrations of phenazines inhibit fungal growth (30).
Here, we demonstrate a new role for bacterial phenazines as modulators of C. albicans metabolism and community behavior. In the presence of phenazine-producing P. aeruginosa or low concentrations of purified phenazines, the fungus grew but no longer developed wrinkled colonies or robust biofilms on plastic surfaces. Phenazines markedly inhibited the yeast-to-filament transition. Consistent with published data indicating that phenazines can impact mitochondrial activity (31, 32), their presence led to increased production of fermentation products and decreased respiratory activity at concentrations that are found in clinical samples (33). Methylene blue (MB), which can also interact with the electron transport chain (ETC) (34, 35), inhibited the development of wrinkled C. albicans colony biofilms. These studies also revealed a link between wrinkles in colony biofilms and respiratory activity, and this finding was supported by the detection of higher oxygen concentrations in wrinkled colonies than in smooth and flat colonies comprised of yeast. Together, our data revealed a previously unidentified role for phenazines as modulators of C. albicans metabolism and, through these effects, on cellular morphology, colony development, and biofilm formation on solid surfaces. Moreover, this work also provides new insights into the links between metabolism, morphogenesis, and community behaviors such as biofilm formation in C. albicans, and this may represent an emerging theme among the microbes.
RESULTS
P. aeruginosa phenazines repress C. albicans biofilm formation and hyphal growth.
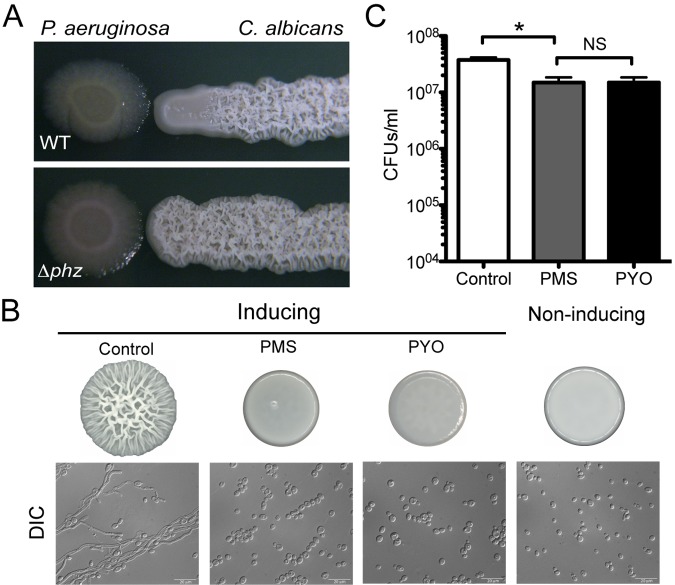
When wild-type (WT) P. aeruginosa strain PA14 and WT C. albicans strain SC5314 were grown adjacent to one another, a striking change in C. albicans colony morphology occurred, with a smooth zone in the region adjacent to the bacterial colony and wrinkling in regions of the fungal streak that were more distant from the bacterial colony (Fig. 1A). In contrast, C. albicans cocultured with a phenazine-deficient (∆phz) P. aeruginosa strain wrinkled robustly up to the edge of the bacterial colony, suggesting that the soluble excreted phenazines played a role in this effect on C. albicans colony morphology (Fig. 1A). When we cocultured C. albicans with P. aeruginosa mutants altered in the synthesis of individual phenazines (see Fig. S1 in the supplemental material for pathway), repression of colony wrinkling still occurred (see Fig. S2). Mutants unable to synthesize pyocyanin (PYO) (phzS::TnM) or 5MPCA (phzM::TnM) but capable of producing phenazine-1-carboxylate (PCA) still produced smooth zones.
FIG 1 .
Effects of phenazines on C. albicans colony development and cellular morphology. (A) C. albicans SC5314 colony morphology when near P. aeruginosa WT or the Δphz strain. (B) Top, C. albicans colonies grown on Glu-AA-GlcNAc at 37°C (inducing conditions) or 30°C in the absence of GlcNAc (noninducing conditions) for 48 h in the absence or presence of 5 µM PMS or 20 µM PYO; bottom, microscopic view of cells from colonies, captured using differential interference contrast (DIC) microscopy. (C) Viable cell counts from C. albicans colonies exposed to phenazines. CFUs from colonies grown on Glu-AA at 30°C without or with 5 µM PMS or 20 µM PYO for 48 h (*, P < 0.05; n = 4).
To determine if phenazines were directly responsible for the change in C. albicans colony morphology, we spot inoculated C. albicans onto medium with and without purified phenazines. Control cultures without phenazines formed wrinkled colony structures, while colonies grown in the presence of low concentrations of any of three phenazines, PYO, PMS, or PCA, were smooth, flat, and undifferentiated (Fig. 1B; see also Fig. S3A in the supplemental material). The effects of different concentrations of PMS and PYO were tested, and complete repression of colony wrinkling was apparent at concentrations as low as 5 µM for PMS and 10 µM for PYO (see Fig. S3B), indicating that the concentrations required to inhibit wrinkled colony development were ~100-fold below those shown previously to be toxic to C. albicans (24, 29, 36).
C. albicans hyphal growth has been associated with both wrinkled colony morphology and biofilm formation on plastic surfaces (5, 37). We found that wrinkled colonies, formed upon growth on medium with GlcNAc at 37°C, exhibited some biofilm-associated characteristics, including cells in hyphal, pseudohyphal, and yeast morphologies that remained associated with one another even upon suspension in liquid (Fig. 1B and data not shown). In contrast, smooth colonies formed in the presence of phenazines were comprised almost exclusively of yeast that dispersed easily upon transfer to liquid (Fig. 1B and data not shown). In addition to the change in cellular morphology, phenazines repressed expression of HWP1, a gene that is strongly correlated with hyphal growth and involved in biofilm formation (38, 39), as shown using a strain harboring a lacZ transcriptional reporter fused to the HWP1 promoter. A strain expressing an actin transcriptional reporter (ACT1::lacZ) did not show differences between colonies grown in the presence or absence of phenazines (see Fig. S3C). Colonies grown at 30°C without GlcNAc (noninducing conditions) were smooth and contained only yeast (Fig. 1B); no phenotypic changes in colony morphology were observed upon inclusion of phenazines in the agar medium under these conditions (see Fig. S3D). The total numbers of CFUs in smooth colonies formed in the presence of either PMS or PYO were slightly but significantly (P < 0.05) lower than those in control colonies (Fig. 1C).
Consistent with the data that indicated that colony wrinkling and hypha formation were repressed by phenazines on agar surfaces, we found that these compounds inhibited C. albicans hyphal growth and biofilm formation in liquid. Planktonic control cultures grown under filament-inducing conditions contained long undulating filaments, whereas cultures exposed to either 5 µM PMS or 20 µM PMS contained small ovoid yeast cells (see Fig. S4A). Biofilm assays on polystyrene surfaces showed a significant reduction in biofilm formation in the presence of PMS or PYO in comparison to that of the untreated controls (see Fig. S4B).
