Abstract
Recombinant, immunodominant antigens derived from Mycobacterium tuberculosis can be used to effectively vaccinate against subsequent infection. However, the efficacy of these recombinant proteins is dependent on the adjuvant used for their delivery. This problem affects many potential vaccines, not just those for tuberculosis, so the discovery of adjuvants that can promote the development of cell-mediated immunity is of great interest. We have previously shown that the combination of the cationic surfactant dimethyl dioctadecyl ammonium bromide and the immunomodulator modified lipid A synergistically potentiates Th1 T-cell responses. Here we report a screening program for other adjuvants with reported Th1-promoting activity and identify a second novel adjuvant formulation that drives the development of Th1 responses with an extremely high efficacy. The combination of dimethyl dioctadecyl ammonium bromide and the synthetic cord factor trehalose dibehenate promotes strong protective immune responses, without overt toxicity, against M. tuberculosis infection in a vaccination model and thus appears to be a very promising candidate for the development of human vaccines.
The obligatory intracellular pathogen Mycobacterium tuberculosis, the causative agent of human tuberculosis (TB), is still among the infectious agents with the most devastating impact globally, despite intense research into the disease. One-third of the world's population is thought to be infected with the bacterium and is at risk of developing the disease, which claims more than two million lives each year (59). The situation is further worsened by the advent of the HIV epidemic and the emergence of multidrug-resistant strains of M. tuberculosis.
The only commercially available vaccine, Mycobacterium bovis BCG, is the most widely used vaccine in the world today, with BCG immunizations being used in 172 countries (16). This tremendous vaccine “success” contrasts sharply with the picture drawn from several clinical trials conducted to test the efficacy of BCG. These studies show an unacceptably large variation in the protective effect of the vaccine, ranging from 0 to 80%, and a meta-analysis of the trials concluded that BCG is ineffective in protecting adults from developing disease (17). Potential explanations for this lack of effectiveness include the increasing attenuation of the BCG strain (9), the waning of vaccine efficacy over time (24, 57), and differences in the genetic backgrounds of the tested populations and their levels of exposure to environmental mycobacteria (12, 25). Whatever the reason, a new and more effective TB vaccine remains an urgent international research priority.
The ability of the host to control infection with M. tuberculosis resides in the ability to mount an effective cellular immune response (28, 42). Thus, for a vaccine to be protective, the induction of a type 1 (Th1) response is essential. In this regard, the key cytokine in mice (18, 26) and also in humans (19, 34) seems to be gamma interferon (IFN-γ), which activates bactericidal effector mechanisms in the mycobacterial host cell, the macrophage. These include the production of reactive oxygen and nitrogen intermediates, such as hydrogen peroxide and nitric oxide (14, 20). Additionally, phagolysosomal fusion gives rise to intense enzymatic activity and acidification of the vacuole. By these mechanisms, the activated macrophage combats the highly resistant intracellular pathogen, and without IFN-γ, effective macrophage function is greatly restricted. IFN-γ thus serves as the primary marker for effective antimycobacterial immunity.
The subunit vaccine approach builds on the concept of boosting the immune response to selected antigens delivered in the form of a well-defined recombinant antigen. Recent research into antigens for use in TB subunit vaccines has been focused on antigens from culture filtrates, and more specifically, on secreted proteins from the culture filtrate (3). This has led to the discovery of immunodominant antigens such as the 6-kDa early secretory antigenic target (ESAT-6) and antigen 85B (Ag85B). When administered as a vaccine, either of these antigens can induce significant immunity against subsequent TB infection, and a fusion molecule containing both is even more effective (4, 11, 47). Compared to live vaccines based on whole mycobacteria, these simple subunit constructs have great advantages when it comes to safety and quality control. However, whereas mycobacterial whole-cell vaccines are often very immunogenic, due to the presence of immunostimulatory molecules such as lipoarabinomannan in the mycobacterial cell wall, subunit vaccines rely on effective adjuvants to elicit the appropriate immunity, since the simplified (synthetic, recombinant, and/or highly purified) antigenic components of the vaccine mostly lack immunogenicity by themselves.
A large amount of research has been devoted to the development of adjuvants for human use. Unfortunately, this has proven to be an extremely difficult task, since the properties of effective immunomodulating components are rarely congruent with the strict international regulations concerning vaccine toxicity. Therefore, only very few of the adjuvant vehicles in testing for subunit delivery today are expected to be used in future human vaccines (48). In light of this, it is disappointing that the only adjuvants currently approved for use in humans (alum and MF59) generally promote Th2 immune responses (40, 55).
Dimethyl dioctadecyl ammonium bromide (DDA) is a cationic, micelle-forming surfactant with a weak Th1-stimulating effect, and it has been reported to promote protective immunity in mice when tested as an adjuvant for a TB subunit vaccine (40). In an attempt to reach higher levels of protection, several immunomodulators have been tested in combination with DDA. The addition of cytokines such as IFN-γ, interleukin-1 (IL-2), and IL-12 did not alter the long-term effect of the vaccine (40, 54). However, a significant improvement was achieved when the inflammation-promoting molecule monophosphoryl lipid A (MPL) was added to a DDA-based TB subunit vaccine (11). Inspired by this finding, we designed the present study to search for other immunomodulators to include in a future TB subunit adjuvant formulation. The work presented here suggests that DDA mixed with trehalose 6,6′-dibehenate (TDB) makes an effective TB subunit adjuvant with the ability to raise a high level of protective memory immunity comparable to that found in BCG-vaccinated mice.
MATERIALS AND METHODS
Animals.
All experiments were performed with female pathogen-free C57BL/6 mice of 6 to 8 weeks of age purchased from M&B, Ry, Denmark. The mice were housed in isolator cages and were allowed a 1-week rest period after delivery before the initiation of experiments. M. tuberculosis-infected animals were kept in a separate biosafety level 3 facility.
Vaccine antigens.
Recombinant ESAT-6 and Ag85B-ESAT-6 were produced in Escherichia coli and were purified as previously described (13, 47). The lipopolysaccharide content in the antigen preparations was measured by the Limulus amoebocyte lysate test and was shown to be below 0.3 ng/μg of protein. This concentration had no influence on cellular activity (data not shown). The antigen preparations were kept at −80°C until use. Both Ag85B-ESAT-6 and ESAT-6 have been titrated across the range of 0.01 to 100 μg and were used in these studies at 1 and 10 μg per immunization, respectively, which are doses repeatedly shown to induce strong immune responses in previous studies by our group (11, 22, 38, 47).
