Abstract
Cannabis remains the most commonly abused illicit drug and is rapidly expanding in quasi-licit use in some jurisdictions under medical marijuana laws. Effects of the psychoactive constituent Δ9tetrahydrocannabinol (Δ9THC) on cognitive function remain of pressing concern. Prior studies in monkeys have not shown consistent evidence of memory specific effects of Δ9THC on recognition tasks and it remains unclear to what extent Δ9THC causes sedative versus specific cognitive effects. In this study, adult male rhesus monkeys were trained on tasks which assess spatial working memory, visuo-spatial associative memory and learning as well as motivation for food reward. Subjects were subsequently challenged with 0.1-0.3 mg/kg Δ9THC, i.m., in randomized order and evaluated on the behavioral measures. The performance of both vsPAL and SOSS tasks was impaired by Δ9THC in a dose and task-difficulty manner. It is concluded that Δ9THC disrupts cognition in a way that is consistent with a direct effect on memory. There was evidence for interference with spatial working memory, visuo-spatial associative memory and incremental learning in the latter task. These results and the lack of specific effect of Δ9THC in prior visual recognition studies imply a sensitivity of spatial memory processing and/or working memory to endocannabinoid perturbation.
Keywords: cannabis, marijuana, Macaca mulatta, memory
1. Introduction
Data from the Monitoring the Future project (Johnston et al. 2011a; Johnston et al. 2011b) show that cannabis remains the most commonly abused illicit drug in the United States with rates of use exceeded only by the legal substances alcohol and nicotine. High rates of exposure to cannabis mean that population prevalence for dependence on cannabis is the highest for any illicit drug (Anthony et al. 1994; Schramm-Sapyta et al. 2009). The longitudinal trends show that cannabis use in high school seniors peaked in the late 1970s, declined to a nadir around 1991-1992, rebounded through the late nineties, declined gradually and has been on the rise 2007-2010. The data also suggest that cannabis use is initiated starting as early as middle school and by the senior year of high school 35% of the population have used cannabis in the past year, including 20% in the past month (Johnston et al. 2011a). Prevalence estimates of cannabis use during young adulthood show about 16% using cannabis monthly and about 4-6% using daily. Exposure to cannabis is therefore quite common in the US and begins in the adolescent period in a large fraction of users. The peak of daily use rates in the young adult ages identifies a critical window overlapping with the period of formal education, which raises concern about the cognitive effects of cannabis use.
It has been established that humans who smoke marijuana or are administered the presumed most-active component of marijuana smoke, Δ9-tetrahydrocannabinol (Δ9THC) exhibit alterations in cognitive performance within multiple domains including memory, attention and motor performance (Braff et al. 1981; Elwan et al. 1997; Fant et al. 1998; Heishman et al. 1997; Heishman et al. 1989; Kurzthaler et al. 1999; Wilson et al. 1994). Cannabis also alters sensitivity to reward in risky choice decision making (Rogers et al. 2007), impairs automobile driving skills (Gjerde and Kinn 1991; Liguori et al. 1998) and degrades flight simulator performance in experienced pilots (Janowsky et al. 1976; Leirer et al. 1989). Impairments are not always reported for all tasks and there is some initial evidence that increased levels of cannabidiol in marijuana may ameliorate detrimental cognitive effects presumably caused by Δ9THC (Morgan et al. 2010b). Further determination of the precise roles played by Δ9THC as well as other constituents of marijuana would be facilitated by animal models which are sensitive to learning and mnemonic effects of Δ9THC. Nonhuman primate behavioral models offer significant advantages for the study of complex cognition.
The distribution of the CB1 receptor in macaque monkey brain is highly similar to that of human brain (Eggan and Lewis 2007). High levels of expression were noted in frontal and temporal lobe regions that have been extensively associated with higher level cognition including memory and executive function in monkeys (Castner et al. 2004; Murray and Wise 2004; Roberts 1996). Results of Eggan and Lewis, as well as others (see (Tanda et al. 1997) for review) also show a high level of CB1 expression in regions of the brain that are critical for reward function and the development of drug abuse. Correspondingly, cannabis or THC exposure has also been demonstrated to disrupt behavior in a variety of nonhuman primate species including spontaneous locomotor activity in baboon (Hienz et al. 1992), schedule responding in macaque and squirrel monkeys (Brady and Balster 1980; Galbicka et al. 1980; Stark and Dews 1980), learning tasks in chimpanzee, orangutan, macaque and squirrel monkeys (Branch et al. 1980; Pieper 1976; Schulze et al. 1988; Schulze et al. 1989), recognition memory tasks in chimpanzee and macaque (Aigner 1988; Ferraro and Grilly 1974; Schulze et al. 1989), spatial delayed response in macaques (Rupniak et al. 1991) and time estimation in macaques, chimpanzee and orangutan (Pieper 1976; Schulze et al. 1988; Schulze et al. 1989; Snyder et al. 1975).
Although behaviors have been disrupted by THC in monkey models, evidence for specific effects on different domains of cognitive function are more ambiguous. The most consistent effect of Δ9THC on behavioral performance of monkeys appears to be a reduction in response rate in various schedule-controlled operant paradigms, e.g., (Beardsley et al. 1987; Brady and Balster 1980; McMahon et al. 2005; Nakamura-Palacios et al. 2000). Even so, one study reports increased response rates in rhesus after 0.1 mg/kg Δ9THC (Stark and Dews 1980). Evidence for a specific effect of Δ9THC on memory is comparatively more equivocal. Minimal (Schulze et al. 1988) or delay-nonspecific (Zimmerberg et al. 1971) effects of THC have been reported on delayed (non)match-to-sample tasks (DNMS), although larger disruptions of DNMS have been reported in chimpanzees administered relatively high (1 mg/kg) doses of Δ9THC (Ferraro and Grilly 1973). Studies also report increased errors on repeated-acquisition tasks in New World and Old World monkeys (Nakamura-Palacios et al. 2000; Winsauer et al. 1999) in which new learning was affected but recall of old sequences was not. Effects in that task, however, occur mostly at doses which produce substantial disruption of responding and appear in a subset of the subjects. Finally, rhesus monkeys were not impaired in learning a 20-pair object discrimination task where DNMS performance was reduced in a delay-independent manner (Aigner 1988), although relatively high doses (2.0-4.0 mg/kg, p.o.) were necessary to see any effect. The existing data are strongest in showing that Δ9THC is sedating and may decrease motivation for appetitive reinforcers, but much weaker in explicating the nature of any specific effects on memory in nonhuman primates.
