Abstract
Adenylate cyclase toxin (CyaA) from Bordetella pertussis can subvert host immune responses allowing bacterial colonization. Here we have examined its adjuvant and immunomodulatory properties and the possible contribution of lipopolysaccharide (LPS), known to be present in purified CyaA preparations. CyaA enhanced antigen-specific interleukin-5 (IL-5) and IL-10 production and immunoglobulin G1 antibodies to coadministered antigen in vivo. Antigen-specific CD4+-T-cell clones generated from mice immunized with antigen and CyaA had cytokine profiles characteristic of Th2 or type 1 regulatory T (Tr1) cells. Since innate immune cells direct the induction of T-cell subtypes, we examined the influence of CyaA on activation of dendritic cells (DC) and macrophages. CyaA significantly augmented LPS-induced IL-6 and IL-10 and inhibited LPS-driven tumor necrosis factor alpha and IL-12p70 production from bone marrow-derived DC and macrophages. CyaA also enhanced cell surface expression of CD80, CD86, and major histocompatibility class II on immature DC. The stimulatory activity of our CyaA preparation for IL-10 production and CD80, CD86, and major histocompatibility complex class II expression was attenuated following the addition of polymyxin B or with the use of DC from Toll-like receptor (TLR) 4-defective mice. However, treatment of DC with LPS alone at the concentration present in the CyaA preparation (0.2 ng/ml) failed to activate DC in vitro. Our findings demonstrate that activation of innate cells in vitro by CyaA is dependent on a second signal through a TLR and that CyaA can promote Th2/Tr1-cell responses by inhibiting IL-12 and promoting IL-10 production by DC and macrophages.
Cells of the innate immune system, especially dendritic cells (DC), direct the differentiation of naive CD4+ T cells into functionally distinct Th1, Th2, or regulatory T (Tr) cell subtypes (35). Activation of immature DC though binding of conserved microbial molecules to pathogen recognition receptors, such as Toll-like receptors (TLR), complement receptors and integrins, is accompanied by maturation and homing to the lymph nodes, where the mature DC presents antigen to the naive T cell (1, 34). Certain pathogen-derived molecules—including lipopolysaccharide (LPS), CpG motifs, double-stranded RNA, and pertussis toxin (PT)—activate DC that drive the differentiation of Th1 cells (8, 23). In contrast, products of helminth parasites (54), cholera toxin (CT) (13), and candida albicans yeast hyphae (9) activate DC that drive the differentiation of Th2 cells. Finally, filamentous hemagglutinin (FHA) from Bordetella pertussis (34) and CT (31) activate DC that promote the differentiation of IL-10-secreting type 1 Tr (Tr1) cells. In addition, cytokines, including interleukin-4 (IL-4), IL-6, IL-10, and IL-12, secreted by innate immune cells in response to pathogen-derived molecules, play a critical role in regulating the differentiation of naive CD4+ T cells into distinct T-cell subtypes (34, 36, 39).
The gram-negative bacterium B. pertussis causes whooping cough, a severe and prolonged infection in young children. A number of virulence factors of B. pertussis—including PT, LPS, FHA, and adenylate cyclase toxin (CyaA)—which are essential for bacterial colonization, can modulate host immune responses (37). Bacteria deficient in CyaA are less pathogenic in mice and fail to stimulate inflammatory cell recruitment into murine lungs (15, 16, 22, 27, 52). CyaA has been shown to subvert immune responses to B. pertussis by interfering with chemotaxis, phagocytosis and superoxide production in host cells through the generation of supraphysiological levels of cyclic AMP (cAMP) (12, 40, 42). Furthermore, CyaA causes lysis and cytotoxicity in a variety of cells (24, 28, 53) and induces apoptosis in macrophages (17, 29). CyaA is encoded by the cyaA gene and is posttranslationally activated through palmitoylation of K983 (20) by the product of the cyaC gene (3), although Escherichia coli-expressed CyaA can also be palmitoylated at K860 (21). The C-terminal 1,306 amino acids contain a series of nonapeptide repeats involved in calcium binding (46) similar to the repeat in toxin (RTX) family of exotoxins which have hemolytic and immune stimulatory properties (5). The N-terminal 400 amino acids contain the catalytic domain (50) that converts ATP to cAMP (4). Upon cell binding, the enzymatic domain is delivered into the cytosol where it must bind eukaryotic calmodulin to become enzymatically active (55).
The invasive nature of CyaA has been employed to deliver antigenic peptides to the endogenous route of antigen processing for presentation to major histocompatibility complex class I (MHC-I)-restricted CD8+ T cells (11). Recently it has been shown that an enzymatically inactive CyaA could also deliver an epitope into the MHC-II processing pathway for activation of CD4+ cells (33). In addition, CyaA has been shown to enhance antibody levels to coadministered ovalbumin (26) and to promote Th1 responses to an expressed viral epitope (7). The adjuvant activity of CyaA may reside in its ability to activate cells of the innate immune system through the upregulation of cAMP (14) and/or the binding to the CD11b/CD18 αMβ2 integrin (19), expressed on innate immune cells, including macrophages and DC. It has been reported that CyaA can promote maturation and suppress inflammatory cytokine production by human monocyte derived DC (2).
In this study we have examined the adjuvant and immunomodulatory activity of CyaA, paying particular attention to a possible contribution of LPS, which is known to be closely associated with RTX molecules (6, 32). We addressed the hypothesis that CyaA may act as an adjuvant to enhance specific subsets of CD4+ T cells by promoting activation of DC. Our findings demonstrate that CyaA has adjuvant activity, promoting Th2 and Tr1 responses, with significant enhancement of antigen-specific IL-10-producing T cells. This effect appears to be mediated in part by its ability to activate cells of the innate immune system, including DC. However, our data also reveal that CyaA can synergize with and modulate TLR4-mediated responses of DC to LPS.
MATERIALS AND METHODS
Plasmid construction.
Genomic DNA of B. pertussis (strain W28) was prepared from a mid-log-phase culture. The 5′ end of cyaA was amplified by PCR with oligonucleotides (MWG Biotech, Ebersberg, Germany) PAB5 (5′-CGCCGGTACCATGCAGCAATCGCATCAGGCT-3′) and PAB6 (5′-TGGTGAATTCGCTCTTGCCCG-3′) (restriction sites are underlined). The resulting product was digested with KpnI and EcoRI (Invitrogen, Carlsbad, Calif.) and inserted in corresponding sites of the cloning vector pBluescript SK− (Stratagene, La Jolla, Calif.), and this plasmid was named pAPB4. The 3′ end of cyaA was amplified by PCR from B. pertussis genomic DNA with oligonucleotides PAB7 (5′-AAGAGCGAATTCACCACATTCGTCG-3′) and PAB2 (5′-CGCGGATCCTCAGCGCCAGTTGACAGCCA-3′). The product was digested with EcoRI and BamHI and ligated into pBluescript SK− at the same restriction sites (underlined). This plasmid was named pAPB5 and was then digested with EcoRI and BamHI, and the 3′ cyaA fragment was subcloned into the corresponding sites of pAPB4, giving a full-length cyaA gene. This plasmid was named pAPB6. cyaC was amplified by PCR from the genomic DNA of B. pertussis with oligonucleotides PAB3 (5′-CGCGGATCCGAGGGCATGTCATGCTTCCGTCCGCC-3′) and PAB4 (5′-CGCGGCGAAGCTTTCAGGCGGTGCCCCGGC-3′). The PCR fragment was digested with BamHI and HindIII and cloned into the pASK-IAB6 (IBA GmbH, Goettingen, Germany) expression vector opened with the same restriction enzymes (restriction sites underlined). This new plasmid was termed pAPB1. The intact cyaA gene was isolated from pAPB6, digested with KpnI and BamHI, and cloned into pAPB1 upstream of the cyaC gene using the KpnI and BamHI sites, and this plasmid was termed pAPB8. pAPB8 was digested with KpnI and HindIII, and the 5.9-kb product containing cyaA and cyaC was cloned into the commercial His-tagged vector pQE-80 (Qiagen, West Sussex, United Kingdom) opened at the same restriction sites. The sequence and orientation of the cloned genes were confirmed by restriction digestion and sequencing (MWG Biotech). This plasmid was named pJR2, from which His-tagged palmitoylated CyaA could be expressed in E. coli.
Purification of CyaA.