Adjuvant formulations.
DDA (Eastman Kodak, Inc., Rochester, N.Y.) was mixed into sterile distilled water (dH2O) to a concentration of 2.5 mg/ml, heated to 80°C while being stirred continuously on a magnetic hot plate for 20 min, and then cooled to room temperature before use. This adjuvant was delivered at 250 μg/dose, based on results from previous titration series (data not shown).
Saponin (Sigma Biochemicals, St. Louis, Mo.) was dissolved in dH2O to a concentration of 1 mg/ml. The solution was mixed by vortexing for 30 s and was kept at 4°C until use. Based on studies of the literature, a titration study was performed and data from the most effective concentration (15 μg of saponin per immunization) are presented here.
1,25-Dihydroxyvitamin D3 (Calcitriol; Sigma Biochemicals) was dissolved in dH2O to a concentration of 10 μg/ml. The mixture was vortexed for 30 s and stored at 4°C until use. This antigen was also titrated, and the best results were obtained with 0.3 μg of Calcitriol per injection.
β-Glucan from Saccharomyces cerevisiae (Sigma Biochemicals) was dissolved in dH2O to different concentrations and vortexed for 30 s just prior to use. Peak responses were found with 7.5 μg of β-glucan per immunization.
n-Hexadecane (Sigma Biochemicals) was dissolved in dH2O to the desired concentration and vortexed for 30 s just prior to use. The data presented are based on the optimal dose for our studies of 300 μg of n-hexadecane per immunization.
TDB (Sigma Biochemicals) was suspended in dH2O containing 2% dimethyl sulfoxide to a concentration of 5 mg/ml by repeated passaging through a fine-tipped pipette followed by 30 s of vortexing. This step was repeated three times before freezing the solution at −20°C until use. TDB was tested in a twofold titration from 10 to 600 μg/dose. The best results were obtained with 300 μg of TDB per immunization.
N-Acetylmuramyl-l-alanyl-d-isoglutamine (or muramyl dipeptide [MDP]) (Sigma Biochemicals) was solubilized in dH2O to a concentration of 1 mg/ml. The solution was then mixed by vortexing for 60 s and was stored at 4°C until use. The highest-level responses were observed when we used 150 μg of MDP per immunization.
MPL (Corixa, Seattle, Wash.) was mixed into dH2O containing 2 μl of triethylamine/ml to a concentration of 1 mg/ml. The mixture was heated in a 65 to 70°C water bath for 30 s and then sonicated for 30 s. The heating and sonication procedure was repeated twice. The solution was stored at 4°C until use. Based on results from prior titration experiments, MPL was used at 25 μg/dose.
Immunization protocol for subunit vaccines.
For immunization, the antigen was diluted in saline, followed by the addition of DDA and the immunomodulator for testing. The mixture was then vortexed for 30 s, kept at room temperature for half an hour, and then vortexed once more immediately prior to vaccination.
Mice were injected subcutaneously (s.c.) just above the base of the tail (200 μl/dose). Since the protocol was intended for the detection of adjuvant effects, in the majority of experiments it was decided to immunize the mice twice (with a 2-week interval between injections) with an antigen of low immunogenicity (ESAT-6). This protocol had previously been tested and found to give significant, but not maximal, immune responses with DDA, thus providing a window in which the effect of any additional components could be detected. Immune responses primed by the vaccines were investigated 1 and 3 weeks after the final immunization. Six weeks after the final vaccination, the remaining mice in each group received an aerosol challenge infection. In some experiments, the immune response at 3 weeks postchallenge was also investigated. Six weeks after challenge, mice were sacrificed and spleens and lungs were removed for bacterial enumeration.
BCG vaccination.
A group of BCG-vaccinated mice was included in each immunization experiment as a control for vaccine efficacy. The mice received 5 × 104 CFU of BCG Danish 1331 s.c. in 0.2 ml of 0.9% saline at the same time that the remaining test groups received their first immunization with experimental subunit vaccines. No booster injections were given.
Spleen and lung lymphocyte cultures.
To obtain single-cell suspensions, each organ was homogenized by maceration through a fine mesh of stainless steel into complete RPMI (RPMI 1640 medium supplemented with 1% penicillin-streptomycin [Gibco BRL Life Technologies], 5 × 10−5 M 2-mercaptoethanol, 5% fetal calf serum, and 1% nonessential amino acids [FLOW; ICN Biochemicals]). Cells were recovered by centrifugation at 500 × g for 5 min and were counted. An aliquot was diluted to 2 × 106 cells/ml, and the cells were dispensed in 100-μl volumes into 96-well flat-bottom plates (Nunc, Roskilde, Denmark) containing twofold serial dilutions in triplicate of either ESAT-6 (from 10 μg/ml), Ag85B-ESAT-6 (from 5 μg/ml), or phytohemagglutinin (at 2 μg/ml) in 100 μl of complete RPMI medium. Cultures were incubated at 37°C in 5% CO2 and 90% humidity for 3 days before the removal of the supernatant for cytokine determination by enzyme-linked immunosorbent assay (ELISA). In the larger screening experiments, a pool of cells from four mice was used. Otherwise, organs from each mouse were tested individually (four mice per group).
Peripheral blood lymphocyte cultures.
For peripheral blood lymphocyte cultures, mice were bled and the blood was transferred to EDTA tubes and diluted 1:1 in 0.9% saline. The blood suspension was carefully layered on top of 5 ml of Lympholyte-Mammal (Cedarlane, Ontario, Canada) in 15-ml tubes (Nunc) that were precoated with 0.9% saline supplemented with 20% fetal calf serum. After layering, the tubes were centrifuged at 750 × g for 20 min with the brake off to separate lymphocytes from erythrocytes and dead cells. Lymphocytes were carefully retrieved, washed once in medium, and resuspended for counting and plating as described previously.
ELISA for IFN-γ.
A double-sandwich ELISA method was used to quantify the levels of IFN-γ in triplicate titrations of culture supernatants. Microtiter plates (96 wells) (Maxisorb; Nunc) were coated with a hamster anti-murine IFN-γ monoclonal antibody (Genzyme, Cambridge, Mass.) in phosphate-buffered saline (PBS) at 4°C. Free binding sites were blocked with 1% (wt/vol) bovine serum albumin-0.05% Tween 20. IFN-γ was detected with a biotin-labeled rat anti-murine monoclonal antibody (clone XMG1.2; Pharmingen, San Diego, Calif.). Concentrations of IFN-γ in the samples were calculated by using a standard curve generated from recombinant IFN-γ (Life Technologies), and results are expressed in nanograms per milliliter. The differences between the triplicate wells were consistently <10% of the mean.