The relatively modest, inconsistent and/or non-specific behavioral effects of Δ9THC reported in monkeys to date may reflect the tasks chosen for study. Recent work with rodents and humans has focused on the effects of Δ9THC on spatial and working memory and indeed one prior study which demonstrated a clear retention-interval dependent effect of THC in monkeys used a spatial delayed-response procedure thought to assess working memory (Rupniak et al. 1991). The present study was therefore conducted to contrast the effects of Δ9THC on two memory tests which feature significant spatial and working-memory demands. The self-ordered spatial search (SOSS) task of the nonhuman primate version of the Cambridge Neuropsychological Test Automated Batter (CANTAB) was designed to assay spatial working memory. It has been shown that performance of this task depends on intact function in frontal regions in marmoset monkeys (Collins et al. 1998) (similar to humans (Owen et al. 1990; Owen et al. 1996)). Furthermore SOSS performance is impaired in a trial difficulty-dependent manner by amnestic drugs such as the muscarinic cholinergic receptor antagonist scopolamine (Taffe et al. 1999), the NMDA receptor, noncompetitive antagonist, ketamine (Taffe et al. 2002a) and the D2-like dopamine receptor antagonist raclopride (Von Huben et al. 2006). The CANTAB visuo-spatial paired-associate learning task (vsPAL) is highly sensitive to early stage Alzheimer's Disease (Blackwell et al. 2004; Fowler et al. 1997; 2002; Swainson et al. 2001) and has likewise been adapted for monkeys (Taffe et al. 2002b; 2004). Prior work has shown it is sensitive to challenge with the amnestic drugs scopolamine, ketamine, raclopride, the nicotinic cholinergic antagonist mecamylamine as well as to chronic alcohol drinking (Crean et al. 2011; Katner et al. 2004; Taffe et al. 2002b; Von Huben et al. 2006). These refined and pharmacologically validated behavioral tests may therefore provide improved sensitivity to mnemonic effects of acute Δ9THC in rhesus monkeys.
The selected model also permits a higher degree of translational inference since parallel human CANTAB tasks are available. For example, one recent study in human adolescents found greater detrimental effects of chronic cannabis use on the human CANTAB SOSS task, but not on the vsPAL task (Harvey et al. 2007). Although users had been abstinent for at least 12 hrs in that study, this provides some indication that these tasks may be sensitive to disruption of endocannabinoid systems. The SOSS task challenges spatial memory and the vsPAL involves some degree of pattern recognition, similar to a DNMS task. Therefore it was predicted that acute effects of Δ9THC will be greatest on the SOSS task in the present study.
2. Materials and Methods
2.1 Animals
Eight male rhesus monkeys (Macaca mulatta) were used in these experiments. Animals were 5-6 years of age, weighed 8.0-13.4 kg at the start of the study. Daily chow (LabDiet® 5038, PMI Nutrition International, Richmond, IN, USA; 3.22 kcal of metabolizable energy (ME) per gram) allocations were supplemented with fruit or vegetables seven days per week and water was available ad libitum in the home cage. Animals on this study had previously been immobilized with ketamine (5-20 mg/kg) no less than semiannually for purposes of routine care and some experimental procedures. Animals also had various acute exposures to challenge drugs in prior studies. The United States National Institutes of Health guidelines for laboratory animal care (Clark et al. 1996) were followed and all protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
2.2 Behavioral Testing
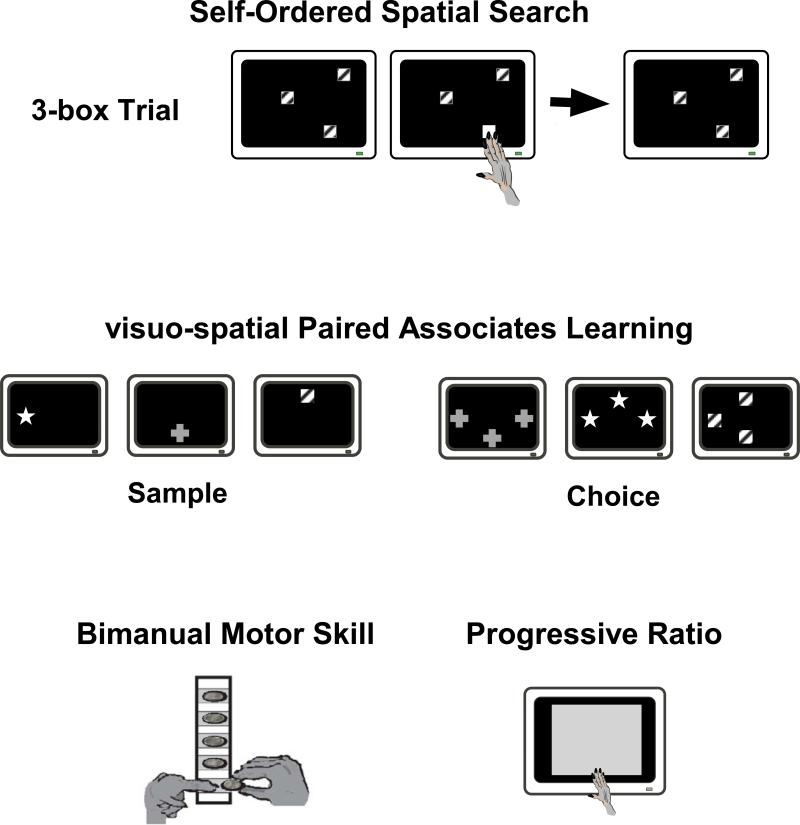
For behavioral testing, a touch-sensitive computer monitor was placed in front of the animal, unrestrained in a cage. All subjects had been trained to reach out of the cage to touch the location on the screen at which visual stimuli were presented to obtain a food pellet reward. The test battery consisted of four behavioral tasks, three of which (vsPAL, SOSS, PR) are part of the non-human primate CAmbridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition, Cambridge, UK). Comprehensive descriptions of the individual tasks and the procedural details have been previously reported (Taffe et al. 2004; Weed et al. 1999) and a task schematic is provided in Figure 1.
Figure 1.
A depiction of the behavioral tasks included in this study including a sample stimulus display for the 3-box difficulty condition in the Self-Ordered Spatial Search task (top) and the 3-stimuli by three choice locations trials in the visuo-spatial Paired Associates Learning task. See Materials and Methods and (Taffe et al. 2002b; 2004; Weed et al. 1999) for further specification of the tasks.
2.2.1 Self-Ordered Spatial Search (SOSS)
Two or more small colored rectangles (boxes) were displayed on the screen in positions randomly allocated from 16 possible locations, as previously depicted (Taffe and Taffe 2011). The animal was required to touch a box within 30 seconds of stimulus onset after which the color of the touched box was briefly (100 ms) changed, the screen blanked and a reinforcer delivered. After a 2 second delay, the boxes were re-displayed and the animal was required to touch a box which had not previously been touched within the trial for a successful trial completion. The trial was completed when the animal either touched all boxes without a repetition (correct), touched a box that had previously been selected in that trial (error) or failed to touch a box within 30 seconds of stimulus presentation (omission). Errors and omissions were followed by a tone and a 4 second timeout with a screen blank. After an inter-trial interval of 5 seconds, another trial was presented with stimuli in new (randomly allocated) positions. Each session consisted of 40 trials grouped into 8 blocks by trial type as follows: 5 (2 boxes), 5 (3 boxes), 5 (4 boxes), 5 (5 boxes), 5(3 boxes), 5(4 boxes), 5(5 boxes), 5 (2 boxes). Accuracy scores were calculated for each trial type by dividing the number of correctly completed trials by the number of trials in which there was at least one response. Six of the eight animals were trained to stable baseline performance on this task.