E. coli XL1-Blue(pJR2) was induced to express CyaA and CyaC by the addition of isopropyl-β-thiogalactopyranoside (IPTG) (Bioline, London, United Kingdom) to an exponentially growing bacterial culture in Luria-Bertani broth supplemented with ampicillin (150 μg/ml) with vigorous shaking at 37°C. The bacterial culture was centrifuged, and the bacterial pellet was resuspended in 50 mM Tris-HCl-0.2 mM CaCl2, pH 8.0, supplemented with protease inhibitor cocktail (catalog no. P-8465; Sigma, Poole, United Kingdom). Bacteria were disrupted with FastPrep Protein Blue beads (QbioGene, Earlsbad, Calif.) in a FastPrep machine at speed 6 for 20 s. The insoluble material containing CyaA was separated by centrifugation, washed with 50 mM Tris-HCl-0.2 mM CaCl2-0.2% Triton X-100, pH 8.0, and incubated in 50 mM Tris-HCl-0.2 mM CaCl2-8 M urea, pH 8.0 (buffer A), for 1 h at room temperature with stirring. The solubilized CyaA was collected following centrifugation. After addition of NaCl to a final concentration of 0.1 M, CyaA was loaded on a DEAE cellulose (Sigma) column equilibrated with buffer A supplemented with 0.1 M NaCl and eluted with buffer A supplemented with 0.2 M NaCl as previously described (50). The protein was further purified on Ni2+ columns (Qiagen) under denaturing conditions by pH adjustment as recommended by the manufacturers and eluted in 100 mM NaHPO4-10 mM Tris-HCl-8 M urea-0.2 mM CaCl2, pH 4.5. LPS removal was attempted using Detoxigel endotoxin removal columns (polymyxin B-conjugated columns; Pierce, Rockford, Ill.) following the manufacturer's protocols. LPS was dissociated from CyaA by dialysis first against Dulbecco's phosphate-buffered saline (PBS) (Sigma)-1 mM EDTA-1 M urea (pH 4.6) and then against Dulbecco's PBS-0.1 mM CaCl2-2 M urea (pH 8.0). The purified protein was stored in aliquots at −20°C. LPS was measured by a colorimetric Limulus amoebocyte lysate assay (QCL-1000; Biowhittaker, Walkersville, Md.), and protein concentrations were determined by Bradford assay (Bio-Rad). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized with Coomassie blue (GelCode Blue Stain Reagent; Pierce). Alternatively, proteins were transferred to a nitrocellulose membrane following sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with anti-His Tag antibodies (Santa Cruz Biotechnologies) or anti-CyaA antibodies (kind gift from Erik Hewlett). The bands were visualized by incubation with secondary anti-rabbit immunoglobulin G (IgG) horseradish peroxidase-conjugated antibodies (Sigma) and chemiluminescent supersignal detection system (Pierce).
In vitro CyaA enzymatic activity.
The enzymatic activity of CyaA was measured as previously described (30). A 0.1-μg aliquot of protein was incubated for 5 min at 30°C in 50 μl of a solution containing 50 mM Tris-HCl, bovine serum albumin (100 μg/ml), 0.1 μM calmodulin, 0.12 mM CaCl2, 6 mM MgCl2, and 2 mM ATP, pH 8.0. The reaction mixture was mixed with lysis reagent 1B of the Amersham Biosciences Biotrak Enzymeimmunoassay system and boiled for 5 min. cAMP was measured by a competitive enzyme-linked immunosorbent assay (ELISA) in the Amersham kit. Results are given in micromoles of cAMP produced per minute at 30°C and pH 8.0.
Intracellular cAMP accumulation.
Macrophages (J774 cell line) and DC were cultured at 106 cells/ml in Dulbecco's modified Eagle medium (DMEM) or RPMI medium, respectively, with CyaA (1 μg/ml). After 30 min, 4 h, and 24 h of incubation, lysis reagent 1B of the Amersham Biosciences Biotrak Enzymeimmunoassay system was added and the samples were processed for quantification by cAMP ELISA.
LDH assay.
The lysis of J774 macrophages was measured by the release of lactose dehydrogenase (LDH) into the culture supernatants using the CytoTox 96 nonradioactive cytotoxicity assay (Promega, Madison, Wisc.). Cells (105/100 μl) were aliquoted into a 96-well plate in DMEM. CyaA was added at the specified concentrations and the plates were incubated at 37°C with 5% CO2 for the indicated amounts of time. 100% lysis wells were prepared by the addition of lysis reagent to the appropriate wells. Supernatants (50 μl) were removed from each well for the LDH assay. Percentage lysis was calculated as follows: [(OD of sample − OD of untreated cells)/(OD of 100% lysis cells − OD of untreated cells)] × 100, where OD is optical density.
Animals and immunization.
Female specific-pathogen-free BALB/c, C3H/HeN and C3H/HeJ mice were purchased from Harlan Olac (Bicester, United Kingdom) and used at 6 to 8 weeks old, with four or five mice per group. Mice were housed in individually ventilated cages and all experiments were performed according to regulations of the Irish Department of Health, the European Union, and the Ethics Committee of Trinity College Dublin. Mice were immunized subcutaneously (s.c.) in the hind footpads once or twice (0 and 21 days) with depyrogenated keyhole limpet hemocyanin (KLH) (5 μg; Calbiochem, La Jolla, Calif.); KLH (5 μg) with CyaA (1 μg); KLH (5 μg) and phosphorothioate-stabilized oligodeoxynucleotide-containing CpG motifs (CpG-ODN) (5′-GCTAGACGTTAGCGT-3′), synthesized by Sigma-Genosys Ltd., Cambridge, United Kingdom; or with Dulbecco's PBS (Sigma, Poole, United Kingdom) in a final volume of 50 μl. Seven days after the first or second immunization mice were sacrificed by cervical dislocation, and serum and popliteal lymph nodes were collected.
Generation of antigen-specific T-cell lines and clones.
Popliteal lymph node cells (106/ml) from immunized mice were cultured with KLH (50 μg/ml). After two rounds of antigen stimulation, T-cell lines were cloned by limiting dilution as described previously (37). T-cell lines and clones were maintained by culture with antigen (KLH at 50 μg/ml) and irradiated splenic antigen-presenting cells (APC) for 4 to 5 days, followed by 5 to 7 days of culture with IL-2. T cells were tested for cytokine production at the end of the starve cycle.
Antigen-specific cytokine production.
Lymph node cells (106 cells/ml) from immunized mice or T-cell lines or clones and APC (irradiated spleen cells, 2 × 106/ml) were cultured at 37°C and 5% CO2 in RPMI medium with KLH (2 to 50 μg/ml) or phorbol 12-myristate 13-acetate (PMA) (25 ng/ml; Sigma) and anti-CD3 (0.5 μg/ml; BD Pharmingen, San Diego, Calif.) or medium only. After 3 days, supernatants were collected for cytokine detection and the medium was replaced. On the following day [3H]thymidine (950 μCi/well; Amersham Pharmacia, United Kingdom) was added and the cells were cultured for a further 5 h, after which cells were harvested and proliferation was assessed by [3H]thymidine incorporation as described previously (38). Concentrations of IL-4, IL-5, and gamma interferon (IFN-γ) were determined by immunoassay using pairs of antibodies and recombinant cytokines (BD Pharmingen) as standards. IL-10 concentrations were determined using a commercially available Duo-Set kit (R&D Systems, Minneapolis, Minn.).
Antibody assays.
Titers of KLH-specific IgG, IgG1, and IgG2a in the serum of immunized mice were determined by ELISA as described previously (48).
Effects of CyaA on cytokine and chemokine release by J774 macrophages and murine bone marrow-derived DC.
Murine bone marrow-derived DC were prepared by culturing bone marrow cells from the femur and tibia of mice in RPMI medium supplemented with 10% supernatant from a granulocyte-monocyte colony-stimulating factor-secreting cell line, J558-GM-CSF. On day 7 of culture, cells were collected, washed, and resuspended in RPMI medium. J774 macrophages were cultured in DMEM supplemented with 8% fetal calf serum at 37°C with 5% CO2 and used before the 20th passage. Macrophages and DC (106 cells/ml) were cultured with CyaA (1 μg/ml), E. coli LPS (1 to 1,000 ng/ml; Sigma), or CyaA followed 2 h later with LPS. Polymyxin B (10 μg/ml) was added where indicated. Supernatants were collected after 2, 4, and 28 h for analysis of cytokine production and cell surface marker expression. Concentrations of IL-10, IL-12p70, and tumor necrosis factor alpha (TNF-α) in cell supernatants were determined using commercially available Duo-Set kits (R&D Systems). Concentrations of IL-6 and macrophage inflammatory protein (MIP)-1α were determined by immunoassay using pairs of antibodies and recombinant cytokines as standards (BD Pharmingen).
Analysis of DC maturation.