FACS analysis of lymphocytes.
Lymphocytes from vaccinated and control mice were isolated as described above from lungs that were perfused with 15 ml of cold PBS containing 50 U of heparin sulfate/ml. Cells were resuspended in complete RPMI medium at 2 × 106 cells/ml and transferred in 100-μl volumes in triplicate to 96-well round-bottom plates with 100 μl of medium containing 5 μg of Ag85B-ESAT-6/ml. Control wells without antigen were also prepared. Cells were cultured with antigen in vitro for 72 h before staining. Although not a direct ex vivo picture of the immune status in the mice, earlier studies had shown that this protocol improved the sensitivity of the assay for detecting even small differences in cell populations among groups (unpublished data) over that of the shorter stimulation that was previously used (22). After 72 h of incubation, cells were stained for the surface expression of CD4 and CD8 in addition to intracellular IFN-γ. All fluorescence-activated cell sorting (FACS) procedures were performed in accordance with the protocol supplied by the manufacturer of the labeled antigens and the kit components used (BD Pharmingen).
Infection.
M. tuberculosis (Erdman) for infection was grown by the culturing of bacteria from infected organs at 37°C in Löwenstein-Jensen medium or in suspension in modified Sauton medium enriched with 0.5% sodium pyruvate and 0.5% glucose as previously described (5). Challenge infections with M. tuberculosis were administered by the aerosol route in a Glas-Col inhalation exposure system with an inoculum of ∼30 to 40 CFU per mouse. The mice were sacrificed 6 weeks after the challenge infection, and bacterial numbers in the lungs were determined by macerating the organs and plating serial threefold titrations on Middlebrook 7H11 agar plates. As controls, organs from unvaccinated and BCG-vaccinated mice were also grown on 7H11 plates supplemented with 2-triophene-carboxylic acid hydrazide to selectively inhibit the growth of BCG. After 2 weeks of incubation at 37°C, the numbers of bacteria were counted and reported in CFU. The protective efficacy of the vaccinations was determined as the log10 reduction in bacterial numbers in vaccinated groups compared to the unvaccinated control group. All results are based on individual analyses of four to six mice per group.
Statistical analysis.
The data obtained were tested by one-way analysis of variance (ANOVA). Differences between means were assessed by Student's t test for IFN-γ assays, whereas Dunnett's method was used to assess log-transformed CFU data. In both instances, P values of <0.05 were considered significant.
RESULTS
Adjuvant screening based on suboptimal immunization principles.
A panel of adjuvants was tested in combination with the well-characterized protein ESAT-6 as a model antigen. While it is an effective vaccine candidate if delivered with a strongly Th1-promoting adjuvant, ESAT-6 has a relatively low inherent immunogenicity and its efficacy is therefore dependent on the adjuvant chosen (11), thereby facilitating screening. Moreover, the dose of antigen was reduced, since high antigen doses could potentially overcome weaknesses among the adjuvants tested. To further increase the sensitivity, only two immunizations were used, as opposed to the three injections normally given to generate an optimal recall response when testing vaccines. Adjuvant formulations were based on DDA mixed with different immunomodulators (Table 1), which were selected based either on their expected activating effect on antigen-presenting cells (APCs) or on literature citations suggesting their ability to promote Th1 responses. Injections were delivered s.c., with a 2-week interval between them, and the magnitudes of the immune responses induced were assessed by ELISA analysis of IL-4 and IFN-γ produced by in vitro restimulation. IL-4 levels were uniformly low (data not shown), so since IFN-γ is essential for protection against infection with M. tuberculosis and is also a good indicator of vaccine immunogenicity, we used this Th1 cytokine as the readout in the screening. It is clear that IFN-γ is not, by itself, predictive of a vaccine's protective efficacy (38). However, IFN-γ production is, by definition, a good measure of a vaccine's ability to induce a Th1 response.
TABLE 1.
Immunomodulators chosen for their expected Th1-promoting abilities and tested in combination with DDA
| Immunomodulator | Biological activity | Reference |
|---|---|---|
| Saponin | Plant-derived extract with delayed type hypersensitivity-inducing qualities | 10 |
| Calcitriol | Biologically active form of vitamin D3 with macrophage-activating abilities | 1, 50 |
| β-Glucan | Effective in activating macrophage functions | 30 |
| n-Hexadecane | Strong inducer of proinflammatory responses | 41 |
| TDB | Synthetic analog of the potent immunostimulator TDM (cord factor); TDB has shorter mycolic acids and is therefore expected to show less toxicity | 46, 49 |
| MDP | The smallest unit of bacterial cell walls that can replace mycobacteria in complete Freund’s adjuvant; has the ability to regulate several functions of cells involved in the immune response, including macrophage activation | 7, 44 |
| MPL | Induces the synthesis and release of cytokines, particularly IL-2 and IFN-γ, thereby promoting Th1 responses | 58 |
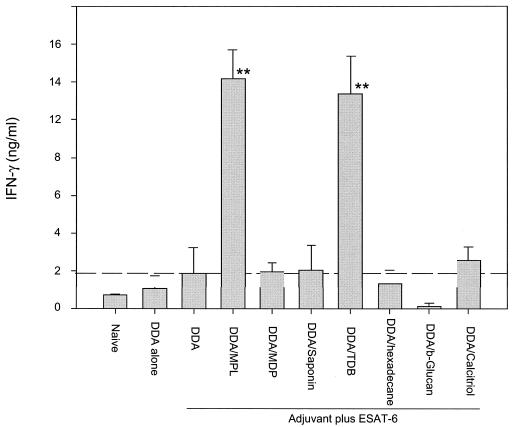
Lymphocytes from mice immunized with either ESAT-6 without adjuvant (data not shown) or DDA alone (Fig. 1) did not produce significantly more IFN-γ in vitro than lymphocytes from unimmunized (naïve) mice. ESAT-6 combined with DDA induced a small but not significant increase in IFN-γ compared to naïve mice. Of the seven immunomodulators tested in combination with DDA, only MPL and the synthetic cord factor TDB were able to increase the IFN-γ response significantly (P < 0.01, in both cases) above that induced by the DDA-ESAT-6 vaccine (Fig. 1). The individual immunomodulators were also tested with ESAT-6, but without DDA, to investigate their own adjuvant effects in this model. While several of them induced increased immune responses (detectable as IFN-γ in the culture supernatants), as would be expected based on the published literature, none of the tested immunomodulators (including MPL and TDB) reproducibly induced higher levels of IFN-γ than the relatively modest levels seen with DDA-ESAT-6 (data not shown). Thus, only TDB and MPL stood out as effective Th1-inducers, and this was only when they were combined with the micelle-forming surfactant DDA.