Inhibition of extracellular alkalinization by phenazines prevents C. albicans filamentation and wrinkling.
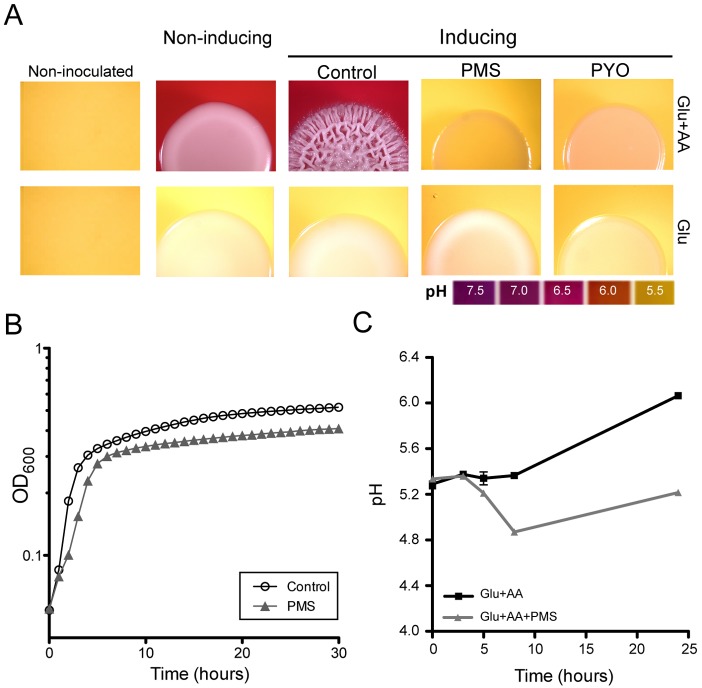
Previous studies demonstrated links between C. albicans morphological transitions and extracellular pH, with yeast growth dominating under acidic conditions and filamentation promoted at neutral pH (40). Seminal studies by Vylkova and colleagues (41) showed that C. albicans amino acid catabolism can raise the extracellular pH in environments that are initially acidic. Since our culture medium was rich in amino acids and had an initial pH of 5.0, we determined the role of alkalinization in the induction of filamentation and wrinkled colony morphology. As expected, alkalinization of the medium was evident only in the presence of amino acids, while medium containing only glucose as a carbon source remained acidic (Fig. 2A). The addition of GlcNAc and incubation at 37°C (conditions that induce filamentation) promoted colony wrinkling on medium with amino acids that had increased pH (Fig. 2A; control). However, GlcNAc and 37°C were not sufficient to support wrinkled colony formation in the absence of amino acids (Fig. 2A). Concentrations of phenazines that inhibited colony wrinkling also had striking effects on the pH changes in the medium. While the medium surrounding control colonies became red by 48 h, indicating a pH greater near 7, the medium surrounding PMS- and PYO-grown colonies remained acidic (Fig. 2A). The addition of PMS or PYO did not change the pH of uninoculated media. These differences in extracellular pH led us to hypothesize that phenazines were impacting C. albicans metabolism.
FIG 2 .
Phenazines modulate C. albicans extracellular alkalinization and morphogenesis. (A) Fungal colonies grown on Glu+AA or Glu alone under filament-inducing and noninducing conditions without or with 5 µM of PMS and 20 µM PYO for 48 h; pH was monitored using bromocresol purple. The images were obtained by using a stereoscope (7.5×). (B and C) Growth (B) and pH (C) in liquid cultures grown with Glu-AA in the presence and absence of 5 µM PMS at 37°C.
Phenazine-induced fermentation correlates with C. albicans acidification of the extracellular milieu.
In liquid culture conditions, as observed on agar (Fig. 1C), PMS caused a small decrease in growth rate and final yield (Fig. 2B), and the pH in the extracellular medium of cultures grown with PMS was lower (Fig. 2C). Extracellular pH can increase upon ammonia release (41, 42), and acidic products can lower the pH. We first evaluated the effects of phenazines on ammonia release using an acid trap methodology (41). In control colonies, 138 ± 19 ppm NH3 was detected in comparison to 290 ± 56 ppm NH3 for colonies grown with PYO and 270 ± 69 ppm NH3 for colonies grown with PMS. Thus, neither PMS nor PYO caused a decrease in ammonia release from colonies, suggesting that differences in amino acid catabolism were likely not the cause of the lower pH values in cultures with PMS or PYO.
To determine whether phenazines altered the production of acidic C. albicans products, we grew the fungus on filters floating on liquid medium with or without phenazines at 37°C (Fig. 3A) and analyzed the culture supernatants. As on agar, the pH of the supernatants decreased in the presence of phenazines, as visualized by bromocresol green addition (Fig. 3A, top). High-performance liquid chromatography (HPLC) analyses of the supernatants found 8- and 17-fold-higher levels of acetic acid produced per unit of optical density at 600 nm in the presence of PMS and PYO, respectively, at 24 h (Fig. 3B) despite the fact that slightly less glucose was consumed compared to control cultures (see Table S2). Other fermentation products, such as ethanol, were also found at higher relative levels upon phenazine exposure. There was 5.5- and 3.4-fold more ethanol (mg/ml/OD600) in cultures grown with PMS and PYO, respectively, than in 24-h supernatants from untreated cultures of C. albicans colonies (Fig. 3B). HPLC analyses of C. albicans supernatants in planktonic cultures at 8 h also showed higher levels of ethanol as well as glycerol, another C. albicans fermentation product, in PMS-containing cultures than in controls (data not shown). Together, these data suggest a shift toward fermentation in the presence of phenazines.
FIG 3 .