2.2.2 Visuo-Spatial Paired Associates Learning (vsPAL)
Colored abstract stimuli were displayed in one of four possible target locations (see (Taffe et al. 2002b)) and the subject was required to touch this sample stimulus, which then disappeared. The same pattern re-appeared during the choice phase in 2, 3 or 4 locations on the screen (the original location plus one or more novel locations) after a one second screen blank. The subject was required to touch the stimulus presented in the same location as the sample item to obtain a reinforce delivery. Subjects were allowed up to 10 additional attempts to successfully complete the set of stimulus-location associations in a given trial, thus measuring incremental learning. Each session consisted of 35 trials in sequential blocks including 5 × 1-stimulus (3 choice locations) trials, 10 × 2-stimuli (2 choice locations) trials, 10 × 3-stimuli (3 choice locations) trials and 10 × 4-stimuli (4 choice locations) trials. Performance was measured by percent correct trials on the initial-attempt to complete a trial and the percent correct of trials successfully completed within the allowed attempts (repeated-attempt completion). Four of the eight subjects had been trained to stable baseline levels of performance on this task.
2.2.3 Progressive-Ratio (PR) Schedule of Reinforcement
Subjects were required to respond to a single colored rectangle presented in the center of the screen for pellet reinforcement. The response requirement started at 1 touch and incremented by arithmetic progression within blocks of 8 reinforcers and by geometric progression between blocks of 8. (I.e., the first successive 8 ratios increase by 1, the second successive 8 increase by 2, the third successive 8 increase by 4, etc.) The session was terminated after 10 minutes, or earlier if 3 minutes elapsed following a response. The primary dependent variable was the number of reinforcers acquired.
2.2.4 Bimanual Motor Skill Task (BMS)
A transparent polycarbonate board (10 cm wide × 25 cm high × 2.75 cm thick) drilled with 15 holes (spaced 13 mm apart in a 3 horizontal × 5 vertical array) was filled with raisins and mounted perpendicular to the door of the transport cage. Subjects acquire a technique wherein they push the raisin out of the hole with one finger before retrieving it with the opposite hand, thus entailing bimanual dexterity. The time elapsed to retrieve all 15 raisins was recorded.
2.2 Drug Challenges
Monkeys were administered acute doses (0.1-0.3 mg/kg, IM) of Δ9THC 45 minutes prior to behavioral testing with active drug challenges being conducted no more than twice per week at 3-4 day intervals. Treatment order was pseudo-randomized across subjects and with respect to behavioral testing combinations (e.g., sessions of PR/SOSS/BMS versus vsPAL/BMS). For injection, Δ9THC was suspended in a vehicle of absolute ethanol, Emulphor and saline in a 1:1:18 ratio. The Δ9THC was provided by the U.S. National Institute on Drug Abuse.
2.3 Data Analysis
Analysis of the behavioral data employed randomized block analysis of variance (ANOVA) with a consistent within-subjects factor of drug treatment condition (baseline, vehicle, 0.1, 0.2, 0.3 mg/kg THC). Analysis of the SOSS data employed an additional repeated measures factor of trial difficulty (2-, 3-, 4- and 5-boxes). Two of the six animals failed to complete sufficient trials for analysis in the SOSS task in the 0.2 mg/kg condition, thus they are included only in the remaining conditions. Three factor repeated-measures analysis of the vsPAL performance was necessary to include a factor of initial versus repeated attempts (to demonstrate improvement with practice, or learning) as well as the trial difficulty (1, 2, 3 or 4 stimuli per trial). Post-hoc analyses of any significant main effects in the multi-factor ANOVAs was conducted using the Fisher LSD test including all pairwise comparisons. Post-hoc evaluation of the single factor PR and BMS tasks was conducted with the Dunnett procedure using the vehicle treatment condition for comparison with all other conditions. The criterion for significance in all tests was p< 0.05. Analyses were conducted with GB-STATv7.0; Dynamic Microsystems, Silver Spring MD
3. Results
3.1. Self-Ordered Spatial Search Task
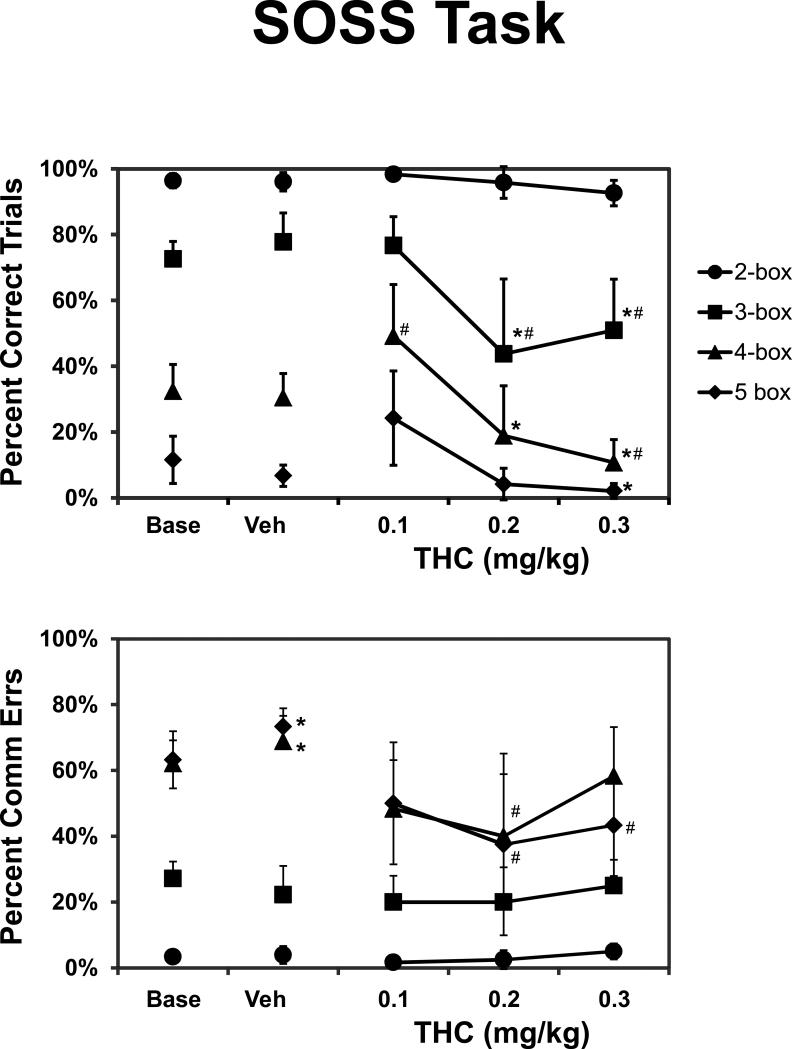
Performance accuracy (percent correct trials) of the Self-Ordered Spatial Search (SOSS) task was ranked by trial difficulty and altered by drug challenge (Figure 2) as was confirmed by significant main effects of trial difficulty [F3,15 = 130.5; p < 0.0001] and drug treatment condition [F4,20 = 6.4; p < 0.005]. Furthermore, the post hoc test confirmed that under baseline, vehicle and 0.1 mg/kg Δ9THC treatment conditions, trial completion success for each trial type was significantly different from every other trial type. Accuracy on the simplest, 2-box trials was significantly better than all other trial types, and the performance of 3-box trials remained better than the 4-box and 5-box trials, following the administration of the two highest doses of Δ9THC as well. Trial completion success of the 4-box and 5-box trial types did not differ from each other after the two highest doses of Δ9THC. The drug challenge also produced detrimental effects when considered within a trial difficulty type. The post-hoc test confirmed that relative to baseline, vehicle and the 0.1 mg/kg condition, 3-box trial success was significantly impaired when either 0.2 or 0.3 mg/kg Δ9THC was injected and 4-box trial success was impaired when 0.3 mg/kg was injected. The 5-box trial completion success was significantly lower after 0.3 mg/kg compared to the 0.1 mg/kg Δ9THC condition, but no differences from either the baseline or vehicle condition were confirmed for 5-box trials after drug administration. Interestingly, the post-hoc test also confirmed that performance of the 4-box trials was improved after 0.1 mg/kg Δ9THC relative to baseline, vehicle and the 0.3 mg/kg treatment conditions.