FIG. 1.
Effective induction of Th1 responses with DDA-based adjuvant formulations is dependent on efficient immunomodulators. IFN-γ (in nanograms per milliliter) from restimulated PBMCs was measured as an indicator of systemic immune responses. Mice (four animals per group) were bled 1 week after the final immunization, and PBMCs were pooled within groups and restimulated with the vaccine antigen in triplicate wells. The figure shows representative data from three separate experiments. Values are the means of triplicate assays ± standard errors of the means. The level of response observed with the DDA-ESAT-6 combination is indicated by the dotted line for ease of comparison. **, values significantly above this level (P < 0.01).
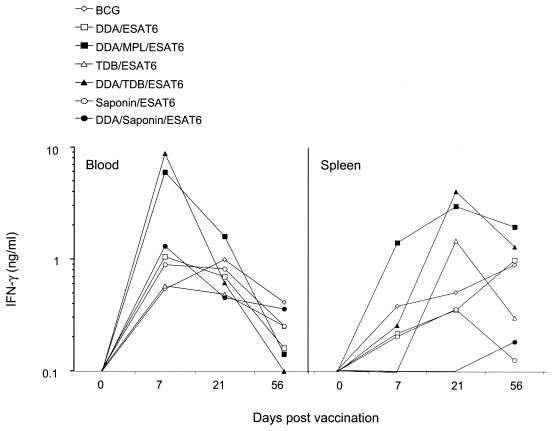
To ensure that the results obtained reflected the overall immunogenicity of the vaccines and not merely differences in kinetics, the ability of the adjuvants to induce IFN-γ responses was investigated in the blood and the spleen 1, 3, and 8 weeks after the final immunization, with multiple combinations of antigen and adjuvant, including both the strongly Th1-promoting DDA-MPL and DDA-TDB combinations in addition to the weakly Th1-promoting DDA-saponin combination. Figure 2 presents representative data showing that for six different combinations, and also for the live BCG vaccine, responses peaked rapidly in the blood after the second vaccination but dropped to low levels by 8 weeks after vaccination. In the spleen, peak responses were obtained at 3 weeks postvaccination, but by 8 weeks postvaccination these had either fallen or remained at a similar level (not significantly different) (Fig. 2). Similar data were obtained with all of the adjuvants tested (data not shown). Thus, peripheral blood mononuclear cell (PBMC) responses 1 week after vaccination were chosen for all further analyses.
FIG. 2.
Kinetics of the IFN-γ response in C57BL/6 mice immunized with subunit vaccines. Mice were immunized with either BCG or ESAT in a variety of adjuvants, and IFN-γ from the restimulation of PBMCs (in nanograms per milliliter) was measured as an indicator of systemic immune responses. Mice (four animals per group) were used for tissue samples either the day before the first vaccination (day −1), 1 week after the final vaccination (day 7), 3 weeks after the final vaccination (day 21), or 8 weeks after the final vaccination (day 56). Cells from blood or spleen were pooled within groups and restimulated in triplicate wells. Kinetic studies were performed with spleen and blood cells for all adjuvants assessed, using at least two time points after vaccination, with comparable results.
While IFN-γ induction after vaccination has proven to be a good marker of immunogenicity (and thus of adjuvant effectiveness), the real test of vaccine efficacy is the ability to restrict bacterial growth in vivo. We therefore exposed animals to an aerosol infection with M. tuberculosis, as previously described (11, 47), 6 weeks after the final immunization. A control group that was vaccinated with BCG was included. Animals were sacrificed 6 weeks after infection, and the numbers of bacteria in the lungs were enumerated. As shown in Table 2, the only adjuvant combinations that stimulated effective immunity when combined with ESAT-6 were DDA-MPL and DDA-TDB. Neither adjuvant nor antigen alone induced significant levels of protection (data not shown). We thus proceeded to further characterize the two most effective adjuvant systems, DDA-MPL and DDA-TDB.
TABLE 2.
Protective efficacy of ESAT-6 subunit vaccine delivered in different adjuvant combinations
| Vaccine | Log10 CFU of M. tuberculosis | Protectiona (log10 reduction in bacterial load |
|---|---|---|
| Naïve | 5.85 ± 0.12 | NA |
| BCG | 5.13 ± 0.09 | 0.71 ± 0.12** |
| DDA | 5.97 ± 0.13 | <0 |
| DDA-ESAT-6 | 5.69 ± 0.08 | 0.16 ± 0.14 |
| DDA-MPL-ESAT-6 | 5.40 ± 0.11 | 0.44 ± 0.12* |
| DDA-MDP-ESAT-6 | 6.08 ± 0.13 | <0 |
| DDA-saponin-ESAT-6 | 5.83 ± 0.13 | 0.02 ± 0.19 |
| DDA-TDB-ESAT-6 | 5.28 ± 0.14 | 0.57 ± 0.16* |
| DDA-hexadecane-ESAT-6 | 5.54 ± 0.11 | 0.31 ± 0.16 |
| DDA-β-glucan-ESAT-6 | 5.57 ± 0.10 | 0.28 ± 0.16 |
| DDA-Calcitrio-ESAT-6 | 5.49 ± 0.08 | 0.36 ± 0.18 |
Protective efficacy of the vaccines, expressed as the log10 reduction in bacterial load in the lung, compared to mice in the unimmunized group (naïve). Results presented are from titrations in triplicate of a minimum of four mice per group (analyzed individually) and represent means from four separate experiments ± standard errors of the means. NA, not applicable. Values significantly different from the unimmunized control are marked (*, P < 0.05 **, P < 0.01).
Comparison of immunomodulators MPL and TDB.
While some concerns have been raised about the potential toxicity of MPL in vivo, until now it has remained the adjuvant which (when mixed with DDA) best potentiates protective Th1 responses against M. tuberculosis infection (11, 47). For determination of whether TDB could function as effectively as MPL as a Th1-inducing component in a DDA-based adjuvant formulation, a comparison was set up in a challenge infection model. Moreover, the potential benefit from combining all three components in one adjuvant formulation was also investigated. Thus, mice were immunized as described above, followed by a rest period of 6 weeks before the animals were exposed to aerosol infection with M. tuberculosis. Also as before, bacterial numbers in the lungs 6 weeks after infection were used as a measure of protective efficacy and IFN-γ produced by PBMC in response to in vitro restimulation 1 and 6 weeks after vaccination was used as a measure of immunogenicity.