DC (106/ml) were cultured for 24 h with CyaA (1 μg/ml), E. coli LPS (10 ng/ml or 1 μg/ml), CyaA and LPS, or CyaA in the presence of polymyxin B (10 μg/ml; Sigma) as indicated. Cells were recovered, and surface marker expression was assessed by flow cytometry using fluorescently labeled antibodies (BD Pharmingen). Cells were incubated for 30 min at 4°C with antibodies specific for mouse CD80 (hamster IgG2, clone 16-10A1), CD86 (rat IgG2a, clone GL1), CD11c (hamster IgG1, clone HL3), MHC-II (mouse IgG2b, I-Ad, clone AMS-32.1), CD40 (rat IgG2a, clone 3/23), or ICAM-1 (hamster IgG1, clone 3E2), followed by washing and incubation with streptavidin-PerCP in the case of biotin-labeled primary antibodies. Cells labeled with appropriate isotype matched antibodies with irrelevant specificity acted as controls. A total of 30,000 cells per sample were analyzed on a FACScalibur flow cytometer. Analysis was performed on CD11c-gated cells using CellQuest software (version 3.3; Becton Dickinson Immunocytmetery Systems, San Jose, Calif.).
Statistics.
Cytokine and chemokine levels were compared by one-way analysis of variance. Where significant differences were found, the Tukey Kramer multiple comparisons test was used to identify differences between individual groups.
RESULTS
Cloning, expression, and purification of CyaA.
cyaA, the gene encoding CyaA, and cyaC, the gene whose product is required to posttranslationally activate CyaA, were cloned from the genomic DNA of B. pertussis W28 into pQE-80 to allow inducible expression of these genes in E. coli. This plasmid, pJR2, expressing six-His-tagged CyaA and CyaC, was introduced into electrocompetent E. coli XL1-Blue cells. Bacteria harboring the recombinant plasmid were recovered and the correct orientation and position of the cloned genes were confirmed by both restriction digestion and sequencing.
Active CyaA was solubilized from bacterial inclusion bodies and purified on DEAE and Ni2+ columns. The purity of the 200-kDa CyaA protein was greater than 95% as estimated by Coomassie staining following gel electrophoresis, and this band was recognized by both anti-His and anti-CyaA antibodies (data not shown). The LPS content of the CyaA protein was monitored throughout the purification process. At this stage the protein preparation contained >2 ng of LPS/μg of protein. A number of procedures were used to attempt to remove the LPS. Passage of the protein preparation through polymyxin B-conjugated columns reduced, but did not eliminate, the LPS. This suggested that CyaA was closely complexed with LPS, as has been previously reported for other RTX toxins (6, 32). LPS can be dissociated from proteins by the addition of EDTA to chelate calcium ions and by lowering the pH to below the pI of the protein (43). After dialysis of the protein in these conditions, the preparation of CyaA contained 220 pg of LPS/μg of protein. This concentration of LPS did not stimulate macrophages or DC in vitro (data not shown; see Fig. 4, 5, and 7). This preparation was used in the adjuvant and immunomodulatory studies described below.
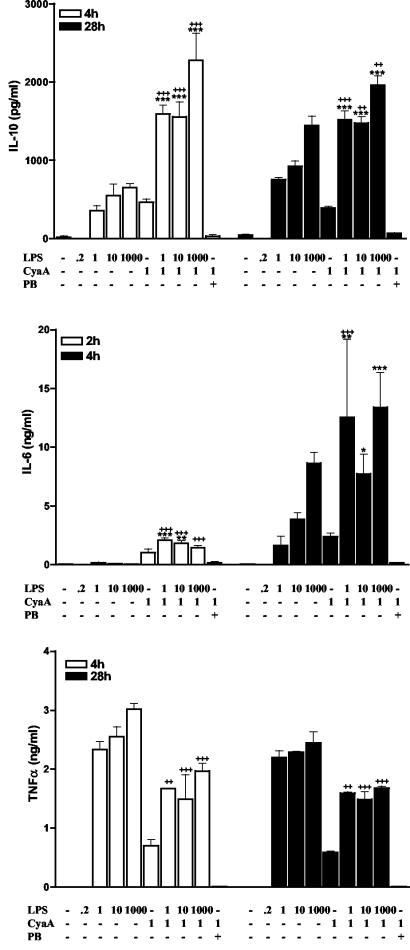
FIG. 4.
CyaA enhances LPS-induced anti-inflammatory cytokines and suppresses proinflammatory cytokines by macrophages. J774 macrophages (106/ml) were incubated with the indicated concentrations of LPS (0 to 1,000 ng/ml), CyaA (1 μg/ml), in the presence or absence of polymyxin B (PB) (10 μg/ml). Supernatants were collected at the indicated times and were tested for IL-10, IL-6, and TNF-α by immunoassay. Results are means (error bars, SD) of triplicate assays and are representative of three experiments. Symbols: *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus CyaA; ++, P < 0.01, +++, P < 0.001 versus LPS alone at the same concentration.
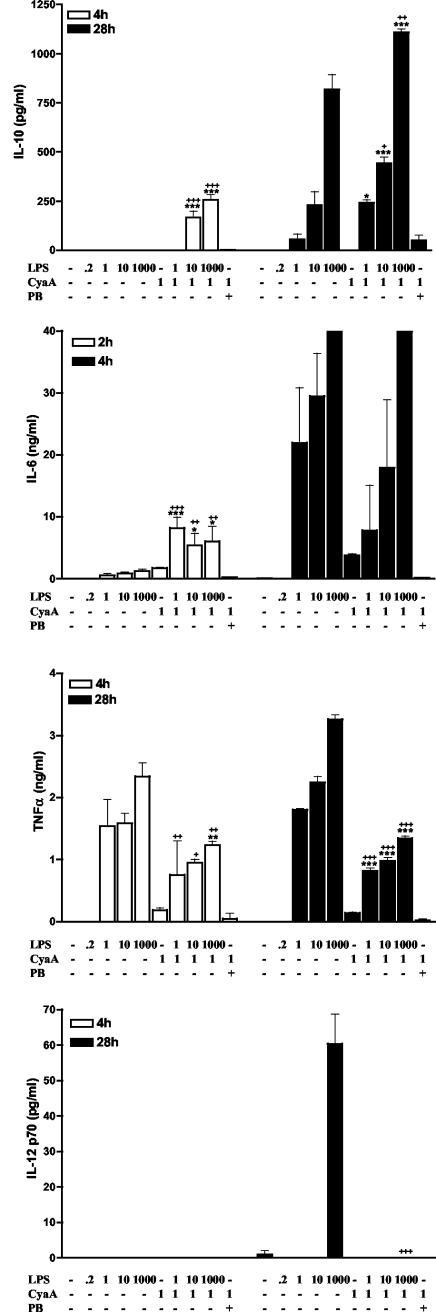
FIG. 5.
CyaA enhances LPS-induced anti-inflammatory cytokines and suppresses LPS-induced proinflammatory cytokines from DC. Murine bone marrow-derived immature DC (106/ml) were incubated with the indicated concentrations of LPS (0 to 1,000 ng/ml), CyaA (1 μg/ml) in the presence or absence of polymyxin B (PB) (10 μg/ml). Supernatants were collected at the indicated times and tested for IL-10, IL-6, TNF-α, and IL-12p70 by immunoassay. Results are means (error bars, SD) of triplicate assays and are representative of three experiments. Symbols: *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus CyaA; +, P < 0.05; ++, P < 0.01; +++, P < 0.01 versus LPS alone at the same concentration.
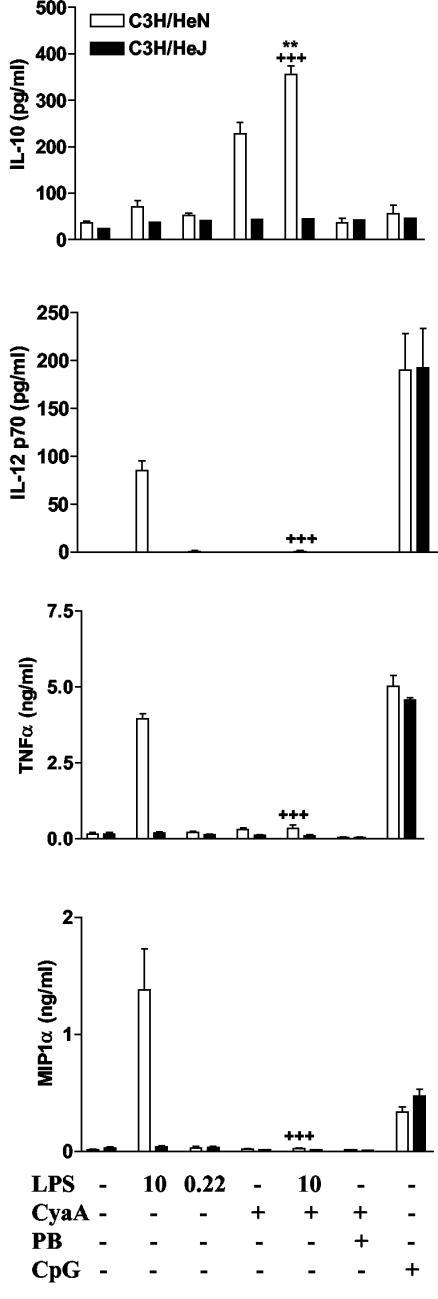
FIG. 7.