Figure 2.
The mean (N=6; ±SEM) trial completion accuracy on the SOSS task is presented by trial-difficulty and drug treatment condition. A significant difference from the vehicle and baseline conditions is indicated by #, and a difference from the 0.1 mg/kg condition by * within a given trial-difficulty level. The percent correct on all trial types differed significantly from all of the other trial types within a treatment condition, save that 4-box and 5-box completion success did not differ after 0.2 and 0.3 mg/kg THC.
3.2. visuo-spatial Paired Associates Learning Task
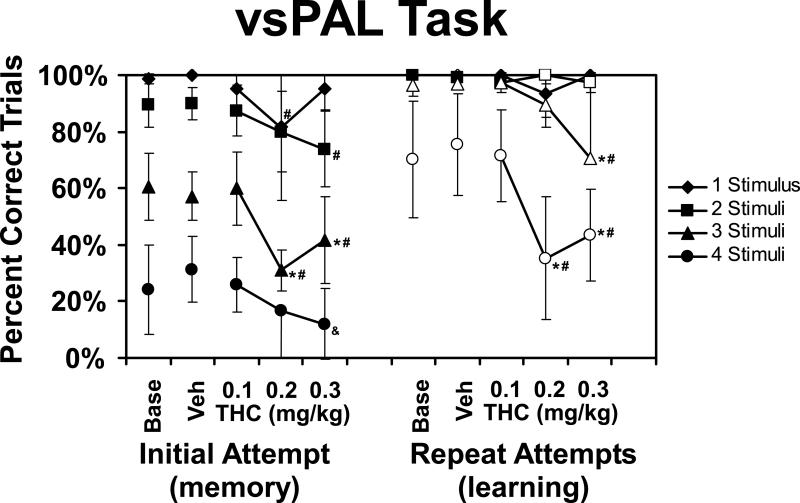
The monkeys’ trial completion success in the visuo-spatial Paired Associates Learning (vsPAL) task was determined by trial difficulty (number of stimuli) and improved with repeated attempts at the same trial (Figure 3). These differences were statistically reliable as was confirmed by main effects of trial difficulty [F3,21 = 306.4; p < 0.0001], of initial versus repeated attempts [F1,7 = 292.6; p < 0.0001] and the interaction between these two factors [F3,21 = 78.8; p < 0.0001]. The post-hoc test confirmed that initial-attempt performance of 3-stimuli and 4-stimuli trial types differed from all other trial types within treatment condition; 1-stimulus and 2-stimuli trial performances only differed after 0.3 mg/kg Δ9THC. The repeated-attempt completion success for 4-stimuli trials differed significantly from all other trial types within each treatment condition as did 3-stimuli trial performance after 0.3 mg/kg Δ9THC.
Figure 3.
The mean (N=4; ±SEM) percentage of trials correctly performed in the vsPAL task on the first attempt, and after a maximum of 6 attempts, are presented for baseline, vehicle and THC treatment conditions. The open symbols indicate significantly improved trial completion after repetition when compared with the initial attempt for a given treatment condition and trial type. Within a given trial-difficulty level, a significant difference from the vehicle and baseline conditions is indicated by #, from the vehicle condition (only) by &, and a difference from the 0.1 mg/kg condition by *.
The performance of vsPAL was impaired by Δ9THC in a dose by difficulty dependent manner. The ANOVA confirmed a significant main effect of drug condition [F4,28 = 71.0; p < 0.0001], of the interaction between drug condition and trial difficulty [F12,84 = 5.8; p < 0.0001] and of all three factors [F12,84 = 14.0; p < 0.0001]; the interaction between initial/repeated attempts and drug condition was not significant. The post-hoc test confirmed that relative to vehicle and baseline initial-attempt performance, 1-stimulus trials were impaired after 0.2 mg/kg and 2-stimuli trials after 0.3 mg/kg Δ9THC was injected. Similarly, the 3-stimuli trial initial-attempt success was lower compared with baseline, vehicle and the 0.1 mg/kg treatment conditions when either 0.2 or 0.3 mg/kg Δ9THC was administered. Finally, the 4-stimuli trial initial-attempt success was impaired relative to vehicle following 0.3 mg/kg Δ9THC. The post-hoc test also confirmed that repeated-attempt trial completion success was impaired relative to baseline, vehicle and the 0.1 mg/kg conditions for 3-stimuli trials after 0.3 mg/kg and for 4-stimuli trials after 0.2 and 0.3 mg/kg Δ9THC.