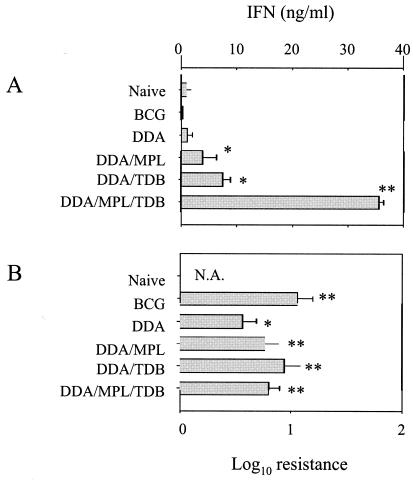
Initial results with the DDA-MPL-TDB combination were very encouraging. One week after vaccination, lymphocytes isolated from mice having received this adjuvant together with ESAT-6 produced very high levels of IFN-γ (Fig. 3A), although, as in the previous experiments, this level had fallen after 6 weeks in all groups (data not shown). Judging from the IFN-γ levels, this triple adjuvant formulation seemed superior to any of the other adjuvant combinations. However, it can be seen that although the DDA-MPL-TDB adjuvant plus ESAT-6 induced a level of protection in the lungs (Fig. 3B), this level was not significantly different from that obtained with DDA-MPL or DDA-TDB, despite the strong initial IFN-γ release induced by the vaccine with DDA-MPL-TDB as adjuvant. This may reflect the limitations of the mouse model: it is not clear how much further the bacterial load can be reduced, even if an optimal immune response is generated. As previously noted, although the induction of IFN-γ seems necessary for good protection, there is not an absolute correlation between levels of vaccine-induced IFN-γ and the level of protection obtained (2, 38). Consistent with this, increasing the number of vaccinations also boosted the level of IFN-γ produced after vaccination, but it did not significantly improve protection (data not shown). This is especially true at higher levels of IFN-γ production, suggesting that once a threshold level of this cytokine is reached, excess IFN-γ may not be beneficial, at least in the short term. Nonetheless, it is clear from these data that the addition of powerful immunomodulators such as TDB or MPL enhances the effect of DDA itself. Moreover, the protection induced by the subunit vaccines was comparable to that induced by BCG at the primary infection site, namely the lungs.
FIG. 3.
Efficacy of MPL and TDB as immunomodulators in an ESAT-6 subunit vaccine. Testing of MPL and TDB in combination revealed a strong additive potential as assessed by IFN-γ production, but this was not reflected in a correspondingly high level of protection. (A) Systemic immune responses determined by IFN-γ production after in vitro restimulation with ESAT-6 of PBMCs harvested from mice (four per group) 1 week after the final immunization (blood was pooled within groups). Results are presented as means of triplicate wells ± standard deviations. (B) Protective efficacy of the vaccines, expressed as the log10 reduction in bacterial load in the lung, compared to the unimmunized group (naïve). Results are from four mice per group, assayed individually. N.A., not applicable. * and **, values significantly different from the naïve control (P < 0.05 and P < 0.01, respectively).
DDA-TDB as a delivery vehicle for the fusion molecule Ag85B-ESAT-6.
Given the demonstrated ability of DDA-TDB to drive a Th1 response against the M. tuberculosis-specific ESAT-6 antigen and to generate a protective immune response, the potential of this adjuvant formulation was tested together with the more immunogenic fusion protein Ag85B-ESAT-6 (47). Groups of mice were immunized twice s.c. with 1 μg of antigen at 2-week intervals. The relative protective potentials of the experimental vaccination regimens were investigated in parallel with a standard BCG vaccination.
As shown in Table 3, IFN-γ levels measured after antigenic restimulation in vitro clearly showed the effective priming of an antigen-specific response when Ag85B-ESAT-6 was delivered in DDA-TDB (P < 0.001). In contrast, no responses were found in mice that received the vaccine with no adjuvant or in mice of the adjuvant control group. While these values (measured after vaccination, but before infection) are a good measure of immunogenicity, measuring IFN-γ levels after infection may be more relevant for protection. Unfortunately, antigen-specific immune responses in the blood after infection are relatively low for the first 7 to 10 days (data not shown), and differentiating between primary and memory responses becomes problematic thereafter. We therefore used the more sensitive FACS analysis to measure IFN-γ production after infection (Table 3) and to investigate if immune responses in the blood correlate with T-cell recruitment to the site of infection.
TABLE 3.
IFN-γ production and number of IFN-γ-producing CD4+ and CD8+ lymphocytes correlated to the level of protection in the lungs
| Vaccine | Amt of IFN-γa (pg/ml) | No. of IFN-γ+ T cells in lungb
|
Log10 resistance (CFU)c | |
|---|---|---|---|---|
| CD4+ | CD8+ | |||
| Naïve (not infected) | 96 | 0.64 | 0.26 | NA (NA) |
| Unvaccinated | 96 | 4.63 | 1.61 | NA (5.07 ± 0.11) |
| BCG | 125 | 2.87 | 2.43 | 0.78* (4.29 ± 0.16) |
| Ag85B-ESAT-6 | 183 | 5.01 | 3.24 | <0 (5.17 ± 0.09) |
| DDA-TDB | 108 | 5.09 | 2.29 | <0 (5.22 ± 0.02) |
| DDA-TDB-Ag85B-ESAT-6 | 15,180** | 14.87 | 5.88 | 0.87* (4.20 ± 0.10) |
PBMCs were assayed for IFN-γ at the peak of the response (1 week after the last vaccination). IFN-γ levels were measured in culture supernatants after 3 days of restimulation with the vaccine antigen (Ag85B-ESAT-6 [2.5 μg/ml]).
Lymphocyte numbers represent the percentages of IFN-γ+ cells in CD4+ and CD8+ gated lymphocyte populations isolated from lungs 3 weeks after aerosol infection. The cells were stimulated with antigen in vitro for 72 h before flow cytometric analysis.