CyaA-induced cytokine and chemokine production is altered in TLR4-defective mice. Bone marrow derived DC from C3H/HeN or C3H/HeJ mice (106/ml) were cultured with the indicated concentrations of LPS (0 to 10 ng/ml), CyaA (1 μg/ml), in the presence or absence of polymyxin B (PB) (10 μg/ml). Supernatants were tested by immunoassay for IL-10 and MIP-1α (4 h) and IL-12p70 and TNF-α (24 h). Results are means (error bars, SD) of triplicate assays and are representative of three experiments. Symbols: **, P < 0.01 versus CyaA; +++, P < 0.001 versus LPS alone at the same concentration.
CyaA biochemical properties.
The CyaA preparation was analyzed biochemically to ensure that both its enzymatic and membrane translocation properties were active. In vitro assays of the adenylate cyclase enzyme function showed that the protein was enzymatically active (mean ± standard deviation [SD], 26.6 ± 0.8 μmol of cAMP/min/mg); this activity is at the lower end of the range reported for other preparations. CyaA at a concentration of 1 μg/ml was able to increase the intracellular concentration of cAMP 80-fold in J774 macrophages (mean ± SD, 72.8 ± 2.9 versus 0.9 ± 0.1 pmol of cAMP/106 cells), showing that the protein has the ability to target and enter eukaryotic cells. The maximal cAMP concentration was reached within 30 min and was maintained for at least 24 h. This concentration of CyaA induced less than 1% cell lysis after 24 h, as measured by LDH release; however, lysis (up to 10%) was observed with CyaA at concentrations of 5 to 10 μg/ml (data not shown). Recombinant CyaA preparations have been shown to be less lytic than B. pertussis derived CyaA (51). To assess its immunomodulatory function CyaA was used at 1 μg/ml, a concentration that induces a large increase in intracellular cAMP, without affecting cell viability.
CyaA generates Th2 and Tr1 cells to coinjected antigen.
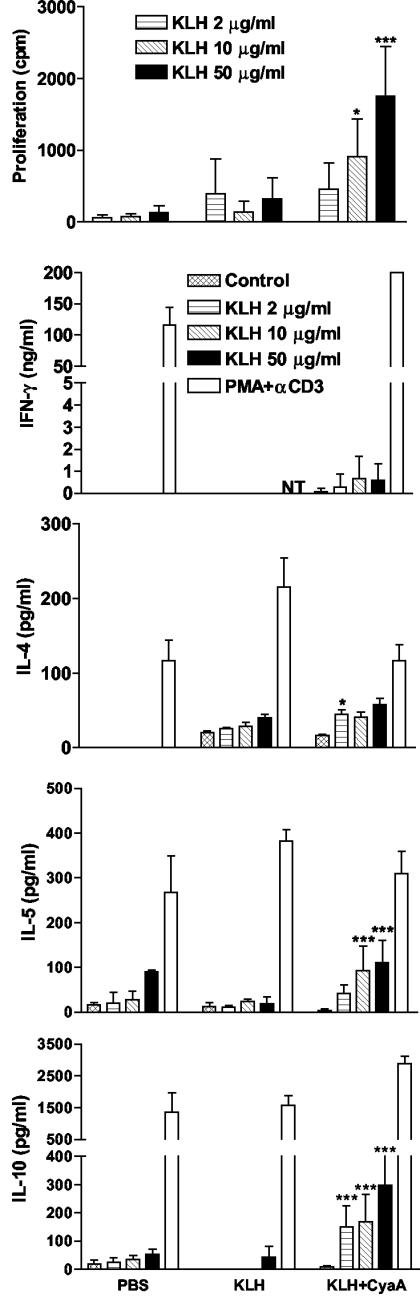
To examine the adjuvant properties of CyaA, mice were immunized s.c. in the hind footpad with KLH (5 μg) alone or with CyaA (1 μg). Seven days after immunization, mice were sacrificed and lymph node cells were restimulated with antigen (KLH at 2 to 50 μg/ml) in vitro. Cytokine concentrations were determined in supernatants removed after 3 days and proliferation was assessed after 4 days. Immunization with KLH alone induced weak cellular immune response; proliferation and IL-4 production was slightly enhanced over that observed in mice immunized with PBS (Fig. 1). The poor immunogenicity of KLH alone probably reflects its high purity; KLH purchased from Sigma, which is contaminated with LPS, generates stronger responses (data not shown), underscoring the role of innate immune activation in the immunogenicity of protein antigens. In contrast, significant antigen-specific proliferation was observed in cells from the restimulated lymph node cells from mice immunized with KLH and CyaA. Furthermore, significantly higher concentrations of KLH-specific IL-10 and IL-5 were detected in lymph node cells from mice immunized with CyaA and KLH compared to those from mice immunized with KLH alone. IL-4 and IFN-γ were also enhanced, but the difference between mice that received KLH alone and KLH and CyaA was, in most cases, not significant. Substantially higher levels of IFN-γ were secreted by lymph nodes cells from mice immunized with KLH and CyaA when polyclonally stimulated with PMA and anti-CD3 (Fig. 1). In contrast, spleen or lymph node cells from mice immunized with KLH in the presence of CpG-ODN secrete IFN-γ at a concentration of 50 to 150 ng/ml in response to restimulation with KLH in vitro (data not shown), which is at least 100-fold higher than that observed with KLH and CyaA (Fig. 1). Similar patterns of cytokine secretion was observed 7 days after a booster immunization (data not shown).
FIG. 1.
CyaA induces Th2/Tr1 responses to a coadministered antigen. BALB/c mice were immunized s.c. in the hind footpad with PBS, KLH (5 μg) alone, or KLH with CyaA (1 μg). After 7 days mice were sacrificed and popliteal lymph node cells were prepared and stimulated with KLH (2 to 50 μg/ml), PMA, and anti-CD3 or medium only. After 3 days supernatants were tested for IFN-γ, IL-4, IL-5, and IL-10 by ELISA. Proliferation was assayed on day 4 by [3H]thymidine incorporation. Results represent means (error bars, SD) of five mice per group and are representative of three experiments. NT, not tested. Symbols: *, P < 0.05; ***, P < 0.001 (KLH versus KLH plus CyaA).
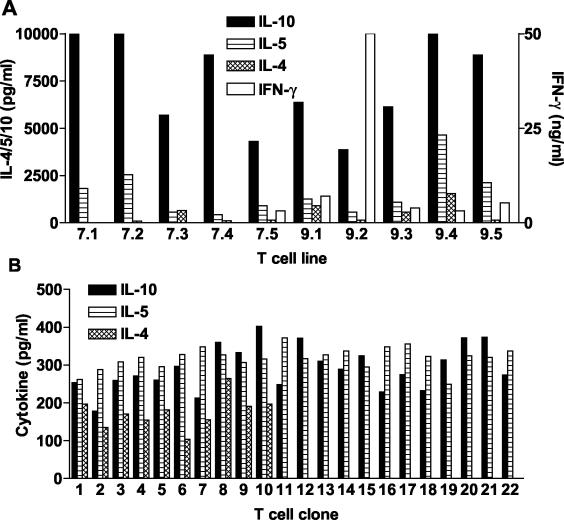
The cytokine profile of antigen-stimulated lymph node cells suggested that CyaA enhanced Th2 and/or Tr1 cells to coadministered antigens. In order to confirm this finding, we generated KLH-specific CD4+-T-cell lines and clones from mice immunized with KLH in the presence of CyaA. Each of the T-cell lines examined secreted high levels of IL-10 and lower levels of IL-5, and a smaller number also secreted IL-4 (Fig. 2A). IFN-γ production was detectable in 6 of 10 T-cell lines examined and at high levels in only one of these T-cell lines (Fig. 2A). In contrast, the majority of T-cell lines generated from mice immunized with KLH in the presence of CpG secrete IFN-γ in the concentration range 50 to 200 ng/ml, with very low or undetectable IL-4 and IL-5 (31; unpublished data). Four of the T-cell lines generated from mice immunized with KLH and CyaA (two that secrete IFN-γ and two that did not secrete this cytokine) were cloned and cytokine production by T-cell clones from one T-cell line is shown in Fig. 2B. These KLH-specific T-cell clones secreted IL-5 and IL-10, or IL-4, IL-5, and IL-10 but secreted undetectable IFN-γ—profiles characteristic of Tr1 and Th2 cells, respectively. A dominance of Th2 or Tr1-type cytokines was detected from the T-cell clones generated from the other T-cell lines examined (data not shown). These findings demonstrate that CyaA promotes the induction of Th2 and Tr1-type cells specific for the coadministered antigen, and that Th1-type T cells may also be generated, but at lower frequency.