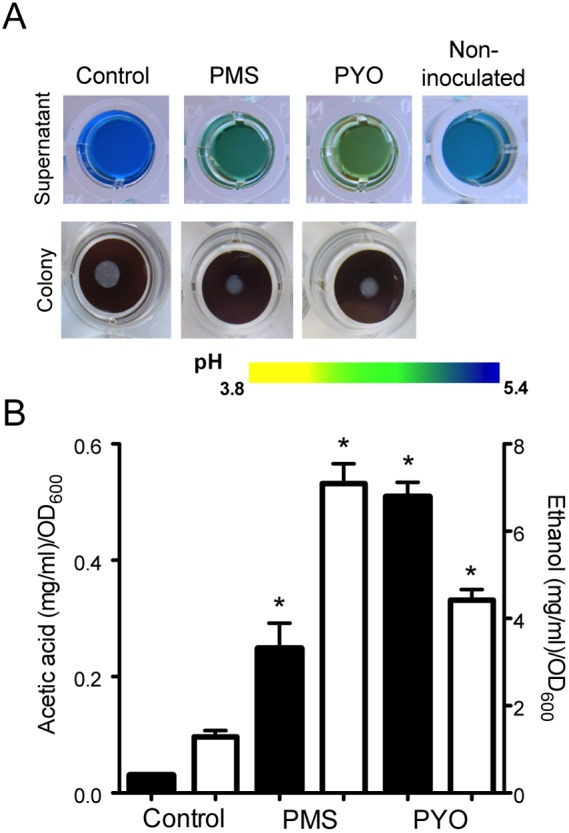
Phenazines promote glucose fermentation. (A) Top, extracellular changes in pH were visualized by addition of bromocresol green; bottom, C. albicans colonies grown for 24 h at 37°C on polycarbonate filters suspended on Glu-AA liquid medium in the presence or absence of 20 µM PYO and 5 µM PMS without bromocresol green. (B) Quantification of acetic acid (black bars) and ethanol (white bars) in supernatants of colonies grown on filters (n = 3; *, P < 0.05).
Phenazines inhibit C. albicans growth on nonfermentable carbon sources.
C. albicans can either ferment or respire glucose, whereas other substrates, like amino acids, alcohols, or organic acids, can be used only for energy generation via respiration (43). In light of the finding that phenazines increased production of C. albicans fermentation products (Fig. 3B), we tested the hypothesis that phenazines inhibited respiration by assessing their effects on growth on nonfermentable carbon sources. Indeed, while growth on glucose was only slightly slower in the presence of PMS (Fig. 4A), this phenazine caused a marked decrease in both growth rates and yield in cultures grown on amino acids (Fig. 4B) or succinate (see Fig. S5).
FIG 4 .
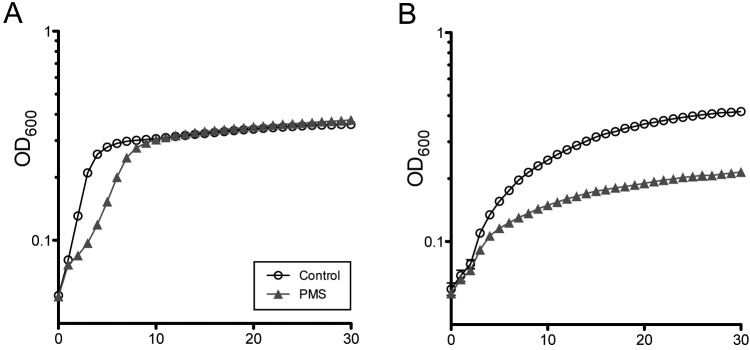
Phenazines alter C. albicans growth in fermentable and nonfermentable carbon sources. Growth curves of liquid cultures on media containing only glucose (A) or amino acids (B) in the presence (triangles) or absence (open circles) of 5 µM PMS at 37°C. The optical density (OD600) of each culture was monitored every hour.
Phenazine-mediated C. albicans colony morphology changes are not due solely to altered extracellular pH.
Phenazines repressed biofilm behaviors both on agar and in liquid medium with glucose (Fig. 1; see also Fig. S4B in the supplemental material) and caused acidification of the medium (Fig. 2A and C). Because of the known role of extracellular pH in C. albicans morphological regulation (40, 41), we tested whether acidification of the extracellular milieu was required for phenazine-mediated inhibition of C. albicans filamentous growth and colony development. The fungus was spot inoculated on medium containing glucose and amino acids that was buffered at either pH 5 or pH 7 (Fig. 5A). As previously described (44), a stable acidic extracellular milieu (pH 5.0) completely inhibited C. albicans filamentation and wrinkled colony formation (Fig. 5A). Colonies formed on medium that was buffered at pH 7.0 were wrinkled in the absence of PMS or PYO. Interestingly, PYO and PMS still repressed filamentation and colony wrinkling on this buffered medium (Fig. 5A). Therefore, our results demonstrate that while phenazine-mediated decreases in pH were sufficient to repress hyphal growth, this change in extracellular pH was not required to prevent inhibition of wrinkled colony morphology formation and filamentation.
FIG 5 .
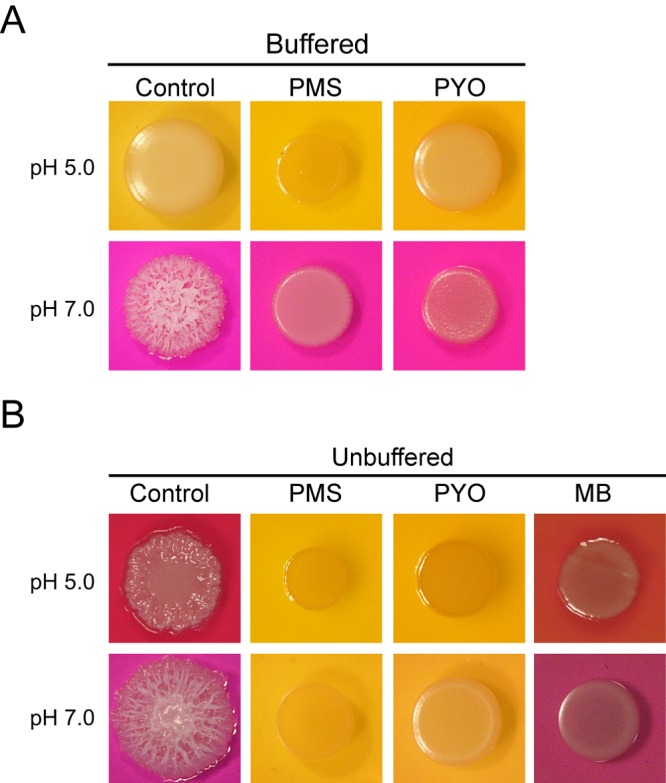
Phenazine-modulated inhibition of C. albicans wrinkled colony morphology is not solely due to decreased extracellular pH. (A and B) C. albicans colonies grown on Glu-AA-GlcNAc with the pH adjusted to 7.0 or 5.0 prior to inoculation. A total of 5 µM of PMS, PYO, or methylene blue (MB) was added to the medium when specified, and the pH indicator bromocresol purple, which yields a red-purple color under alkaline conditions and a yellow-orange color under acidic conditions, was included. In panel A, the medium was buffered with 40 mM MOPS (pH 7) or 100 mM citrate (pH 5) as indicated. Colonies were incubated for 48 h at 37°C.
Phenazines alter C. albicans respiratory activity.