Protective efficacy of the vaccines is expressed as the log10 reduction in bacterial load in the lungs compared to mice in the unimmunized group (naïve) from a minimum of four mice per group, assayed individually at 6 weeks postinfection. NA, not applicable. Values significantly different from mice in the naïve group are marked (*, P < 0.05, **, P < 0.001). Data are means ± standard errors of the means.
The immune responses induced by the vaccines were also investigated directly in the lungs by monitoring the expression of the T-cell surface markers CD4 and CD8 plus the intracellular expression of IFN-γ in lymphocytes. Based on earlier kinetics studies (22), lungs were taken 3 weeks after infection and blood lymphocytes were cleared by perfusion with cold, heparin-containing PBS in order to obtain only lung-infiltrating T cells for restimulation with antigen and FACS analysis. Table 3 shows the percentages of IFN-γ+ cells found within populations of such restimulated lymphocytes gated for CD4 or CD8 expression. These data showed that two immunizations with the fusion antigen delivered in DDA-TDB increased the percentages of CD4+ IFN-γ+ and CD8+ IFN-γ+ cells in the lung cultures more than threefold compared to unvaccinated mice after infection and more than 20 times compared to uninfected control animals. Neither the adjuvant control nor Ag85B-ESAT-6 alone seemed to stimulate a notable enlargement of the IFN-γ-producing CD4+ or CD8+ population, although a slight increase in IFN-γ+ CD8+ cells was observed in the lungs of mice immunized with only the fusion antigen compared to unvaccinated animals. The reason for the relatively low numbers found in the BCG-vaccinated group despite its protective efficacy is not clear, but we have made this observation repeatedly (unpublished data). Moreover, the numbers correlate with the low IFN-γ response in this group of mice. As seen in Table 3, mice that received two immunizations of Ag85B-ESAT-6 in DDA-TDB or a vaccination with BCG were significantly better protected than unvaccinated animals (P < 0.05). While the level of protection in the lung was highest in DDA-TDB-Ag85B-plus-ESAT-6-vaccinated mice, it was not significantly different from that observed in BCG-vaccinated animals.
DISCUSSION
Much effort has been expended on the development of a TB subunit vaccine with a higher protective efficacy than the controversial BCG vaccine. However, since several potential vaccine antigens have already been identified and are in the process of being approved for human clinical trials (21), the vaccine component mostly needed today is an effective adjuvant. Thus, this study was set up to test a panel of adjuvant formulations built on a combination of DDA and selected immunomodulators, thereby taking one step further towards the development of a useful Th1-inducing delivery system.
Built upon knowledge from the past decade of adjuvant research and the many years of research into TB immunology, this study aimed to combine potent Th1-inducing immunomodulators with a surface-active agent (DDA) that forms depots to retain the TB subunit antigen(s). The rationale was to optimize antigen acquisition by APCs in an environment that would potentiate their ability to produce Th1-promoting cytokines. The goal was to achieve a high level of protective immunity against an aerosol M. tuberculosis infection based on a strong cell-mediated immune response in immunized mice. Although both MPL and DDA have been tested as human adjuvants in the past, the doses required to attain effective immunity have caused lingering concerns over toxicity, making the synergy shown here extremely attractive.
Since the potential of DDA as an adjuvant in TB subunit vaccines has already been demonstrated (11, 40), this micelle-forming, weak Th1 adjuvant was selected for the present study as the foundation of the adjuvant formulations that were designed and tested. When injected subcutaneously, DDA forms depots which last up to 60 days in mice without any evidence of overt toxicity (data not shown). It is believed that the antigen and DDA interact in a way that ensures the long-term release of antigen, perhaps by incorporation of the antigen into DDA micelles, thereby ensuring that APCs activated by the adjuvant are also exposed to the associated antigen. It is also possible that the surfactant ability of DDA accelerates the uptake of the antigen and immunomodulator. Cationic lipids have long been used as an aid to transfection for this reason (32, 52). Several reports have provided good evidence for the benefits of such depot effects in other adjuvant delivery systems (15, 27, 31). Since DDA by itself is only a weak Th1 promoter (11, 40) (also confirmed by data presented here), the potential for developing a more effective delivery system based on the principle of an uptake-promoting matrix or depot of fine particles (provided by the DDA micelles) carrying both antigen and an immunomodulator was therefore investigated. The present study clearly shows that Th1-promoting immunomodulators mixed with an antigen were unable to elicit strong antigen-specific responses unless they were coadministered with DDA. In light of this, the exact interaction between the antigen, immunomodulator, and DDA is now being investigated in our laboratory.
Seven different immunomodulators were tested for the ability to improve the adjuvanticity of DDA. One of the modulators was a plant-derived extract, saponin, with proven cell-mediated immunity-promoting qualities (10). However, when tested in combination with the poorly immunogenic ESAT-6, either alone (Fig. 2) or mixed with DDA (Fig. 1 and 2), saponin was unable to induce an IFN-γ response above the level seen in mice immunized with only DDA and ESAT-6. Recently, purified fractions of saponin derived from the bark of Quillaja saponaria have been produced. The unique feature of these substances is their ability to facilitate the development of cytotoxic T lymphocyte responses (45). The most promising saponin fraction described so far, QS-21, has been found to stimulate Th1 cytokines (IL-2 and IFN-γ) and antibodies of the IgG2a isotype in addition to strong cytotoxic T lymphocyte responses (35, 56). While saponin did not induce notable immune responses when combined with ESAT-6, very potent IFN-γ responses were evoked with Ag85B-ESAT-6 (data not shown), making it tempting to speculate that the fusion molecule holds peptides for major histocompatibility complex (MHC) class I presentation which are not found on the ESAT-6 antigen alone. However, the specific epitopes to which CD8+ T cells respond are thus far poorly characterized, although a recent study emphasized the importance of CD8+ T cells in protective memory responses against M. tuberculosis (53). The identification of potential CD8+-T-cell epitopes has lately gained much interest and could lead to the discovery of new antigens for a future TB subunit vaccine. Thus, in addition to peptide and epitope studies, two purified saponin fractions, one of which is an analogue to QS-21, are now being tested in adjuvant studies in the hope of developing an effective delivery system which would also promote MHC class I presentation.
Although Calcitriol, the biologically active form of vitamin D, has been shown to possess macrophage-activating qualities (1, 37, 50), this immunomodulator only stimulated weak IFN-γ responses in combination with DDA and ESAT-6 (Fig. 1) and failed to induce significant protection against a subsequent challenge with M. tuberculosis (Table 2). However, DDA combined with Calcitriol at a very high dose did produce slightly higher levels of IFN-γ than DDA alone (data not shown), suggesting that there is some Th1-promoting effect of this molecule. Nonetheless, the data presented here do not suggest Calcitriol as a promising candidate for inclusion in an adjuvant formulation designed to promote Th1 responses.