FIG. 2.
KLH-specific Tr1- and Th2-cell lines and clones generated from mice immunized with KLH in the presence of CyaA. (A) CD4+-T-cell lines were generated from lymph nodes of 10 individual mice immunized with KLH and CyaA. (B) T-cell line 7.2 was cloned by limiting dilution. T-cell lines or clones were stimulated with KLH (50 μg/ml) in the presence of autologous APC and cytokine concentrations tested in the supernatants after 3 days. Results are cytokine profiles for lines or clones cultured in individual wells of 96- or 24-well plates and are representative of three different assays. IFN-γ was at background levels in each of the T-cell clones.
CyaA enhances IgG1 responses to coadministered antigen.
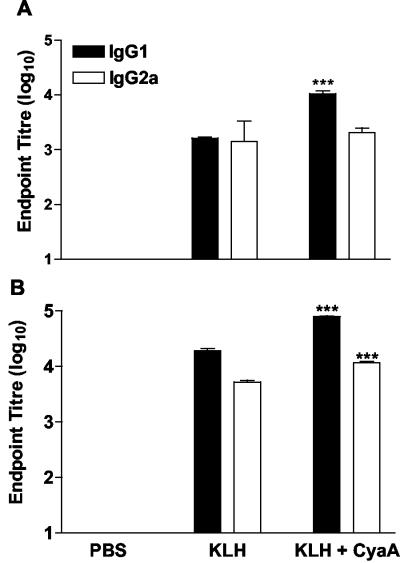
We examined the adjuvant effect of CyaA for antibody responses to coinjected antigen by assessing KLH-specific IgG and IgG subclasses in mice immunized with KLH alone or with CyaA. Significantly higher levels of KLH-specific IgG1 were found in the serum of mice immunized with KLH and CyaA compared with mice that received antigen alone. In contrast, CyaA did not enhance IgG2a levels above those observed in mice immunized with KLH alone (Fig. 3A). Following a second immunization the serum IgG titers were increased over those observed after a single immunization and the responses to KLH in the presence of CyaA were significantly greater than those in mice immunized with KLH alone (Fig. 3B). Comparable with the data after a single immunization, IgG1 was the dominant subclass of the antibody response. These data clearly demonstrate that CyaA acts an adjuvant for antibody, as well as T-cell responses in vivo.
FIG. 3.
CyaA enhances IgG1 antibodies to coadministered antigens in vivo. Mice were immunized s.c. in the hind footpad with PBS, KLH (5 μg) alone or with CyaA (1 μg) and boosted 21 days later. Serum samples taken 7 days after one (A) or two (B) immunizations and KLH-specific IgG, IgG1, and IgG2a determined by ELISA. Results are mean titers (error bars, SD) for five mice per group and are representative of two experiments. ***, P < 0.001 KLH versus KLH plus CyaA.
CyaA modulates cytokine production from innate cells.
Cells of the innate immune system, including DC and macrophages, direct the adaptive immune response by presenting antigens and secreting regulatory cytokines. To investigate the effect of CyaA on these cells, J774 macrophages and immature bone marrow-derived DC were incubated with CyaA (1 μg/ml), E. coli LPS (1 to 1,000 ng/ml), or CyaA and LPS. Although LPS from B. pertussis differs in structure, we found that it activates innate immune cells through TLR4 in an identical fashion to that observed for E. coli LPS (25 and unpublished data). Since the CyaA protein is associated with LPS, which was reduced but not completely eliminated during purification, it was important to determine the role, if any, of this LPS in the immunomodulatory effects of CyaA. Therefore, cells were also stimulated with CyaA in the presence of polymyxin B. Supernatants were collected 2, 4, and 28 h after stimulation and assayed for cytokines. The purified CyaA, which included residual LPS (220 pg/ml), stimulated low levels of IL-6, IL-10, and TNF-α production from J774 cells (Fig. 4) and low levels of IL-6 and TNF-α (but no IL-10) secretion from DC (Fig. 5). This cytokine production was completely inhibited in the presence of polymyxin B. However, stimulation with LPS alone at the dose present in the CyaA preparation (220 pg/ml) did not induce production of these cytokines (Fig. 4 and 5). Furthermore, polymyxin B alone did not affect cytokine production by macrophages or DC. These data suggest that CyaA activates innate cells only in the presence of LPS. We also examined the effect of CyaA on cytokine production in response to increasing doses of LPS. CyaA synergized with LPS in promoting IL-6 and IL-10 production from macrophages and DC. IL-10 production from macrophages stimulated with CyaA and LPS (1 to 1,000 ng/ml) was significantly higher than that of macrophages stimulated with the corresponding dose of LPS alone at all time points examined (Fig. 4). IL-10 could not be detected in DC supernatants 4 h after stimulation with LPS (1 to 1,000 ng/ml) alone, whereas significant levels of IL-10 were produced following addition of CyaA (Fig. 5). LPS-induced IL-6 production by macrophages and DC was also significantly enhanced by the addition of CyaA, but this was only observed at early time points. In contrast to the positive effect on IL-6 and IL-10 production, CyaA suppressed TNF-α secretion from macrophages and DC and IL-12p70 production from DC. These inhibitory effects were observed at the three time points examined and over a range of doses of LPS. Furthermore, CyaA enhanced IL-10 production by DC stimulated with oligodeoxynucleotide-containing CpG motifs (data not shown). These data demonstrate that CyaA alone has little enhancing effect on cytokine production by cells of the innate immune system, but can synergize with a TLR ligand, even at very low concentrations, in promoting IL-10 production, but also inhibiting TNF-α and IL-12 production.
Effect of CyaA on maturation of DC.
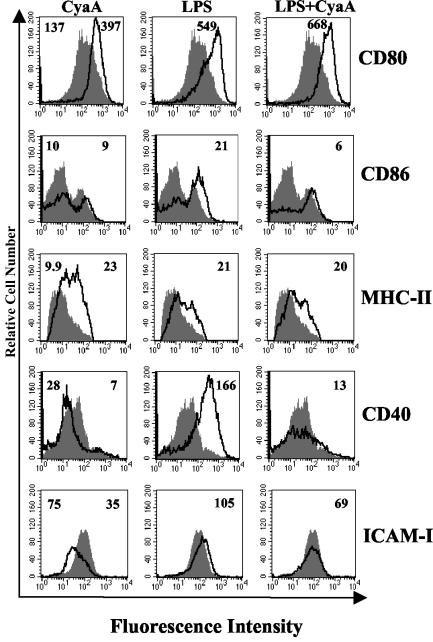
Several pathogen-derived molecules that bind to TLR, induce maturation of immature DC (1, 8, 23), thereby enhancing their capacity to activate naive T cells. Therefore, we examined the ability of CyaA to stimulate the maturation of DC and/or to modulate LPS-induced maturation. Immature DC were stimulated with CyaA, LPS, or LPS with CyaA, and the expression of surface markers associated with maturation was examined by immunofluorescence analysis 24 h later. As expected, LPS (1 μg/ml), enhanced surface expression of CD80, CD86, and MHC-II, CD40, and ICAM-1 (Fig. 6). Stimulation of immature DC with CyaA (in the presence of polymyxin B) also resulted in upregulation of surface expression of CD80 and MHC-II, and to a lesser extent CD86. In contrast, expression of CD40 and ICAM-1 was downregulated following incubation with CyaA. Furthermore, CyaA inhibited LPS-induced upregulation of CD40, ICAM-1, and CD86. In contrast, treatment of cells with LPS at the level present in the CyaA preparation (220 pg/ml) had no effect on DC surface marker expression (data not shown). These findings demonstrate that CyaA treatment results in partial maturation of the DC, upregulating CD80 and MHC-II, but inhibiting CD40 and ICAM-1, a phenotype distinct from mature DC that drive the differentiation of Th1 cells, but similar to those that promote the induction of Tr1 cells (34, 37).
FIG. 6.
CyaA enhances CD80, CD86, and MHC-II but inhibits CD40 and ICAM-1 expression on DC. DC were stimulated with CyaA (1 μg/ml) in the presence of polymyxin B (10 μg/ml), LPS (1 μg/ml), CyaA and LPS, or medium only. After 24 h of incubation, cells were washed and stained with antibodies specific for CD80, CD86, MHC-II, CD40, and ICAM-1 or with isotype matched controls. Results of immunofluorescence analysis are shown for treated (black line) compared to untreated (grey histogram) DC. The numbers on the right of each histogram refer to the mean fluorescence intensity of the treated cells for the relevant fluorescent antibody, the value for cells treated with medium only is shown on the left of the first histogram in each case. Profiles are shown for a single experiment and are representative of four experiments.