3.3. Progressive Ratio and Bimanual Motor Skill Tasks
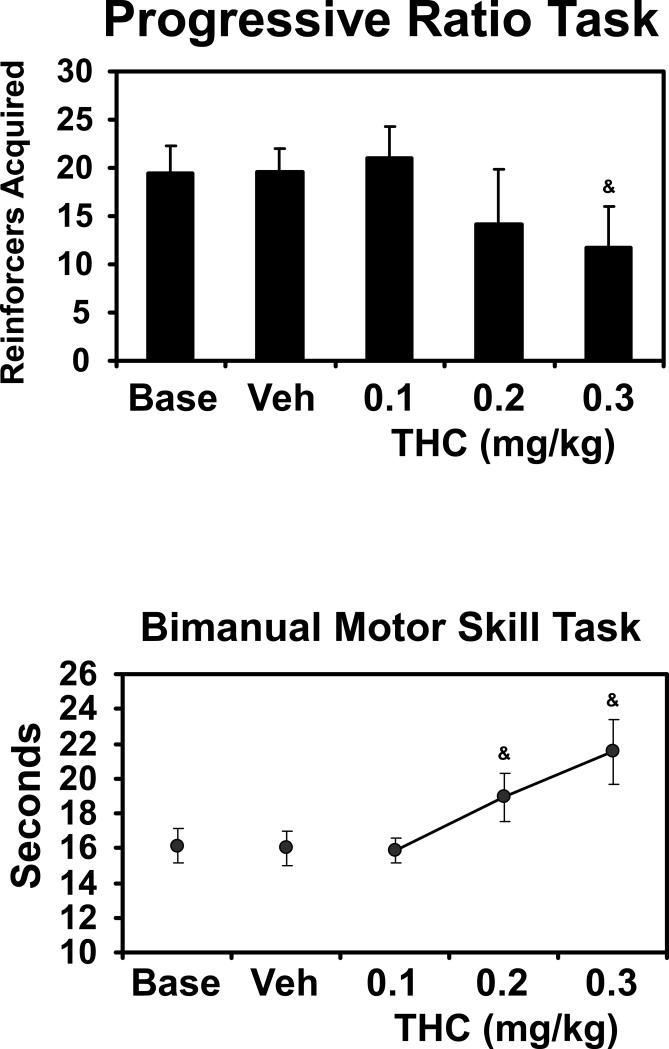
The remaining tasks were also affected detrimentally by Δ9THC as is shown in Figure 4. The ANOVAs confirmed that Δ9THC significantly changed the number of reinforcers acquired on the progressive ratio task [F4,24 = 6.11; p < 0.01] and slowed raisin retrieval in the bimanual motor skill task [F4,28 = 9.41; p < 0.0001]. The post-hoc analysis further confirmed that compared with the vehicle condition, PR performance was lower after 0.3 mg/kg Δ9THC and raisin retrieval was slower after either 0.2 or 0.3 mg/kg Δ9THC.
Figure 4.
Mean (Bars indicate SEM) performance on the Progressive Ratio (N=7) and Bimanual Motor Skill (N=8) tasks. A significant difference from the vehicle condition is indicated by &.
3. Discussion
This study shows that the administration of Δ9-tetrahydrocannabinol (Δ9THC) interferes with the performance of two spatial memory tasks in rhesus monkeys. The detrimental effects of Δ9THC were task specific within both the Self-Ordered Spatial Search (SOSS) and visuo-spatial Paired Associates Learning (vsPAL) tasks of the nonhuman primate CANTAB battery (Taffe et al. 2004; Weed et al. 1999) since the monkeys’ performance was degraded in a dose by trial difficulty manner. Although the simpler tasks that are intended to index motivated responding (PR task) and psychomotor function (bimanual motor skills task) were also affected these effects were incremental rather than a complete disruption of behavior at the doses administered (0.1-0.3 mg/kg i.m.). Similarly, performance of the easiest trial types within both SOSS and vsPAL tasks was minimally affected, or unaffected, by Δ9THC. Additional evidence that motivation does not explain the pattern of effects on the memory tasks is provided by a prior demonstration that degrading the motivation for the food reinforcer disrupts SOSS and vsPAL performance in a trial-difficulty independent manner (Taffe 2004). Therefore the present data are most consistent with an interpretation of a specific disruptive effect of Δ9THC on spatial working memory, visuo-spatial associative memory and incremental learning.
Prior cognitive studies in Old World monkeys have not generally found effects of Δ9THC on memory procedures that are clear demonstrations of a highly specific impact. That is, drug effects were not task-difficulty dependent and in some cases were observed at doses which produced significant general behavioral disruption. For example Aigner found that Δ9THC (2-4 mg/kg p.o.) impaired overall performance on a sequential-list version of Delayed Non-Match to Sample (DNMS) but no retention-interval or list-length dependent effects were reported (Aigner 1988). Similarly, Schulze and colleagues found that Δ9THC did not impair a traditional sample/response DNMS (with a 7-stimulus set) task in a retention-interval dependent manner (Schulze et al. 1988). Interestingly this same task was impaired in a retention-interval dependent manner by the inhalation of marijuana smoke by monkeys (Schulze et al. 1989). Winsauer and colleagues showed that Δ9THC impaired the acquisition of a novel stimulus-response chain but not the expression of an already-learned chain of responses (Winsauer et al. 1999; Winsauer et al. 2011). In this procedure, however, there was no report of within-task difficulty levels for either the acquisition or performance components, the drug effects on accuracy were observed in about half of the subject population and following doses that significantly suppressed responding.
As is obvious from the present findings on the PR and BMS tasks, Δ9THC is very likely to have behavioral effects that may not be specific to learning or memory. It was also the case that choice latency was altered in the complex tasks. For example in the vsPAL task choice latency was slowed from about 800 ms in the vehicle condition (for all trial types) to 1500 ms for 2-stimuli trials and 2630 ms for 4-stimuli trials after 0.3 mg/kg Δ9THC. Interestingly, mean correct response latency in the SOSS task was slowed only slightly (1864-2169 ms vs 1410 under vehicle conditions) in the easy, 2-box trials after 0.2-0.3 mg/kg Δ9THC, however response latencies were faster for the more difficult trial types under baseline and vehicle conditions (~900-1100 ms) and were not slowed any more than the 2-box trials under Δ9THC challenge. We have shown previously that experimentally extending the retention interval in these tasks (Crean et al. 2011; Weed et al. 1999) by a duration similar to the slowing induced by Δ9THC does not introduce an accuracy cost similar to the one produced by Δ9THC in this study. This again supports the interpretation of a selective effect of Δ9THC on the mnemonic aspects of the SOSS and vsPAL tasks.
One prior report that did identify detrimental effects of Δ9THC on monkey performance (Rupniak et al. 1991) using a spatial delayed-response task, raising the possibility of a cognitive domain selective, and potentially regionally-selective, effect. That is, spatial working memory tasks are often characterized as depending primarily on frontal mechanisms whereas recognition memory procedures such as DNMS are thought to depend primarily on the medial temporal lobe memory systems. The present SOSS task has been shown to depend on intact function of prefrontal cortex in marmoset monkeys (Collins et al. 1998) and therefore this commonality may suggest a specific or enhanced sensitivity of frontal cognitive mechanisms to Δ9THC. The visuo-spatial associations that are intrinsic to the vsPAL procedure likely involve frontal-temporal circuitry (Browning and Gaffan 2008), perhaps with a dominance of orbital regions and excluding dorsolateral prefrontal areas (Baxter et al. 2008). Nevertheless dominant contribution to pattern/spatial associative memory appears to be in the temporal lobe systems (Browning and Gaffan 2008; Malkova and Mishkin 2003). Furthermore, a recent study of humans with Mild Cognitive Impairment found functional Magnetic Resonance Imaging differences in hippocampal regions, but not frontal regions, during the performance of a human analog of vsPAL (de Rover et al. 2011). This is consistent with reports of relatively high density of the CB1 endocannabinoid receptor in the dentate gyrus and CA fields of the hippocampus, entorhinal cortex, Areas 9 and 46 (dorsolateral prefrontal cortex) and Areas 10 and 11 (anterior prefrontal, orbitofrontal) in the macaque brain (Eggan and Lewis 2007).