The same is true for n-hexadecane, although Lorentzen found this hydrocarbon to stimulate strong proinflammatory responses in Lewis rats (41). In the present study, n-hexadecane evoked only weak ESAT-6-specific IFN-γ responses when combined with DDA (Fig. 1), and no significant protection was found in mice immunized with vaccines including this component (Table 2).
Four of the immunomodulators tested were of microbial origin and all of these were derived from cell walls (β-glucan, MDP, MPL, and TDB). Such immunogenic microbial derivatives, also referred to as pathogen-associated molecular patterns (PAMPs), are recognized by evolutionarily conserved receptors such as scavenger receptors, the mannose receptor, CD14, and Toll-like receptors, collectively named pattern recognition receptors, on the surfaces of phagocytes (33). With inclusion of such PAMPs in a subunit vaccine, it is speculated that the pattern recognition receptors, in addition to triggering innate immune responses, would also mimic signaling to lymphocytes that the vaccine antigen is associated with a microorganism and would thereby stimulate a more effective immune response (23). This probably reflects the fact that these PAMP immunomodulators are recognized by the innate immune system as danger signals and are thus able to effectively stimulate the release of proinflammatory cytokines and the up-regulation of costimulatory signals and MHC class II molecules on the surfaces of APCs.
β-Glucan binds to a lectin domain within complement receptor type 3 (also known as Mac-1 or CD11b/CD18) (51) and has been shown to be an activator of macrophage functions (30). Nevertheless, in this model, β-glucan, whether alone or with DDA, did not enhance responses above those seen with DDA and ESAT-6 (Fig. 1). Moreover, vaccines containing this immunomodulator were unable to protect animals from infection when they were challenged 6 weeks after vaccination (Table 2). Likewise, the mycobacterial cell wall component MDP did not improve the effect of DDA when tested with ESAT-6. This finding was unexpected, since MDP has proven its Th1-promoting qualities in numerous studies and has been included in several adjuvant formulations that were tested in clinical trials (55). These results may be due to the deliberately stringent screening protocol chosen to facilitate adjuvant screening, using a weak immunogen and only two immunizations, whereas other studies have naturally optimized their delivery systems. Nonetheless, based on this study, neither MDP nor β-glucan shows the same degree of promise for future TB subunit adjuvant formulations as does TDB or MPL.
The combination of DDA with either immunomodulator MPL or TDB produced consistently strong IFN-γ responses as well as high levels of protection throughout this study, even when tested with the less immunogenic antigen ESAT-6. Here we have extended the data of Brandt et al. (11), which showed the synergistic effect of the combination of DDA and MPL, and in addition present the entirely new finding that TDB, but not the other adjuvants tested, can function in a similar fashion. When the two were tested in parallel, the data indicated that TDB is at least as effective an immunomodulator as MPL when included in a DDA-based TB subunit vaccine. In addition, the DDA-TDB combination has been found to be extremely effective in the more sensitive guinea pig model of TB infection (A. W. Olsen, A. Williams, L. M. Okkels, G. Hatch, and P. Andersen, submitted for publication).
In a number of studies, MPL has been shown to induce the synthesis and release of cytokines, particularly IL-2 and IFN-γ, thereby promoting the development of Th1 responses (58). However, no immunological data of that kind have so far been published for TDB. Since cancer research in the early 1970s indicated a possible correlation between some infections and tumor suppression (8), much focus has been put on the identification of single components responsible for this effect. These studies led to the identification of the mycobacterial cell wall component trehalose dimycolate (TDM) as a tumor suppressive molecule (49). In later years, the immunomodulating effect of this highly active molecule has been determined and it has been found to activate macrophages and to stimulate a broad panel of cytokines, including IL-12, tumor necrosis factor alpha, and IFN-γ, thus driving a Th1 response (39, 60). This immunostimulatory effect has been utilized in several adjuvant formulations (36, 43), but indications are that TDM is likely to be too toxic for use in prophylactic vaccines (6, 29). Since the synthetically produced TDB, which has shorter, less saturated carbon chain fatty acids, is less toxic than TDM but has retained much of the bioactivity of the native form (46, 49), TDB was tested as a novel Th1-promoting adjuvant component in the present study. Certainly the potent IFN-γ-promoting qualities of this immunomodulator when tested in vivo suggest that TDB, like native TDM, is able to stimulate APCs to synthesize and secrete IFN-γ-promoting molecules such as IL-12 and IL-18. Further studies are needed to clarify the mechanism involved.
The adjuvant formulation DDA-TDB is now being subjected to more extensive analysis and is currently being tested in multiple mouse strains in addition to the highly susceptible guinea pig model. However, the work presented here shows that a TB subunit vaccine based on Ag85B-ESAT-6 delivered in DDA-TDB is able to induce a level of protection in aerosol-challenged mice that is comparable to that by the standard BCG vaccine. Future studies need to determine the long-term protective effect of a DDA-TDB-based vaccine as well as confirm its seemingly low toxicity, since these are both demands that need to be fulfilled for a modern TB vaccine.
Acknowledgments
This study has been supported by the Danish Research Council and The European Commission (contract number QLRT-PL1999-01093).
We thank Lene Rasmussen and Tina Lerche for excellent technical assistance.