Effect of CyaA on DC from TLR4-defective mice.
To further address the role of LPS in the mechanism of action of CyaA as an adjuvant and immunomodulator, we examined innate cytokine and chemokine production and maturation of DC from TLR4-defective mice. DC from TLR4-defective C3H/HeJ and immunocompetent C3H/HeN mice were treated with CyaA, LPS, LPS plus CyaA, or CyaA and polymyxin B, and supernatants were recovered after 4 h. CyaA (that included residual LPS [220 pg/ml]) stimulated IL-10 production by DC from C3H/HeN, but not from C3H/HeJ mice (Fig. 7). Furthermore, IL-10 production by DC from C3H/HeN mice was inhibited by polymyxin B. However, LPS alone at the concentration present in the CyaA preparation (220 pg/μg) did not induce IL-10, IL-12p70, TNF-α, or MIP-1α production by DC from C3H/HeN mice. Addition of a higher dose of exogenous LPS (10 ng/ml) did not induce significant IL-10 at the 4-h time point but synergized with CyaA in promoting IL-10 production. Furthermore, CyaA suppressed LPS induced IL-12p70, TNF-α and MIP-1α production by DC from C3H/HeN mice (Fig. 7), but had no effect on cytokine production by DC from C3H/HeJ mice (Fig. 7). In contrast, CpG, a TLR9 ligand, activated cytokine production by C3H/HeN and C3H/HeJ DC in a similar fashion (Fig. 7). These data provide further evidence that CyaA, although having no direct effect on cytokine production by DC, modulates LPS-induced responses, synergizing with LPS to induce IL-10 and suppressing LPS induced production of IL-12p70, TNF-α, and MIP-1α.
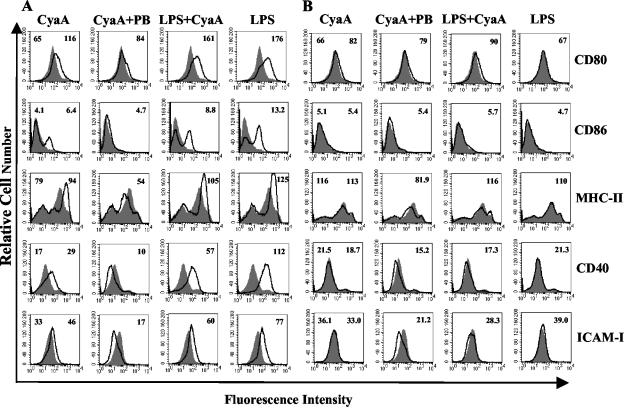
We also examined the effects of CyaA on maturation of DC from C3H/HeN and C3H/HeJ mice. As shown for BALB/c mice, CyaA induced maturation of DC from C3H/HeN mice, specifically CyaA enhanced expression of CD80, CD86, MHC-II, CD40, and ICAM-1 (Fig. 8A). In the presence of polymyxin B these effects were diminished, in particular CD40, ICAM-1, and MHC-II, which were expressed at lower levels than those seen on medium-treated control DC. LPS-induced expression of CD86, CD40, and ICAM-1 was also inhibited by CyaA, though not to the same extent as that observed in DC from BALB/c mice. In contrast to the modulatory effects in C3H/HeN mice, LPS, CyaA, or LPS with CyaA had no enhancing effects on CD86, MHC-II, CD40, or ICAM-1 and did have a modest, but reproducible, effect on CD80 on DC from C3H/HeJ mice (Fig. 8B). However CyaA marginally reduced CD40 and ICAM-1 expression on DC from C3H/HeJ mice in the presence of polymyxin B. These findings suggest that upregulation of maturation markers on DC by CyaA is dependent on a second signal, such as LPS even at very low doses, but that inhibition of endogenous expression of CD40 and ICAM-1 can occur in the absence of LPS.
FIG. 8.
CyaA-induced DC activation is altered in TLR4-defective mice. Bone marrow derived DC from C3H/HeN (A) or C3H/HeJ (B) mice (106/ml) were cultured with CyaA (1 μg/ml) either alone or with polymyxin B (PB) (10 μg/ml) or LPS (10 ng/ml) or with medium. After 24 h of incubation, cells were washed and stained with antibodies specific for CD80, CD86, MHC-II, CD40, and ICAM-1 or with isotype matched control antibodies. Immunofluorescence analyses are shown for treated (black line) compared to untreated (grey histogram) DC. The numbers on the right of each histogram refer to the mean fluorescence intensity of the treated cells for the relevant fluorescent antibody, the value for cells treated with medium only is shown on the left of the first histogram in each case. Profiles are shown for a single experiment and are representative of three experiments.
DISCUSSION
This study demonstrates that CyaA can selectively promote the induction of T-cell responses to coadministered antigens in vivo through its modulatory effect on DC, but that the effects on DC were facilitated by on a second signal provided by LPS signaling through TLR4. It was previously reported that CyaA could enhance antibody responses to coinjected antigen (26) and when used as a vector for viral peptide antigens it resulted in the stimulation of CD8+ cytotoxic T lymphocytes and Th1 responses against the viral peptide (7, 11). We found that CyaA enhanced IgG1 antibodies and IL-5 and IL-10 production by antigen-stimulated lymph node cells, but had very little effect on IFN-γ production. Furthermore, antigen-specific CD4+ T-cell clones generated from mice immunized with KLH in the presence of CyaA secreted IL-5 and IL-10 or IL-4, IL-5, and IL-10, suggesting that it selectively promoted the generation of Th1 and Th2 cells in vivo.
A number of bacterial toxins and virulence factors have been shown to modulate immune responses as a strategy to evade protective immunity and prolong their survival in the host (37). Moreover, certain of these molecules, including CT, E. coli heat labile enterotoxin and LPS can also act as adjuvants enhancing immune responses to coadministered antigens (47). The adjuvant properties of CyaA may be related to its ability to bind to CD11b/CD18 on innate cells (18, 19). CyaA binding to CD11b/CD18, expressed on DC, macrophages and other innate cells, has been exploited as a means of targeting foreign peptide antigens to MHC-I and -II processing pathways (10, 33). FHA from B. pertussis also binds to CD11b/CD18 (as well as to CD47/CD61) and it has been demonstrated that FHA can stimulate IL-10 production from DC and macrophages and activate maturation of DC into a phenotype that promotes the differentiation of naive T cells into Tr1 cells (34). Furthermore CT has been shown to promote the activation of Tr1 cells, as well as Th2 cells, by modulating DC activation (31).
The induction of antigen-specific Tr cells by pathogens or pathogen-derived molecules and their role in infectious diseases is only beginning to be understood. It appears that these cells are generated during chronic or persistent infection, either as an evasion strategy by the pathogen to inhibit protective immune responses or as a host protective mechanism to limit collateral damage mediated by inflammatory Th1 or Th2 cells (25, 35). Tr cells also have an important role in preventing autoimmune diseases and allergy mediated by Th1 cells and Th2 cells, respectively, therefore their induction in vivo may be a useful therapeutic strategy for immune mediated diseases. Current evidence suggests that IL-10, and possibly IL-4 and IFN-α, may play a role in promoting the induction of Tr1 cells from naive T cells in vivo (35, 36). In contrast, innate IL-12 and IL-27 enhance Th1 responses, while IL-4 and IL-6 have been implicated in directing the induction of Th2 cells (39, 44, 45). It has also been suggested that IL-6 induced by microbial products through the TLR pathway may inhibit DC activation of CD4+ CD25+ natural Tr cells (41). The results of the present study support a role for macrophage- and DC-derived IL-10 and IL-6 directing the induction of Tr1 and Th2 cells. CyaA, in synergy with low doses of LPS, enhances IL-6 and IL-10 production from DC, but inhibits LPS-induced IL-12 and TNF-α production.
In addition to regulatory cytokine secretion, the maturation status of the DC also influences T-cell subtype induction. Many pathogen-derived molecules that bind to TLR promote the maturation of DC that direct the induction of Th1 cells. Emerging evidence indicates that DC which promote activation of Tr cells are not immature or fully mature but have an intermediate phenotype (31, 35). We found that CyaA enhanced CD80, but reduced CD40 and ICAM-1 expression on murine DC when LPS effects were abolished from our CyaA preparation with polymyxin B. It had previously been reported that commercially available E. coli-expressed CyaA enhances CD80, CD86, CD83, and MHC-II on human DC (2). We failed to detect significant enhancement of CD86 on murine DC and in fact LPS-induced CD86 was suppressed by incubation with CyaA, as was LPS-induced CD40 and ICAM-1.