Our data led us to propose that phenazines altered C. albicans respiratory activity. To assess whether perturbation of respiratory activity was sufficient to repress colony biofilm development, we determined if methylene blue (MB), another well-known electron acceptor capable of altering mitochondrial activity and with structural similarity to phenazines (34, 35), also repressed colony wrinkling. Similar to the effects of PMS, PCA, and PYO (Fig. 1B; see also Fig. S3A in the supplemental material), micromolar concentrations of MB also caused C. albicans formation of smooth colonies on unbuffered medium (Fig. 5B), and the effects were dose dependent, with completely smooth colonies observed at 5 µM (see Fig. S6). Unlike phenazines, which promoted extracellular acidification (Fig. 2A and C and 3A), cultures with MB alkalinized the medium when it was at an initial pH of 5 and did not acidify the extracellular milieu when the initial pH was adjusted to 7 (Fig. 5B). These data highlight that compounds that impact respiration can inhibit colony wrinkling and that extracellular pH changes were not required for the repression of hyphal growth and the development of wrinkled, structured colonies.
We next used TTC, a dye indicative of mitochondrial activity, in order to test the idea that phenazines could impair respiration directly. Reduction of TTC by the electron transport chain (ETC) leads to the formation of a red product that accumulates within cells (45). Colony biofilms formed without phenazines exhibited a high level of TTC reduction as evidenced by the strong red pigmentation (Fig. 6A and B). However, PMS- or PYO-grown colonies showed decreased TTC conversion in this assay and, thus, were much less metabolically active (Fig. 6A). As observed with phenazine-exposed C. albicans, colonies grown on agar with MB also demonstrated a reduced red pigmentation (see Fig. S6B).
FIG 6 .
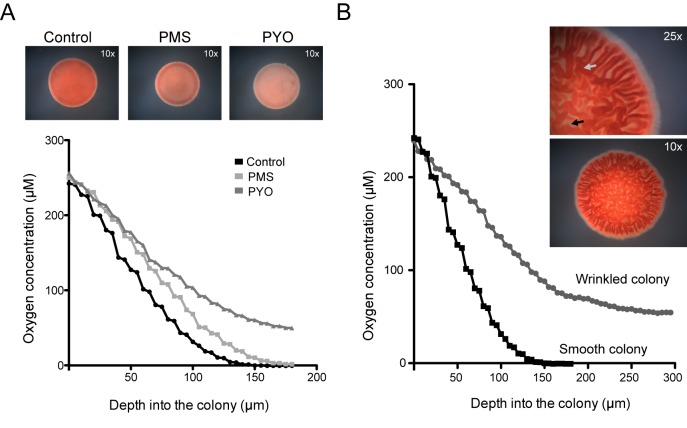
Effects of phenazines on respiratory metabolism and oxygen consumption. (A) C. albicans colonies grown in Glu-AA medium alone or with 5 µM PMS or 20 µM PYO under noninducing conditions. The respiratory activity of colonies was assessed using TTC, and images were captured using a stereoscope at 10× (top). Internal oxygen measurements in similarly grown colonies were made from the top to the bottom of control colonies (black), with PMS (light gray) or PYO (dark gray). (B) Oxygen gradients in colonies grown under noninducing (smooth) and inducing conditions (wrinkled) were measured at room temperature. The respiratory activity in wrinkled colonies was greater in wrinkles (gray arrow) than in valleys (black arrow) (inset).
To complement these studies, we used an electrode probe to measure the oxygen gradients as a function of depth from the top of the colony as an indicator of oxygen utilization within the colony interior. Under noninducing conditions, smooth colonies grown with PMS or PYO demonstrated higher concentrations of oxygen than control colonies when measured at comparable depths from the surface (Fig. 6A). These data are consistent with oxygen utilization being repressed by phenazines. Together, these findings indicate that phenazines cause a disruption of the normal respiratory patterns in C. albicans cells.
The wrinkles of C. albicans colonies have elevated oxygen concentrations and exhibit a high respiratory metabolism.
When TTC was added to C. albicans wrinkled colonies formed in inducing conditions in the absence of phenazines, stronger red pigmentation was observed than in colonies grown under noninducing conditions (Fig. 6A and B), suggesting that wrinkled colony biofilms exhibited a more active respiratory metabolism than colonies with a smooth morphology. Consistent with this observation, phenazine-treated colonies grown under inducing conditions also showed decreased TTC-derived coloration than untreated wrinkled controls (data not shown). Strikingly, while there was even, red pigmentation from TTC conversion in smooth colonies (Fig. 6A), wrinkled colonies had higher levels of pigmentation in the wrinkled areas (higher regions of the colony). The “valleys” appeared to have less respiratory activity (Fig. 6B, inset). Based on these observations and in light of our previous results demonstrating that C. albicans wrinkled colony formation was repressed by phenazines and MB (Fig. 1B and 5B), we proposed that wrinkling was linked to respiration.
To determine whether the topology of the wrinkles promoted access to oxygen within dense colonies, we used an electrode to quantify the oxygen concentration within the wrinkles of colonies grown under inducing conditions compared to noninducing conditions at similar distances from the colony edge. Wrinkled colony areas had shallow oxygen gradients, and moderately high oxygen remained detectable throughout the structure (Fig. 6B). In sharp contrast, the gradients for smooth yeast colonies formed in noninducing conditions were much steeper and showed significantly lower oxygen concentrations at all levels within the colony. Thus, our results suggest a model in which wrinkling promotes aerobic respiration as part of fungal colony biofilm development.
DISCUSSION
Our work reveals a new role for P. aeruginosa phenazines as modulators of C. albicans metabolism and biofilm development. We found that low, nonlethal concentrations of bacterial phenazines inhibited fungal morphogenesis and prevented the development of wrinkled colony biofilms, and subsequent analyses indicated that these effects were due to alteration of C. albicans respiratory activity. Several lines of evidence indicated that phenazines inhibited normal fungal respiratory activity: (i) fermentation products were at higher concentrations in the presence of phenazines (Fig. 3B), (ii) growth on nonfermentable carbon sources was severely impaired with only slight growth inhibition on glucose (Fig. 4B; see also Fig. S5 in the supplemental material), (iii) metabolic activity was lower in the presence of phenazines (Fig. 6A), and (iv) oxygen concentrations were higher in yeast colonies grown with phenazines than in yeast colonies grown in their absence (Fig. 6A). The increased secretion of acetic acid (Fig. 3B) (46) in the presence of phenazines likely reduced the extracellular pH (Fig. 2A and C); low pH was not the only mechanism responsible for the phenazine-mediated effects since buffering the medium at pH 7 did not ameliorate the effects of phenazines (Fig. 5A). Importantly, MB, another compound that alters the cellular respiratory activity (34, 35), also repressed hyphal growth and colony biofilm formation but did not lead to a pH change, suggesting that a different spectrum of fermentation products may have been produced (Fig. 5B). The different effects of MB and phenazines with respect to altering the extracellular pH may be explained by their respective redox properties (47), which could lead to different interactions with the ETC, or due to differences in other targets within the cell. Our data suggest that decreased ability to transfer electrons to oxygen led to decreased colony wrinkling and filamentation, and this model is supported by the fact that C. albicans wrinkled colony formation was inhibited (see Fig. S7) and biofilm formation on plastic in liquid medium does not occur in anoxic environments despite the fact that cells form hyphae (48, 49) (see Fig. S7).