The current data also provide further insight into potential species differences in responses to Δ9THC when considered in combination with a recent report from this laboratory showing that the same dose range reduces the body temperature of freely moving monkeys (Harvey et al. 2007). Prior studies have shown that while doses of about 0.5-1.0 mg/kg, i.p. disrupt cognition in rats (Egerton et al. 2005; Han and Robinson 2001), the considerably higher doses (~10-30 mg/kg) required to decrease body temperature in the rat also produce hypolocomotion and/or catalepsy (Smirnov and Kiyatkin 2008; Whitlow et al. 2002). In macaque monkeys, doses that are higher than the 0.1-0.3 mg/kg used in the current study produce behavioral disruption, sedation, task refusal and other non-cognitive effects (McMahon et al. 2005; Winsauer et al. 1999), consistent with pilot studies conducted to establish the appropriate dose range for the current investigation. With the dose range of 0.1-0.3 mg/kg, monkeys were visibly intoxicated (most frequently evidenced by drooping eyelids, slowed reaction to conspecifics and investigative staff) within about 15-20 minutes of injection. Nevertheless they still responsive in the behavioral tasks, completing sufficient numbers of trials for analysis across the difficulty conditions and generating response latencies that were quantitatively slower but did not reflect total task disruption, as noted above.
The present results can also be compared in magnitude with prior pharmacological challenge of these same memory tasks with drugs more generally established to have amnestic effects. For example, the SOSS task is impaired in a difficulty- and dose-dependent manner by the muscarinic antagonist scopolamine (Taffe et al. 1999) and the NMDA noncompetitive antagonist ketamine (Taffe et al. 2002b) but not by the nicotinic antagonist mecamylamine (Katner et al. 2004) or the D1-like dopamine receptor antagonist SCH23390 (Von Huben et al. 2006). SOSS performance may be improved by a rapid tryptophan depletion approach which decrements central serotonin function (Taffe et al. 2003). It has also been shown that ketamine, mecamylamine and the D2-like dopamine receptor antagonist raclopride impair the initial memory component of vsPAL while leaving the learning component intact (Katner et al. 2004; Taffe et al. 2002b; Von Huben et al. 2006). In contrast scopolamine impairs both initial memory and incremental learning (Taffe et al. 2002b). The present results therefore put Δ9THC on a similar scope and scale of effect as more canonical amnestic drugs. Within this CANTAB model of NHP cognition, Δ9THC had an impact comparable to that of scopolamine, which is one of the few drugs that has been shown to disrupt incremental learning as well as initial memory in the NHP vsPAL task.
In summary, this study used refined and validated memory procedures to assess the cognitive effects of the primary psychoactive constituent of marijuana, Δ9-tetrahydrocannabinol, in a nonhuman primate model. The results show that while there is a general behavioral impact (as with many prior studies in which operant responding was disrupted) there do appear to be selective effects on spatial working memory, visuo-spatial associative memory and incremental learning. This selective effect was evidenced in both vsPAL and SOSS tasks by preservation of the easiest trial conditions and dose-dependent impact on the harder trial conditions. These studies confirm that cognitive/behavioral models in the macaque monkey are likely translatable to the human condition and identify cannabinoid sensitive assays that will be of further utility in additional studies of exo- and/or endocannabinoid regulation of cognitive function and complex behavior. For example a recent study of human adolescent regular cannabis users, tested at least 12 hrs after last use, on human CANTAB which were impaired on SOSS (but not on vsPAL) compared with the occasional-use controls (Taylor and Fennessy 1978). The present nonhuman primate model can now be used to explore effects of chronic exposure to Δ9THC and/or other marijuana constituents (Morgan et al. 2010a; Morgan et al. 2010b), withdrawal and degree of recovery from chronic Δ9THC dosing.
Acknowledgements
The author is grateful to the other principal investigators of the Scripps Center for Cannabis Addiction Neurobiology, Drs. Barbara J. Mason, Susan F. Tapert and Loren H. Parsons, for many helpful discussions as well as to Sophia A. Vandewater (Davis) and Amber J. Kirsten for technical contributions. Portions of this work were presented in preliminary form at the 2001 annual meeting of the College on Problems of Drug Dependence. This work was supported by US Public Health Service / National Institutes of Health grants DA013390, DA018418 and DA024194. This is publication #21504 from The Scripps Research Institute.
Footnotes
Conflict of Interest Statement : The author declares that there is no conflict of interest for this study.
REFERENCES
- Aigner TG. Delta-9-tetrahydrocannabinol impairs visual recognition memory but not discrimination learning in rhesus monkeys. Psychopharmacology (Berl) 1988;95:507–11. doi: 10.1007/BF00172964. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances and inhalants: Basic findings from the national comorbidity survey. Exp Clin Pyschopharm. 1994;2:244–268. [Google Scholar]
- Baxter MG, Gaffan D, Kyriazis DA, Mitchell AS. Dorsolateral prefrontal lesions do not impair tests of scene learning and decision-making that require frontal-temporal interaction. Eur J Neurosci. 2008;28:491–9. doi: 10.1111/j.1460-9568.2008.06353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Scimeca JA, Martin BR. Studies on the agonistic activity of delta 9-11-tetrahydrocannabinol in mice, dogs and rhesus monkeys and its interactions with delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1987;241:521–6. [PubMed] [Google Scholar]
- Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, Hodges JR. Detecting dementia: novel neuropsychological markers of preclinical Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;17:42–8. doi: 10.1159/000074081. [DOI] [PubMed] [Google Scholar]
- Brady KT, Balster RL. The effects of delta 9-tetrahydrocannabinol alone and in combination with cannabidiol on fixed-interval performance in rhesus monkeys. Psychopharmacology (Berl) 1980;72:21–6. doi: 10.1007/BF00433803. [DOI] [PubMed] [Google Scholar]
- Braff DL, Silverton L, Saccuzzo DP, Janowsky DS. Impaired speed of visual information processing in marijuana intoxication. Am J Psychiatry. 1981;138:613–7. doi: 10.1176/ajp.138.5.613. [DOI] [PubMed] [Google Scholar]
- Branch MN, Dearing ME, Lee DM. Acute and chronic effects of delta 9-tetrahydrocannabinol on complex behavior of squirrel monkeys. Psychopharmacology. 1980;71:247–56. doi: 10.1007/BF00433059. [DOI] [PubMed] [Google Scholar]