Studies on immunomodulation with purified native or recombinant pathogen-derived molecules are complicated by the possible contamination with low levels of other molecules, which may also have immunomodulatory activities. This is a problem with molecules expressed in E. coli or purified from Gram-negative bacteria, since LPS is often difficult to remove or dissociate from protein molecules, especially RTX toxins (6, 32). The primary objective of our studies was to examine the immunomodulatory effects of CyaA and to try to devolve these effects from that generated with the protein in the presence of LPS. Since the immunotherapeutic applications of CyaA are more likely to employ recombinant molecules, we choose to work with a recombinant E. coli expressed protein. However, during B. pertussis infection host cells will respond to CyaA in the context of high concentrations of LPS. Furthermore, CyaA, in addition to being secreted by B. pertussis, can be delivered to the host epithelial cells upon contact with B. pertussis. Although it has been considered that B. pertussis cells are primarily localized at the epithelial surface, there is also evidence that they can infect human macrophages (37). Furthermore, immature myeloid DC, which express CD11b and other pathogen recognition receptors, are present in respiratory airways (unpublished observations) and will be activated by different B. pertussis virulence factors, including CyaA and LPS, before migrating to the lymph node, where they direct the induction of naive T cells. Indeed we have detected B. pertussis-specific Tr1 and Th1 cells in lymph nodes and spleen from mice infected with B. pertussis (25, 34). We have also detected circulating IFN-γ-secreting Th1 cells in children infected with B. pertussis (49).
The assumption that low levels of contaminating LPS do not contribute to immunological activity of a protein because the same dose of LPS alone has no demonstrable effect or that denaturing the protein inhibits the activity may not take into account possible cooperative or synergistic effects. Our findings demonstrate that CyaA does have adjuvant and immunomodulatory properties, but that this effect may be partly dependent on additional signaling induced by LPS through TLR4. Purified CyaA with low levels of contaminating LPS (220 pg/μg of protein) enhanced certain aspects of DC maturation, including upregulation of CD80 and MHC-II expression and stimulation of low levels of IL-6, IL-10, and TNF-α production from DC and macrophages. Many of the activities of the purified CyaA preparation, including induction of TNF-α, IL-10, and IL-6 production and enhancement of CD80 and MHC-II expression on DC were inhibited following the addition of polymyxin B or through use of TLR4-defective mice. However, LPS at a concentration equivalent to that present in our CyaA preparation (220 pg/μg CyaA protein) did not itself induce innate cytokine production or DC maturation, but did significantly augment the effect of the CyaA. Furthermore, addition of increasing amounts of exogenous LPS (1 to 1,000 ng/ml) significantly augmented IL-10 and IL-6 production by DC and macrophages. Synergistic effects between CyaA and LPS were particularly evident at early time points after cell stimulation, when the same dose of LPS alone had little effect.
The concept that two signals are required for immune activation is fully recognized for T cells, but there is growing evidence for this phenomenon for cells of the innate immune system. PT has been shown to synergize with LPS to promote IL-12 production from DC (8). Furthermore, CT synergizes with LPS, or other TLR ligands, including CpG motifs or poly(I:C), to stimulate IL-10 production from DC and macrophages (31). Elevation of intracellular cAMP and activation of protein kinase A have been implicated in the immunomodulatory effects of CT and of CyaA (2; unpublished observations), suggesting that cAMP-dependent signaling pathways are involved in innate cell activation by CyaA. However, the present study, combined with our previous reports on synergy between CT and different TLR ligands (30) and the demonstration that innate IL-10 production and induction of Tr1 cells following B. pertussis infection is compromised in TLR4-defective mice (25), suggest that signaling pathways leading to DC maturation and IL-10 and IL-6 production following CyaA recognition of CD11b/CD18 may be dependent on cooperative activation through a TLR.
Acknowledgments
This material is based upon works supported by the Science Foundation Ireland under grant 00/PI.I/B045. Pádraig J. Ross also receives support from Enterprise Ireland.
We thank Erik Hewlett, University of Virginia School of Medicine, for the kind gift of the anti-CyaA antibody.
Editor: D. L. Burns
REFERENCES
- 1.Akira, S. 2003. Toll-like receptor signaling. J. Biol. Chem. 278:38105-38108. [DOI] [PubMed] [Google Scholar]
- 2.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, T. R. Fouts, and G. K. Lewis. 2002. Pertussis toxin and the adenylate cyclase toxin from Bordetella pertussis activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cAMP-dependent pathway. J. Leukoc. Biol. 72:962-969. [PubMed] [Google Scholar]
- 3.Barry, E. M., A. A. Weiss, I. E. Ehrmann, M. C. Gray, E. L. Hewlett, and M. S. Goodwin. 1991. Bordetella pertussis adenylate cyclase toxin and hemolytic activities require a second gene, cyaC, for activation. J. Bacteriol. 173:720-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Confer, D. L., and J. W. Eaton. 1982. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science 217:948-950. [DOI] [PubMed] [Google Scholar]
- 5.Coote, J. G. 1992. Structural and functional relationships among the RTX toxin determinants of gram-negative bacteria. FEMS Microbiol. Rev. 8:137-161. [DOI] [PubMed] [Google Scholar]
- 6.Czuprynski, C. J., and R. A. Welch. 1995. Biological effects of RTX toxins: the possible role of lipopolysaccharide. Trends Microbiol. 3:480-483. [DOI] [PubMed] [Google Scholar]
- 7.Dadaglio, G., Z. Moukrim, R. Lo-Man, V. Sheshko, P. Sebo, and C. Leclerc. 2000. Induction of a polarized Th1 response by insertion of multiple copies of a viral T-cell epitope into adenylate cyclase of Bordetella pertussis. Infect. Immun. 68:3867-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong, E. C., P. L. Vieira, P. Kalinski, J. H. Schuitemaker, Y. Tanaka, E. A. Wierenga, M. Yazdanbakhsh, and M. L. Kapsenberg. 2002. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J. Immunol. 168:1704-1709. [DOI] [PubMed] [Google Scholar]
- 9.d'Ostiani, C. F., G. Del Sero, A. Bacci, C. Montagnoli, A. Spreca, A. Mencacci, P. Ricciardi-Castagnoli, and L. Romani. 2000. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191:1661-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fayolle, C., D. Ladant, G. Karimova, A. Ullmann, and C. Leclerc. 1999. Therapy of murine tumors with recombinant Bordetella pertussis adenylate cyclase carrying a cytotoxic T cell epitope. J. Immunol. 162:4157-4162. [PubMed] [Google Scholar]
- 11.Fayolle, C., P. Sebo, D. Ladant, A. Ullmann, and C. Leclerc. 1996. In vivo induction of CTL responses by recombinant adenylate cyclase of Bordetella pertussis carrying viral CD8+ T cell epitopes. J. Immunol. 156:4697-4706. [PubMed] [Google Scholar]
- 12.Friedman, R. L., R. L. Fiederlein, L. Glasser, and J. N. Galgiani. 1987. Bordetella pertussis adenylate cyclase: effects of affinity-purified adenylate cyclase on human polymorphonuclear leukocyte functions. Infect. Immun. 55:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagliardi, M. C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M. T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur. J. Immunol. 30:2394-2403. [DOI] [PubMed] [Google Scholar]
- 14.Gentile, F., L. G. Knipling, D. L. Sackett, and J. Wolff. 1990. Invasive adenylyl cyclase of Bordetella pertussis. Physical, catalytic, and toxic properties. J. Biol. Chem. 265:10686-10692. [PubMed] [Google Scholar]