Our observations that colony wrinkling was repressed under conditions that limit respiration suggest that these structures may allow cells to increase their access to oxygen in dense populations. Several findings support this model. First, while the absence of oxygen inhibited wrinkled colony formation (see Fig. S7), hypoxic conditions strongly promote colony wrinkling (10) (data not shown). Second, we found that wrinkled regions had higher rates of respiration than nonwrinkled regions of the colony (Fig. 6B) and that wrinkled colonies had more respiratory activity than colonies that lacked wrinkling (Fig. 6). Lastly, mutants locked in a highly respiratory state, such as strains lacking Ace2 and Tye2, have increased colony wrinkling (10) and filamentation within biofilms (50). We speculate that the C. albicans wrinkled phenotype is related to biofilm phenotypes on submerged, plastic surfaces, as both structures involve spatially distributed filamentation and cells that adhere tightly to one another. In multiple bacterial species, the study of colony wrinkling has led to the discovery of genes crucial for biofilm formation on solid surfaces (51–53).
Links between biofilm formation and oxygen availability may emerge as a common theme in diverse microbial species, including both fungi and bacteria. For example, wrinkled P. aeruginosa colonies have increased oxygen concentrations within the colony compared to smooth counterparts, and it has been hypothesized that biofilm structure enables access to oxygen by increasing surface area (54). In P. aeruginosa, endogenously produced phenazines serve as alternate electron acceptors, thereby facilitating P. aeruginosa survival in low-oxygen conditions (55, 56). Phenazines, likely in part through modulation of intracellular redox, led to decreased surface-to-volume ratios in both flow cell and colony biofilms (57, 58) in a manner that is reminiscent to what we observed with C. albicans. In addition, C. albicans biofilm development may also be influenced by ethanol, a metabolite that has been shown to inhibit submerged biofilm formation in this fungus (59).
Previous studies have also shown links between C. albicans respiratory metabolism and cellular and colony morphology. For instance, C. albicans strains that lack the mitochondrial NADH dehydrogenase were unable to filament (13), and other ETC inhibitors such as rotenone (13) and thenoyl trifluoro acetone (16) repressed C. albicans filamentation. However, it is important to note that antimycin A, another respiratory chain inhibitor, promoted filamentation (10), suggesting that the inhibition of filamentation in response to altered ETC activity is likely due to more than decreased energy generation, such as a response to a specific metabolic signal (10). Biochemical studies that have examined the interactions between phenazines and respiratory electron transport systems in eukaryotic and bacterial cells have found that these compounds and related molecules can either inhibit or stimulate the flux of electrons through the ETC (31, 60, 61). Future work will determine what signal, such as ratios of intracellular electron carriers, the state of the ETC components, intracellular pH, or ATP levels, is sensed by the cell and which regulators respond to these important intracellular signals. Furthermore, additional studies will be required to explain the differences in respiration seen in the “folds” of wrinkled colonies compared to smooth regions. Possible explanations include regulated differences in metabolism or differences in cell density due to morphology or matrix production. While phenazines are likely too toxic for use as therapeutic agents, our findings with MB may have implications for antifungal therapy. MB is nontoxic in mammals and has been used as an antimalarial drug, a neurotoxin antidote, and a potentiating factor in photodynamic therapy (34, 35). Thus, there is potential for this or similar compounds to be used to inhibit C. albicans biofilm infections in vivo.
This work highlights that phenazines have different biological effects at different concentrations. Kerr and colleagues (27, 36) and our laboratory (24, 29) have demonstrated the anti-Candida properties of P. aeruginosa phenazines at high concentrations (>500 µM). Here, we show that respiration is inhibited at concentrations that are 25- to 200-fold lower than those required to stop growth (29, 36) (Fig. 1B). In the lungs of cystic fibrosis (CF) patients, where P. aeruginosa and C. albicans are often found together (21, 62), PYO and PCA were found to be between 5 and 80 µM (33). We show here that these concentrations repressed C. albicans filamentation and biofilm development but did not kill the fungus. A 1989 study by Bhargava et al. (63) that assessed fungal morphology in the CF lung suggests that this fungus exists as yeast in CF airways. Our findings also suggest the possibility that by releasing phenazines, P. aeruginosa causes C. albicans to secrete more fermentation products that are readily used by P. aeruginosa to enhance its own growth and survival.
MATERIALS AND METHODS
Strains, media, and culture conditions.
See Table S1 for strain descriptions. C. albicans and P. aeruginosa were inoculated from overnight cultures onto yeast nitrogen base synthetic medium (YNB salts with ammonium sulfate that was supplemented with 10 mM glucose [Glu], 0.2% [wt/vol] amino acids from yeast synthetic dropout medium supplement without tryptophan [AA], both Glu and AA, or succinate [10 mM]). Where stated, 40 mM MOPS (morpholinepropanesulfonic acid; pH 7) or 100 mM citrate buffer (pH 5) was added. Noninducing conditions that promoted growth as yeast and the formation of smooth colonies were defined as Glu or Glu-AA and incubation at 30°C. In inducing conditions that cause colony wrinkling and filamentation, 5 mM GlcNAc was added and cultures were incubated at 37°C. When indicated, media were supplemented with phenazines or methylene blue as described in the supplemental information. To monitor pH, bromocresol purple (0.01% [vol/vol]) was added to the agar medium. C. albicans-P. aeruginosa cocultures were performed on yeast extract-peptone-dextrose (YPD) agar.
Analysis of extracellular pH and ammonia production.
See Methods in Text S1 in the supplemental material.
Respiratory activity analyses and oxygen tension analyses.
To analyze the respiratory activity of colonies, we employed a TTC overlay technique (64) in which 2 ml of the TTC-agar solution was applied to 48-h-old spot-inoculated colonies (see Methods in Text S1 in the supplemental material). Oxygen tensions within the colonies were measured at room temperature using an automated micro Clark-type oxygen sensor (Unisense) in three areas per colony from the top to bottom after growth for 72 h.