- Browning PG, Gaffan D. Impairment in object-in-place scene learning after uncinate fascicle section in macaque monkeys. Behav Neurosci. 2008;122:477–82. doi: 10.1037/0735-7044.122.2.477. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS, Williams GV. Animal models of working memory: insights for targeting cognitive dysfunction in schizophrenia. Psychopharmacology (Berl) 2004;174:111–25. doi: 10.1007/s00213-003-1710-9. [DOI] [PubMed] [Google Scholar]
- Clark JD, Baldwin RL, Bayne KA, Brown MJ, Gebhart GF, Gonder JC, Gwathmey JK, Keeling ME, Kohn DF, Robb JW, Smith OA, Steggarda J-AD, Vandenbergh JG, White WJ, Williams-Blangero S, VandeBerg JL. Guide for the Care and Use of Laboratory Animals. Institute of Laboratory Animal Resources, National Research Council; Washington D.C.: 1996. p. 125. [Google Scholar]
- Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW. Perseveration and Strategy in a Novel Spatial Self-Ordered Sequencing Task for Nonhuman Primates. Effects of Excitotoxic Lesions and Dopamine Depletions of the Prefrontal Cortex. J Cogn Neurosci. 1998;10:332–354. doi: 10.1162/089892998562771. [DOI] [PubMed] [Google Scholar]
- Crean RD, Vandewater SA, Katner SN, Huitron-Resendiz S, Taffe MA. Chronic alcohol consumption impairs visuo-spatial associative memory in periadolescent rhesus monkeys. Drug Alcohol Depend. 2011;114:31–40. doi: 10.1016/j.drugalcdep.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rover M, Pironti VA, McCabe JA, Acosta-Cabronero J, Arana FS, Morein-Zamir S, Hodges JR, Robbins TW, Fletcher PC, Nestor PJ, Sahakian BJ. Hippocampal dysfunction in patients with mild cognitive impairment: a functional neuroimaging study of a visuospatial paired associates learning task. Neuropsychologia. 2011;49:2060–70. doi: 10.1016/j.neuropsychologia.2011.03.037. [DOI] [PubMed] [Google Scholar]
- Egerton A, Brett RR, Pratt JA. Acute delta9-tetrahydrocannabinol-induced deficits in reversal learning: neural correlates of affective inflexibility. Neuropsychopharmacology. 2005;30:1895–905. doi: 10.1038/sj.npp.1300715. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical Distribution of the Cannabinoid CB1 Receptor in the Primate Neocortex: A Regional and Laminar Analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Elwan O, Hassan AA, Abdel Naseer M, Elwan F, Deif R, El Serafy O, El Banhawy E, El Fatatry M. Brain aging in a sample of normal Egyptians cognition, education, addiction and smoking. J Neurol Sci. 1997;148:79–86. doi: 10.1016/s0022-510x(96)05336-1. [DOI] [PubMed] [Google Scholar]
- Fant RV, Heishman SJ, Bunker EB, Pickworth WB. Acute and residual effects of marijuana in humans. Pharmacol Biochem Behav. 1998;60:777–84. doi: 10.1016/s0091-3057(97)00386-9. [DOI] [PubMed] [Google Scholar]
- Ferraro DP, Grilly DM. Lack of tolerance to 9 -tetrahydrocannabinol in chimpanzees. Science. 1973;179:490–2. doi: 10.1126/science.179.4072.490. [DOI] [PubMed] [Google Scholar]
- Ferraro DP, Grilly DM. Effects of chronic exposure to delta9-tetrahydrocannabinol on delayed matching-to-sample in chimpanzees. Psychopharmacologia. 1974;37:127–38. doi: 10.1007/BF00437419. [DOI] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Computerized neuropsychological tests in the early detection of dementia: prospective findings. J Int Neuropsychol Soc. 1997;3:139–46. [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT. J Int Neuropsychol Soc. 2002;8:58–71. [PubMed] [Google Scholar]
- Galbicka G, Lee DM, Branch MN. Schedule-dependent tolerance to behavioral effects of delta 9-tetrahydrocannabinol when reinforcement frequencies are matched. Pharmacol Biochem Behav. 1980;12:85–91. doi: 10.1016/0091-3057(80)90420-7. [DOI] [PubMed] [Google Scholar]
- Gjerde H, Kinn G. Impairment in drivers due to cannabis in combination with other drugs. Forensic Sci Int. 1991;50:57–60. doi: 10.1016/0379-0738(91)90133-4. [DOI] [PubMed] [Google Scholar]
- Han CJ, Robinson JK. Cannabinoid modulation of time estimation in the rat. Behav Neurosci. 2001;115:243–6. doi: 10.1037/0735-7044.115.1.243. [DOI] [PubMed] [Google Scholar]
- Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug and alcohol review. 2007;26:309–19. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Arasteh K, Stitzer ML. Comparative effects of alcohol and marijuana on mood, memory, and performance. Pharmacol Biochem Behav. 1997;58:93–101. doi: 10.1016/s0091-3057(96)00456-x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Yingling JE. Effects of tetrahydrocannabinol content on marijuana smoking behavior, subjective reports, and performance. Pharmacol Biochem Behav. 1989;34:173–9. doi: 10.1016/0091-3057(89)90369-9. [DOI] [PubMed] [Google Scholar]
- Hienz RD, Turkkan JS, Spear DJ, Sannerud CA, Kaminski BJ, Allen RP. General activity in baboons measured with a computerized, lightweight piezoelectric motion sensor: effects of drugs. Pharmacol Biochem Behav. 1992;42:497–507. doi: 10.1016/0091-3057(92)90145-6. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Meacham MP, Blaine JD, Schoor M, Bozzetti LP. Simulated flying performance after marihuana intoxication. Aviat Space Environ Med. 1976;47:124–8. [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Scheulenberg JE. Monitoring the Future national survey results on drug use, 1975-2010. Volume I, Secondary school students. University of Michigan; Ann Arbor: 2011a. p. 734. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2010. Volume II, College students and adults ages 19-45. University of Michigan; Ann Arbor: 2011b. p. 312. [Google Scholar]
- Katner SN, Davis SA, Kirsten AJ, Taffe MA. Effects of nicotine and mecamylamine on cognition in rhesus monkeys. Psychopharmacology (Berl) 2004;175:225–40. doi: 10.1007/s00213-004-1804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzthaler I, Hummer M, Miller C, Sperner-Unterweger B, Gunther V, Wechdorn H, Battista HJ, Fleischhacker WW. Effect of cannabis use on cognitive functions and driving ability. J Clin Psychiatry. 1999;60:395–9. doi: 10.4088/jcp.v60n0609. [DOI] [PubMed] [Google Scholar]
- Leirer VO, Yesavage JA, Morrow DG. Marijuana, aging, and task difficulty effects on pilot performance. Aviat Space Environ Med. 1989;60:1145–52. [PubMed] [Google Scholar]
- Liguori A, Gatto CP, Robinson JH. Effects of marijuana on equilibrium, psychomotor performance, and simulated driving. Behav Pharmacol. 1998;9:599–609. doi: 10.1097/00008877-199811000-00015. [DOI] [PubMed] [Google Scholar]
- Malkova L, Mishkin M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J Neurosci. 2003;23:1956–65. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Amin MR, France CP. SR 141716A differentially attenuates the behavioral effects of delta9-THC in rhesus monkeys. Behav Pharmacol. 2005;16:363–72. doi: 10.1097/00008877-200509000-00008. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Freeman TP, Schafer GL, Curran HV. Cannabidiol attenuates the appetitive effects of Delta 9-tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacology. 2010a;35:1879–85. doi: 10.1038/npp.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected]. Br J Psychiatry. 2010b;197:285–90. doi: 10.1192/bjp.bp.110.077503. [DOI] [PubMed] [Google Scholar]