- 15.Goodwin, M. S., and A. A. Weiss. 1990. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect. Immun. 58:3445-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross, M. K., D. C. Au, A. L. Smith, and D. R. Storm. 1992. Targeted mutations that ablate either the adenylate cyclase or hemolysin function of the bifunctional cyaA toxin of Bordetella pertussis abolish virulence. Proc. Natl. Acad. Sci. USA 89:4898-4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gueirard, P., A. Druilhe, M. Pretolani, and N. Guiso. 1998. Role of adenylate cyclase-hemolysin in alveolar macrophage apoptosis during Bordetella pertussis infection in vivo. Infect. Immun. 66:1718-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guermonprez, P., C. Fayolle, M. J. Rojas, M. Rescigno, D. Ladant, and C. Leclerc. 2002. In vivo receptor-mediated delivery of a recombinant invasive bacterial toxoid to CD11c + CD8 alpha-CD11bhigh dendritic cells. Eur. J. Immunol. 32:3071-3081. [DOI] [PubMed] [Google Scholar]
- 19.Guermonprez, P., N. Khelef, E. Blouin, P. Rieu, P. Ricciardi-Castagnoli, N. Guiso, D. Ladant, and C. Leclerc. 2001. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18). J. Exp. Med. 193:1035-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hackett, M., L. Guo, J. Shabanowitz, D. F. Hunt, and E. L. Hewlett. 1994. Internal lysine palmitoylation in adenylate cyclase toxin from Bordetella pertussis. Science 266:433-435. [DOI] [PubMed] [Google Scholar]
- 21.Hackett, M., C. B. Walker, L. Guo, M. C. Gray, S. Van Cuyk, A. Ullmann, J. Shabanowitz, D. F. Hunt, E. L. Hewlett, and P. Sebo. 1995. Hemolytic, but not cell-invasive activity, of adenylate cyclase toxin is selectively affected by differential fatty-acylation in Escherichia coli. J. Biol. Chem. 270:20250-20253. [DOI] [PubMed] [Google Scholar]
- 22.Harvill, E. T., P. A. Cotter, M. H. Yuk, and J. F. Miller. 1999. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect. Immun. 67:1493-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 24.Hewlett, E. L., M. C. Gray, I. E. Ehrmann, N. J. Maloney, A. S. Otero, L. Gray, M. Allietta, G. Szabo, A. A. Weiss, and E. M. Barry. 1993. Characterization of adenylate cyclase toxin from a mutant of Bordetella pertussis defective in the activator gene, cyaC. J. Biol. Chem. 268:7842-7848. [PubMed] [Google Scholar]
- 25.Higgins, S. C., E. Lavelle, C. McCann, B. Keogh, E. McNeela, P. Byrne, B. O'Gorman, A. Jarnicki, P. McGuirk, and K. H. G. Mills. 2003. Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J. Immunol. 17:3119-3127. [DOI] [PubMed] [Google Scholar]
- 26.Hormozi, K., R. Parton, and J. Coote. 1999. Adjuvant and protective properties of native and recombinant Bordetella pertussis adenylate cyclase toxin preparations in mice. FEMS. Immunol. Med. Microbiol. 23:273-282. [DOI] [PubMed] [Google Scholar]
- 27.Khelef, N., C. M. Bachelet, B. B. Vargaftig, and N. Guiso. 1994. Characterization of murine lung inflammation after infection with parental Bordetella pertussis and mutants deficient in adhesins or toxins. Infect. Immun. 62:2893-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khelef, N., P. Gounon, and N. Guiso. 2001. Internalization of Bordetella pertussis adenylate cyclase-haemolysin into endocytic vesicles contributes to macrophage cytotoxicity. Cell. Microbiol. 3:721-730. [DOI] [PubMed] [Google Scholar]
- 29.Khelef, N., A. Zychlinsky, and N. Guiso. 1993. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect. Immun. 61:4064-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ladant, D., C. Brezin, J. M. Alonso, I. Crenon, and N. Guiso. 1986. Bordetella pertussis adenylate cyclase. Purification, characterization, and radioimmunoassay. J. Biol. Chem. 261:16264-16269. [PubMed] [Google Scholar]
- 31.Lavelle, E., E. McNeela, M. E. Armstrong, O. Leavy, S. C. Higgins, and K. H. G. Mills. 2003. Cholera toxin promotes the induction of regulatory T cells as well as Th2 cells specific for bystander antigens by modulating dendritic cell activation. J. Immunol. 171:2384-2392. [DOI] [PubMed] [Google Scholar]
- 32.Li, J., and K. D. Clinkenbeard. 1999. Lipopolysaccharide complexes with Pasteurella haemolytica leukotoxin. Infect. Immun. 67:2920-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loucka, J., G. Schlecht, J. Vodolanova, C. Leclerc, and P. Sebo. 2002. Delivery of a MalE CD4+-T-cell epitope into the major histocompatibility complex class II antigen presentation pathway by Bordetella pertussis adenylate cyclase. Infect. Immun. 70:1002-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGuirk, P., C. McCann, and K. H. Mills. 2002. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 195:221-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGuirk, P., and K. H. Mills. 2002. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 23:450-455. [DOI] [PubMed] [Google Scholar]
- 36.Mendel, I., and E. M. Shevach. 2002. The IL-10-producing competence of Th2 cells generated in vitro is IL-4 dependent. Eur. J. Immunol. 32:3216-3224. [DOI] [PubMed] [Google Scholar]
- 37.Mills, K. H. G. 2001. Immunity to Bordetella pertussis. Microbes Infect. 3:655-677. [DOI] [PubMed] [Google Scholar]
- 38.Mills, K. H. G. 1999. Murine T cell culture, p. 95-134. In S. L. Rowland-Jones and A. McMichael (ed.), Lymphocytes: a practical approach. IRL Press, Oxford, United Kingdom.
- 39.Moser, M., and K. M. Murphy. 2000. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1:199-205. [DOI] [PubMed] [Google Scholar]
- 40.Njamkepo, E., F. Pinot, D. Francois, N. Guiso, B. S. Polla, and M. Bachelet. 2000. Adaptive responses of human monocytes infected by Bordetella pertussis: the role of adenylate cyclase hemolysin. J. Cell Physiol. 183:91-99. [DOI] [PubMed] [Google Scholar]
- 41.Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 299:1033-1036. [DOI] [PubMed] [Google Scholar]
- 42.Pearson, R. D., P. Symes, M. Conboy, A. A. Weiss, and E. L. Hewlett. 1987. Inhibition of monocyte oxidative responses by Bordetella pertussis adenylate cyclase toxin. J. Immunol. 139:2749-2754. [PubMed] [Google Scholar]
- 43.Petsch, D., and F. B. Anspach. 2000. Endotoxin removal from protein solutions. J. Biotechnol. 76:97-119. [DOI] [PubMed] [Google Scholar]
- 44.Rincon, M., J. Anguita, T. Nakamura, E. Fikrig, and R. A. Flavell. 1997. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J. Exp. Med. 185:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson, D. S., and A. O'Garra. 2002. Further checkpoints in Th1 development. Immunity 16:755-758. [DOI] [PubMed] [Google Scholar]
- 46.Rose, T., P. Sebo, J. Bellalou, and D. Ladant. 1995. Interaction of calcium with Bordetella pertussis adenylate cyclase toxin. Characterization of multiple calcium-binding sites and calcium-induced conformational changes. J. Biol. Chem. 270:26370-26376. [DOI] [PubMed] [Google Scholar]
- 47.Ryan, E. J., L. M. Daly, and K. H. Mills. 2001. Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol. 19:293-304. [DOI] [PubMed] [Google Scholar]
- 48.Ryan, E. J., E. McNeela, M. Pizza, R. Rappuoli, L. O'Neill, and K. H. Mills. 2000. Modulation of innate and acquired immune responses by Escherichia coli heat-labile toxin: distinct pro- and anti-inflammatory effects of the nontoxic AB complex and the enzyme activity. J. Immunol. 165:5750-5759. [DOI] [PubMed] [Google Scholar]
- 49.Ryan, M., G. Murphy, L. Gothefors, L. Nilsson, J. Storsaeter, and K. H. Mills. 1997. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J. Infect. Dis. 175:1246-1250. [DOI] [PubMed] [Google Scholar]
- 50.Sakamoto, H., J. Bellalou, P. Sebo, and D. Ladant. 1992. Bordetella pertussis adenylate cyclase toxin. Structural and functional independence of the catalytic and hemolytic activities. J. Biol. Chem. 267:13598-13602. [PubMed] [Google Scholar]
- 51.Sebo, P., P. Glaser, H. Sakamoto, and A. Ullmann. 1991. High-level synthesis of active adenylate cyclase toxin of Bordetella pertussis in a reconstructed Escherichia coli system. Gene 104:19-24. [DOI] [PubMed] [Google Scholar]
- 52.Weiss, A. A., E. L. Hewlett, G. A. Myers, and S. Falkow. 1984. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J. Infect. Dis. 150:219-222. [DOI] [PubMed] [Google Scholar]
- 53.Westrop, G., K. Hormozi, N. da Costa, R. Parton, and J. Coote. 1997. Structure-function studies of the adenylate cyclase toxin of Bordetella pertussis and the leukotoxin of Pasteurella haemolytica by heterologous C protein activation and construction of hybrid proteins. J. Bacteriol. 179:871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whelan, M., M. M. Harnett, K. M. Houston, V. Patel, W. Harnett, and K. P. Rigley. 2000. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J. Immunol. 164:6453-6460. [DOI] [PubMed] [Google Scholar]
- 55.Wolff, J., G. H. Cook, A. R. Goldhammer, and S. A. Berkowitz. 1980. Calmodulin activates prokaryotic adenylate cyclase. Proc. Natl. Acad. Sci. USA 77:3841-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]