SUPPLEMENTAL MATERIAL
Supplemental Methods. Download
Biosynthetic pathway for pyocyanin from phenazine-1-carboxylic acid in P. aeruginosa. The chemical structure of PMS, an analog of the 5MPCA intermediate, is also shown. Download
C. albicans grown with P. aeruginosa mutants altered in phenazine production. C. albicans SC5314 was streaked adjacent to P. aeruginosa phzM::TnM, which secretes PCA (phzM−), and the phzS::TnM (phzS−) strain, which is capable of producing PCA and 5MPCA. Download
C. albicans colonies in the presence of low concentrations of phenazines. The fungus was spot inoculated on Glu-AA medium, and pictures of colonies were taken after 48 h. (A and B) C. albicans colonies grown under filament-inducing conditions in the presence or in the absence of 60 µM PCA (A) or different concentrations of PMS or PYO (B). (C) Colonies of C. albicans reporter strains with HWP1 or ACT1 promoter fusions to lacZ grown on Glu-AA agar containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) without and with 5 µM PMS for 48 h under filament-inducing conditions. (D) Colony morphology of C. albicans-grown under noninducing conditions on medium alone or medium with PMS (5 µM) and PYO (20 µM). Download
Phenazines modulate C. albicans filamentation and biofilm formation in liquid medium. (A) Microscopic images of C. albicans morphologies of cells removed from the glass bottom of petri dishes that were incubated during 24 h at 37°C in the presence or absence of 5 µM PMS or 20 µM PYO in liquid Glu-AA medium under filament-inducing conditions. Fungal cells were treated for 1 min with Calcofluor white (CFW) prior to visualization with differential interference contrast (DIC) and DAPI (4′,6-diamidino-2-phenylindole) channels. Data are representative of two independent experiments. (B) In vitro biofilm formation assays of phenazine-treated and untreated cultures. C. albicans was allowed to attach onto 24-well plates for 90 min (37°C). After this phase, new Glu-AA liquid medium without GluNAc and supplemented with or without 5 or 20 µM PMS and PYO, respectively, was added. After 18 h, biofilms were visualized and quantified using crystal violet. Data are representative of two independent experiments (*, P < 0.05; n = 3). Download
Phenazines alter C. albicans growth in succinate, a nonfermentable carbon source. Growth curves of liquid cultures on media containing succinate in the presence (triangles) or absence (open circles) of 5 µM PMS at 37°C. The optical density (OD600) of each culture was monitored every hour. Download
Effects of methylene blue on C. albicans colony morphology. (A) C. albicans cells (10 µl) of a YPD overnight culture were spotted on unbuffered Glu-AA media without (control) or with increasing concentrations of methylene blue (MB). Colonies were photographed after 48 h of incubation under inducing conditions (with 5 mM GlcNAc at 37°C). (B) C. albicans colonies grown in Glu-AA medium alone or with 5 µM MB under noninducing conditions for 48 h. The respiratory activity of colonies was assessed by conversion of TTC supplied in an agar overlay, and images were obtained with stereoscope at 16×. The intensity of red pigmentation is proportional to the respiratory activity. Download
C. albicans wrinkled colony development varies with oxygen availability and carbon source. Pictures of drop-inoculated fungal colonies grown in modified Glu-AA with 5 mM GlcNAc medium (with 20 µM oleic and 80 µM nicotinic acid) supplemented with 10 or 100 mM of Glu, incubated under anoxic conditions for 48 h at 37°C. For reference, a colony growth on YNB Glu-AA with 5 mM GlcNAc and 10 mM glucose at 48 h is shown. Download
Bacterial and fungal strains used in this study.
Effect of phenazines on final concentration of acetate, ethanol, and glucose. Cultures were grown in liquid YNB with glucose (10 mM) and amino acids for 24 h. These are all the data used to calculate the results shown in Fig. 3 shown in the main text.
ACKNOWLEDGMENTS
This project was supported in part by grants from the National Center for Research Resources (5P20RR018787-10), the National Institute of General Medical Sciences (8 P20 GM103413-10), and National Institute of Dental and Craniofacial Research (K22 DE016542) from the National Institutes of Health.
We acknowledge Julie Paye and Lee Lynd for the HPLC analysis of culture supernatants and thank Judy Jacobs and Juan R. Cubillos for their input on the manuscript.
Footnotes
Citation Morales DK, Grahl N, Okegbe C, Dietrich LEP, Jacobs NJ, Hogan DA. 2013. Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa phenazines. mBio 4(1):e00526-12. doi:10.1128/mBio.00526-12.
REFERENCES
- 1. Odds FC. 1987. Candida infections: an overview. Crit. Rev. Microbiol. 15:1–5 [DOI] [PubMed] [Google Scholar]
- 2. Sudbery PE. 2011. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 16:737–748 [DOI] [PubMed] [Google Scholar]
- 3. Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2:1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xie Z, Thompson A, Sobue T, Kashleva H, Xu H, Vasilakos J, Dongari-Bagtzoglou A. 2012. Candida albicans biofilms do not trigger reactive oxygen species and evade neutrophil killing. J. Infect. Dis. 15:1936–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nett J, Andes D. 2006. Candida albicans biofilm development, modeling a host-pathogen interaction. Curr. Opin. Microbiol. 9:340–345 [DOI] [PubMed] [Google Scholar]
- 6. Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nobile CJ, Schneider HA, Nett JE, Sheppard DC, Filler SG, Andes DR, Mitchell AP. 2008. Complementary adhesin function in C. albicans biofilm formation. Curr. Biol. 18:1017–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim J, Sudbery P. 2011. Candida albicans, a major human fungal pathogen. J. Microbiol. 49:171–177 [DOI] [PubMed] [Google Scholar]
- 9. Whiteway M, Bachewich C. 2007. Morphogenesis in Candida albicans. Annu. Rev. Microbiol. 61:529–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mulhern SM, Logue ME, Butler G. 2006. Candida albicans transcription factor Ace2 regulates metabolism and is required for filamentation in hypoxic conditions. Eukaryot. Cell 5:2001–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim SY, Kim J. 2010. Roles of dihydrolipoamide dehydrogenase Lpd1 in Candida albicans filamentation. Fungal Genet. Biol. 47:782–788 [DOI] [PubMed] [Google Scholar]
- 12. Land GA, McDonald WC, Stjernholm RL, Friedman L. 1975. Factors affecting filamentation in Candida albicans: changes in respiratory activity of Candida albicans during filamentation. Infect. Immun. 12:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McDonough JA, Bhattacherjee V, Sadlon T, Hostetter MK. 2002. Involvement of Candida albicans NADH dehydrogenase complex I in filamentation. Fungal Genet. Biol. 36:117–127 [DOI] [PubMed] [Google Scholar]