- Murray EA, Wise SP. What, if anything, is the medial temporal lobe, and how can the amygdala be part of it if there is no such thing? Neurobiol Learn Mem. 2004;82:178–98. doi: 10.1016/j.nlm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Nakamura-Palacios EM, Winsauer PJ, Moerschbaecher JM. Effects of the cannabinoid ligand SR 141716A alone or in combination with delta9-tetrahydrocannabinol or scopolamine on learning in squirrel monkeys. Behav Pharmacol. 2000;11:377–86. doi: 10.1097/00008877-200008000-00003. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Owen AM, Morris RG, Sahakian BJ, Polkey CE, Robbins TW. Double dissociations of memory and executive functions in working memory tasks following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Brain. 1996;119:1597–1615. doi: 10.1093/brain/119.5.1597. [DOI] [PubMed] [Google Scholar]
- Pieper WA. Great apes and rhesus monkeys as subjects for psychopharmacological studies of stimulants and depressants. Fed Proc. 1976;35:2254–7. [PubMed] [Google Scholar]
- Roberts AC. Comparison of cognitive function in human and non-human primates. Cogn Brain Res. 1996;3:319–327. doi: 10.1016/0926-6410(96)00017-1. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Wakeley J, Robson PJ, Bhagwagar Z, Makela P. The effects of low doses of delta-9 tetrahydrocannabinol on reinforcement processing in the risky decision-making of young healthy adults. Neuropsychopharmacology. 2007;32:417–28. doi: 10.1038/sj.npp.1301175. [DOI] [PubMed] [Google Scholar]
- Rupniak NM, Samson NA, Steventon MJ, Iversen SD. Induction of cognitive impairment by scopolamine and noncholinergic agents in rhesus monkeys. Life Sci. 1991;48:893–9. doi: 10.1016/0024-3205(91)90036-b. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze GE, McMillan DE, Bailey JR, Scallet A, Ali SF, Slikker W, Jr., Paule MG. Acute effects of delta-9-tetrahydrocannabinol in rhesus monkeys as measured by performance in a battery of complex operant tests. J Pharmacol Exp Ther. 1988;245:178–86. [PubMed] [Google Scholar]
- Schulze GE, McMillan DE, Bailey JR, Scallet AC, Ali SF, Slikker W, Jr., Paule MG. Acute effects of marijuana smoke on complex operant behavior in rhesus monkeys. Life Sci. 1989;45:465–75. doi: 10.1016/0024-3205(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Smirnov MS, Kiyatkin EA. Behavioral and temperature effects of delta 9-tetrahydrocannabinol in human-relevant doses in rats. Brain Res. 2008;1228:145–60. doi: 10.1016/j.brainres.2008.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EW, Lewis EG, Dustman RE, Beck EC. Sustained ingestion of delta 9-tetrahydrocannabinol and the operant behavior of stump-tailed macaques. Pharmacol Biochem Behav. 1975;3:1129–32. doi: 10.1016/0091-3057(75)90028-3. [DOI] [PubMed] [Google Scholar]
- Stark P, Dews PB. Cannabinoids. I. Behavioral effects. J Pharmacol Exp Ther. 1980;214:124–30. [PubMed] [Google Scholar]
- Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Iddon JL, Robbins TW, Sahakian BJ. Early detection and differential diagnosis of Alzheimer's disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001;12:265–80. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Effects of parametric feeding manipulations on behavioral performance in macaques. Physiol Behav. 2004;81:59–70. doi: 10.1016/j.physbeh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Davis SA, Gutierrez T, Gold LH. Ketamine impairs multiple cognitive domains in rhesus monkeys. Drug Alcohol Depend. 2002a;68:175–87. doi: 10.1016/s0376-8716(02)00194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Huitron-Resendiz S, Schroeder R, Parsons LH, Henriksen SJ, Gold LH. MDMA exposure alters cognitive and electrophysiological sensitivity to rapid tryptophan depletion in rhesus monkeys. Pharmacol Biochem Behav. 2003;76:141–52. doi: 10.1016/s0091-3057(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Taffe WJ. Rhesus monkeys employ a procedural strategy to reduce working memory load in a self-ordered spatial search task. Brain Res. 2011;1413:43–50. doi: 10.1016/j.brainres.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gold LH. Scopolamine alters rhesus monkey performance on a novel neuropsychological test battery. Brain Res Cogn Brain Res. 1999;8:203–12. doi: 10.1016/s0926-6410(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Differential muscarinic and NMDA contributions to visuo-spatial paired-associate learning in rhesus monkeys. Psychopharmacology (Berl) 2002b;160:253–62. doi: 10.1007/s00213-001-0954-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Modeling a task that is sensitive to dementia of the Alzheimer's type: individual differences in acquisition of a visuo-spatial paired-associate learning task in rhesus monkeys. Behav Brain Res. 2004;149:123–33. doi: 10.1016/s0166-4328(03)00214-6. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–50. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Taylor DA, Fennessy MR. “Antagonist”-precipitated withdrawal in the rat after chronic delta9-tetrahydrocannabinol treatment. J Pharm Pharmacol. 1978;30:654–6. doi: 10.1111/j.2042-7158.1978.tb13353.x. [DOI] [PubMed] [Google Scholar]
- Von Huben SN, Davis SA, Lay CC, Katner SN, Crean RD, Taffe MA. Differential contributions of dopaminergic D(1)- and D(2)-like receptors to cognitive function in rhesus monkeys. Psychopharmacology (Berl) 2006 doi: 10.1007/s00213-006-0347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LH. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res Cogn Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Freedland CS, Porrino LJ. Metabolic mapping of the time-dependent effects of delta 9-tetrahydrocannabinol administration in the rat. Psychopharmacology (Berl) 2002;161:129–36. doi: 10.1007/s00213-002-1001-x. [DOI] [PubMed] [Google Scholar]
- Wilson WH, Ellinwood EH, Mathew RJ, Johnson K. Effects of marijuana on performance of a computerized cognitive- neuromotor test battery. Psychiatry Res. 1994;51:115–25. doi: 10.1016/0165-1781(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Lambert P, Moerschbaecher JM. Cannabinoid ligands and their effects on learning and performance in rhesus monkeys. Behav Pharmacol. 1999;10:497–511. doi: 10.1097/00008877-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Molina PE, Amedee AM, Filipeanu CM, McGoey RR, Troxclair DA, Walker EM, Birke LL, Stouwe CV, Howard JM, Leonard ST, Moerschbaecher JM, Lewis PB. Tolerance to chronic delta-9-tetrahydrocannabinol (Delta-THC) in rhesus macaques infected with simian immunodeficiency virus. Exp Clin Psychopharmacol. 2011;19:154–72. doi: 10.1037/a0023000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg B, Glick SD, Jarvik ME. Impairment of recent memory by marihuana and THC in rhesus monkeys. Nature. 1971;233:343–5. doi: 10.1038/233343a0. [DOI] [PubMed] [Google Scholar]