- 14. Setiadi ER, Doedt T, Cottier F, Noffz C, Ernst JF. 2006. Transcriptional response of Candida albicans to hypoxia: linkage of oxygen sensing and Efg1p-regulatory networks. J. Mol. Biol. 361:399–411 [DOI] [PubMed] [Google Scholar]
- 15. Vellucci VF, Gygax SE, Hostetter MK. 2007. Involvement of Candida albicans pyruvate dehydrogenase complex protein X (Pdx1) in filamentation. Fungal Genet. Biol. 44:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watanabe T, Ogasawara A, Mikami T, Matsumoto T. 2006. Hyphal formation of Candida albicans is controlled by electron transfer system. Biochem. Biophys. Res. Commun. 348:206–211 [DOI] [PubMed] [Google Scholar]
- 17. Peleg AY, Hogan DA, Mylonakis E. 2010. Medically important bacterial-fungal interactions. Nat. Rev. Microbiol. 8:340–349 [DOI] [PubMed] [Google Scholar]
- 18. Morales DK, Hogan DA. 2010. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 6:e1000886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shirtliff ME, Peters BM, Jabra-Rizk MA. 2009. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol. Lett. 299:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lynch AS, Robertson GT. 2008. Bacterial and fungal biofilm infections. Annu. Rev. Med. 59:415–428 [DOI] [PubMed] [Google Scholar]
- 21. Leclair LW, Hogan DA. 2010. Mixed bacterial-fungal infections in the CF respiratory tract. Med. Mycol. 48:S125–S132 [DOI] [PubMed] [Google Scholar]
- 22. Brand A, Barnes JD, Mackenzie KS, Odds FC, Gow NA. 2008. Cell wall glycans and soluble factors determine the interactions between the hyphae of Candida albicans and Pseudomonas aeruginosa. FEMS Microbiol. Lett. 287:48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hogan DA, Kolter R. 2002. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 296:2229–2232 [DOI] [PubMed] [Google Scholar]
- 24. Gibson J, Sood A, Hogan DA. 2009. Pseudomonas aeruginosa-Candida albicans interactions: localization and fungal toxicity of a phenazine derivative. Appl. Environ. Microbiol. 75:504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cugini C, Calfee MW, Farrow JM, III, Morales DK, Pesci EC, Hogan DA. 2007. Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa. Mol. Microbiol. 65:896–906 [DOI] [PubMed] [Google Scholar]
- 26. Hogan DA, Vik A, Kolter R. 2004. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 54:1212–1223 [DOI] [PubMed] [Google Scholar]
- 27. Kerr JR. 1994. Suppression of fungal growth exhibited by Pseudomonas aeruginosa. J. Clin. Microbiol. 32:525–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cugini C, Morales DK, Hogan DA. 2010. Candida albicans-produced farnesol stimulates Pseudomonas quinolone signal production in LasR-defective Pseudomonas aeruginosa strains. Microbiology 156:3096–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morales DK, Jacobs N, Rajamani S, Krishnamurthy M, Cubillos-Ruiz J, Hogan D. 2010. Antifungal mechanisms by which a novel Pseudomonas aeruginosa phenazine toxin kills Candida albicans in biofilms. Mol. Microbiol. 78:1379–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mavrodi DV, Parejko JA, Mavrodi OV, Kwak YS, Weller DM, Blankenfeldt W, Thomashow LS. 2012. Recent insights into the diversity, frequency and ecological roles of phenazines in fluorescent Pseudomonas spp. Environ. Microbiol. [Epub ahead of print.] http://dx.doi.org/10.1111/j.1462-2920.2012.02846.x [DOI] [PubMed]
- 31. O’Malley YQ, Abdalla MY, McCormick ML, Reszka KJ, Denning GM, Britigan BE. 2003. Subcellular localization of Pseudomonas pyocyanin cytotoxicity in human lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 284:L420–L430 [DOI] [PubMed] [Google Scholar]
- 32. French SW, Palmer DS, Sim WA. 1973. Phenazine methosulfate uptake by rat liver mitochondria. Can. J. Biochem. 51:235–240 [DOI] [PubMed] [Google Scholar]
- 33. Hunter RC, et al. 2012. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am. J. Respir. Cell Mol. Biol. 47:738–745 [DOI] [PubMed] [Google Scholar]
- 34. Kasozi DM, Gromer S, Adler H, Zocher K, Rahlfs S, Wittlin S, Fritz-Wolf K, Schirmer RH, Becker K. 2011. The bacterial redox signaller pyocyanin as an antiplasmodial agent: comparisons with its thioanalog methylene blue. Redox Rep. 16:154–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schirmer RH, Adler H, Pickhardt M, Mandelkow E. 2011. Lest we forget you—methylene blue. Neurobiol. Aging 32:e7–16 [DOI] [PubMed] [Google Scholar]
- 36. Kerr JR, et al. 1999. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J. Clin. Pathol. 52:385–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martinez JP, et al. 1990. Wall mannoproteins in cells from colonial phenotypic variants of Candida albicans. J. Gen. Microbiol. 136:2421–2432 [DOI] [PubMed] [Google Scholar]
- 38. Ene IV, Bennett RJ. 2009. Hwp1 and related adhesins contribute to both mating and biofilm formation in Candida albicans. Eukaryot. Cell 8:1909–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Staab JF, Bradway SD, Fidel PL, Sundstrom P. 1999. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 283:1535–1538 [DOI] [PubMed] [Google Scholar]
- 40. El Barkani A, Kurzai O, Fonzi WA, Ramon A, Porta A, Frosch M, Muhlschlegel FA. 2000. Dominant active alleles of RIM101 (PRR2) bypass the pH restriction on filamentation of Candida albicans. Mol. Cell. Biol. 20:4635–4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vylkova S, Carman AJ, Danhof HA, Collette JR, Zhou H, Lorenz MC. 2011. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. mBio 2:e00055-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zikánová B, Kuthan M, Ricicová M, Forstová J, Palková Z. 2002. Amino acids control ammonia pulses in yeast colonies. Biochem. Biophys. Res. Commun. 294:962–967 [DOI] [PubMed] [Google Scholar]
- 43. Brown AJ. 2006. Integration of metabolism with virulence in Candida albicans, p 185–203 In Brown AJ, Fungal genomics. Mycota, vol XIII Springer-Verlag, Heidelberg, Germany [Google Scholar]
- 44. Davis D, Wilson RB, Mitchell AP. 2000. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 20:971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rich PR, Mischis LA, Purton S, Wiskich JT. 2001. The sites of interaction of triphenyltetrazolium chloride with mitochondrial respiratory chains. FEMS Microbiol. Lett. 202:181–187 [DOI] [PubMed] [Google Scholar]
- 46. Wolfe AJ. 2005. The acetate switch. Microbiol. Mol. Biol. Rev. 69:12–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hassan HM, Fridovich I. 1979. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch. Biochem. Biophys. 196:385–395 [DOI] [PubMed] [Google Scholar]
- 48. Biswas SK, Chaffin WL. 2005. Anaerobic growth of Candida albicans does not support biofilm formation under similar conditions used for aerobic biofilm. Curr. Microbiol. 51:100–104 [DOI] [PubMed] [Google Scholar]
- 49. Szawatkowski M, Hamilton-Miller J. 1978. Anaerobic growth and sensitivity of Candida albicans. Microbios Lett. 5:61–66 [Google Scholar]
- 50. Bonhomme J, et al. 2011. Contribution of the glycolytic flux and hypoxia adaptation to efficient biofilm formation by Candida albicans. Mol. Microbiol. 80:995–1013 [DOI] [PubMed] [Google Scholar]
- 51. Römling U. 2005. Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell. Mol. Life Sci. 62:1234–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yildiz FH, Schoolnik GK. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. U. S. A. 96:4028–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Friedman L, Kolter R. 2004. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J. Bacteriol. 186:4457–4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dietrich LE, Okegbe C, Price-Whelan A, Sakhtah H, Hunter RC, Newman DK. 2013. Bacterial community morphogenesis is intimately linked to the intracellular redox state. J. Bacteriol. [Epub ahead of print.] http://dx.doi.org/10.1128/JB.02273-12 [DOI] [PMC free article] [PubMed]
- 55. Wang Y, Kern SE, Newman DK. 2010. Endogenous phenazine antibiotics promote anaerobic survival of Pseudomonas aeruginosa via extracellular electron transfer. J. Bacteriol. 192:365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Price-Whelan A, Dietrich LE, Newman DK. 2007. Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa PA14. J. Bacteriol. 189:6372–6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dietrich LE, Teal TK, Price-Whelan A, Newman DK. 2008. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science 321:1203–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ramos I, Dietrich LE, Price-Whelan A, Newman DK. 2010. Phenazines affect biofilm formation by Pseudomonas aeruginosa in similar ways at various scales. Res. Microbiol. 161:187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mukherjee PK, Mohamed S, Chandra J, Kuhn D, Liu S, Antar OS, Munyon R, Mitchell AP, Andes D, Chance MR, Rouabhia M, Ghannoum MA. 2006. Alcohol dehydrogenase restricts the ability of the pathogen Candida albicans to form a biofilm on catheter surfaces through an ethanol-based mechanism. Infect. Immun. 74:3804–3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Visarius TM, Stucki JW, Lauterburg BH. 1997. Stimulation of respiration by methylene blue in rat liver mitochondria. FEBS Lett. 412:157–160 [DOI] [PubMed] [Google Scholar]
- 61. Hassan HM, Fridovich I. 1980. Mechanism of the antibiotic action pyocyanine. J. Bacteriol. 141:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chotirmall SH, O'Donoghue E, Bennett K, Gunaratnam C, O'Neill SJ, McElvaney NG. 2010. Sputum Candida albicans presages FEV1 decline and hospitalized exacerbations in cystic fibrosis. Chest 138:1186–1195 [DOI] [PubMed] [Google Scholar]
- 63. Bhargava V, Tomashefski JF, Jr, Stern RC, Abramowsky CR. 1989. The pathology of fungal infection and colonization in patients with cystic fibrosis. Hum. Pathol. 20:977–986 [DOI] [PubMed] [Google Scholar]
- 64. Ogur M, St. John R, Nagai S. 1957. Tetrazolium overlay technique for population studies of respiration deficiency in yeast. Science 125:928–929 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Methods. Download
Biosynthetic pathway for pyocyanin from phenazine-1-carboxylic acid in P. aeruginosa. The chemical structure of PMS, an analog of the 5MPCA intermediate, is also shown. Download
C. albicans grown with P. aeruginosa mutants altered in phenazine production. C. albicans SC5314 was streaked adjacent to P. aeruginosa phzM::TnM, which secretes PCA (phzM−), and the phzS::TnM (phzS−) strain, which is capable of producing PCA and 5MPCA. Download
C. albicans colonies in the presence of low concentrations of phenazines. The fungus was spot inoculated on Glu-AA medium, and pictures of colonies were taken after 48 h. (A and B) C. albicans colonies grown under filament-inducing conditions in the presence or in the absence of 60 µM PCA (A) or different concentrations of PMS or PYO (B). (C) Colonies of C. albicans reporter strains with HWP1 or ACT1 promoter fusions to lacZ grown on Glu-AA agar containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) without and with 5 µM PMS for 48 h under filament-inducing conditions. (D) Colony morphology of C. albicans-grown under noninducing conditions on medium alone or medium with PMS (5 µM) and PYO (20 µM). Download
Phenazines modulate C. albicans filamentation and biofilm formation in liquid medium. (A) Microscopic images of C. albicans morphologies of cells removed from the glass bottom of petri dishes that were incubated during 24 h at 37°C in the presence or absence of 5 µM PMS or 20 µM PYO in liquid Glu-AA medium under filament-inducing conditions. Fungal cells were treated for 1 min with Calcofluor white (CFW) prior to visualization with differential interference contrast (DIC) and DAPI (4′,6-diamidino-2-phenylindole) channels. Data are representative of two independent experiments. (B) In vitro biofilm formation assays of phenazine-treated and untreated cultures. C. albicans was allowed to attach onto 24-well plates for 90 min (37°C). After this phase, new Glu-AA liquid medium without GluNAc and supplemented with or without 5 or 20 µM PMS and PYO, respectively, was added. After 18 h, biofilms were visualized and quantified using crystal violet. Data are representative of two independent experiments (*, P < 0.05; n = 3). Download
Phenazines alter C. albicans growth in succinate, a nonfermentable carbon source. Growth curves of liquid cultures on media containing succinate in the presence (triangles) or absence (open circles) of 5 µM PMS at 37°C. The optical density (OD600) of each culture was monitored every hour. Download
Effects of methylene blue on C. albicans colony morphology. (A) C. albicans cells (10 µl) of a YPD overnight culture were spotted on unbuffered Glu-AA media without (control) or with increasing concentrations of methylene blue (MB). Colonies were photographed after 48 h of incubation under inducing conditions (with 5 mM GlcNAc at 37°C). (B) C. albicans colonies grown in Glu-AA medium alone or with 5 µM MB under noninducing conditions for 48 h. The respiratory activity of colonies was assessed by conversion of TTC supplied in an agar overlay, and images were obtained with stereoscope at 16×. The intensity of red pigmentation is proportional to the respiratory activity. Download
C. albicans wrinkled colony development varies with oxygen availability and carbon source. Pictures of drop-inoculated fungal colonies grown in modified Glu-AA with 5 mM GlcNAc medium (with 20 µM oleic and 80 µM nicotinic acid) supplemented with 10 or 100 mM of Glu, incubated under anoxic conditions for 48 h at 37°C. For reference, a colony growth on YNB Glu-AA with 5 mM GlcNAc and 10 mM glucose at 48 h is shown. Download
Bacterial and fungal strains used in this study.
Effect of phenazines on final concentration of acetate, ethanol, and glucose. Cultures were grown in liquid YNB with glucose (10 mM) and amino acids for 24 h. These are all the data used to calculate the results shown in Fig. 3 shown in the main text.