Editor: D. L. Burns
REFERENCES
- 1.Abu-Amer, Y., and Z. Bar-Shavit. 1994. Modulation of vitamin D increased H2O2 production and MAC-2 expression in the bone marrow-derived macrophages by estrogen. Calcif. Tissue Int. 55:29-32. [DOI] [PubMed] [Google Scholar]
- 2.Agger, E. M., and P. Andersen. 2001. Tuberculosis subunit vaccine development: on the role of interferon-gamma. Vaccine 19:2298-2302. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, P. 1994. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect. Immun. 62:2536-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen, P., A. B. Andersen, A. L. Sorensen, and S. Nagai. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 154:3359-3372. [PubMed] [Google Scholar]
- 5.Andersen, P., D. Askgaard, L. Ljungqvist, M. W. Bentzon, and I. Heron. 1991. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect. Immun. 59:1558-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azuma, I., T. Sakurai, H. Ishida, T. Kitajima, and M. Yamazaki. 1991. Chemical synthesis and biological activities of 6,6′-di-O-mycoloyl-beta,beta- and -alpha,beta-trehalose. Carbohydr. Res. 212:47-53. [DOI] [PubMed] [Google Scholar]
- 7.Bahr, G. M., and L. Chedid. 1986. Immunological activities of muramyl peptides. Fed. Proc. 45:2541-2544. [PubMed] [Google Scholar]
- 8.Bast, R. C., Jr., B. Zbar, G. B. Mackaness, and H. J. Rapp. 1975. Antitumor activity of bacterial infection. I. Effect of Listeria monocytogenes on growth of a murine fibrosarcoma. J. Natl. Cancer Inst. 54:749-756. [PubMed] [Google Scholar]
- 9.Behr, M. A., and P. M. Small. 1997. Has BCG attenuated to impotence? Nature 389:133-134. [DOI] [PubMed] [Google Scholar]
- 10.Bomford, R. 1980. The comparative selectivity of adjuvants for humoral and cell-mediated immunity. II. Effect on delayed-type hypersensitivity in the mouse and guinea pig, and cell-mediated immunity to tumour antigens in the mouse of Freund's incomplete and complete adjuvants, alhydrogel, Corynebacterium parvum, Bordetella pertussis, muramyl dipeptide and saponin. Clin. Exp. Immunol. 39:435-441. [PMC free article] [PubMed] [Google Scholar]
- 11.Brandt, L., M. Elhay, I. Rosenkrands, E. B. Lindblad, and P. Andersen. 2000. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandt, L., J. Feino Cunha, A. Weinreich Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt, L., T. Oettinger, A. Holm, A. B. Andersen, and P. Andersen. 1996. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J. Immunol. 157:3527-3533. [PubMed] [Google Scholar]
- 14.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen, S., T. Yoshioka, M. Lucarelli, L. H. Hwang, and R. Langer. 1991. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm. Res. 8:713-720. [DOI] [PubMed] [Google Scholar]
- 16.Cohn, D. L. 1997. Use of the bacille Calmette-Guerin vaccination for the prevention of tuberculosis: renewed interest in an old vaccine. Am. J. Med. Sci. 313:372-376. [DOI] [PubMed] [Google Scholar]
- 17.Colditz, G. A., C. S. Berkey, F. Mosteller, T. F. Brewer, M. E. Wilson, E. Burdick, and H. V. Fineberg. 1995. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 96:29-35. [PubMed] [Google Scholar]
- 18.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jong, R., F. Altare, I. A. Haagen, D. G. Elferink, T. Boer, P. J. van Breda Vriesman, P. J. Kabel, J. M. Draaisma, J. T. van Dissel, F. P. Kroon, J. L. Casanova, and T. H. Ottenhoff. 1998. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280:1435-1438. [DOI] [PubMed] [Google Scholar]
- 20.Denis, M., E. O. Gregg, and E. Ghandirian. 1990. Cytokine modulation of Mycobacterium tuberculosis growth in human macrophages. Int. J. Immunopharmacol. 12:721-727. [DOI] [PubMed] [Google Scholar]
- 21.Doherty, T. M., and P. Andersen. 2002. Tuberculosis vaccine development. Curr. Opin. Pulm. Med. 8:183-187. [DOI] [PubMed] [Google Scholar]
- 22.Doherty, T. M., A. W. Olsen, L. van Pinxteren, and P. Andersen. 2002. Oral vaccination with subunit vaccines protects animals against aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 70:3111-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fearon, D. 1997. Happy coupling: recruiting both antigen and effector function. Nat. Biotechnol. 15:618-619. [DOI] [PubMed] [Google Scholar]
- 24.Fine, P. E. 2001. BCG: the challenge continues. Scand. J. Infect. Dis. 33:243-245. [DOI] [PubMed] [Google Scholar]
- 25.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 26.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta, R. K., B. E. Rost, E. Relyveld, and G. R. Siber. 1995. Adjuvant properties of aluminum and calcium compounds. Pharm. Biotechnol. 6:229-248. [DOI] [PubMed] [Google Scholar]
- 28.Hahn, H., and S. H. Kaufmann. 1981. The role of cell-mediated immunity in bacterial infections. Rev. Infect. Dis. 3:1221-1250. [DOI] [PubMed] [Google Scholar]
- 29.Hamasaki, N., K. Isowa, K. Kamada, Y. Terano, T. Matsumoto, T. Arakawa, K. Kobayashi, and I. Yano. 2000. In vivo administration of mycobacterial cord factor (trehalose 6,6′-dimycolate) can induce lung and liver granulomas and thymic atrophy in rabbits. Infect. Immun. 68:3704-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hetland, G., M. Lovik, and H. G. Wiker. 1998. Protective effect of beta-glucan against Mycobacterium bovis, BCG infection in BALB/c mice. Scand. J. Immunol. 47:548-553. [DOI] [PubMed] [Google Scholar]
- 31.Hilleman, M. R., A. Woodhour, A. Friedman, R. E. Weibel, and J. Stokes, Jr. 1972. The clinical application of adjuvant 65. Ann. Allergy 30:152-158. [PubMed] [Google Scholar]
- 32.Hofland, H. E., L. Shephard, and S. M. Sullivan. 1996. Formation of stable cationic lipid/DNA complexes for gene transfer. Proc. Natl. Acad. Sci. USA 93:7305-7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janeway, C. A., Jr., and R. Medzhitov. 1998. Introduction: the role of innate immunity in the adaptive immune response. Semin. Immunol. 10:349-350. [DOI] [PubMed] [Google Scholar]
- 34.Jouanguy, E., F. Altare, S. Lamhamedi, P. Revy, J. F. Emile, M. Newport, M. Levin, S. Blanche, E. Seboun, A. Fischer, and J. L. Casanova. 1996. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 335:1956-1961. [DOI] [PubMed] [Google Scholar]
- 35.Kensil, C. R., U. Patel, M. Lennick, and D. Marciani. 1991. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 146:431-437. [PubMed] [Google Scholar]
- 36.Koike, Y., Y. C. Yoo, M. Mitobe, T. Oka, K. Okuma, S. Tono-oka, and I. Azuma. 1998. Enhancing activity of mycobacterial cell-derived adjuvants on immunogenicity of recombinant human hepatitis B virus vaccine. Vaccine 16:1982-1989. [DOI] [PubMed] [Google Scholar]
- 37.Kreutz, M., R. Andreesen, S. W. Krause, A. Szabo, E. Ritz, and H. Reichel. 1993. 1,25-Dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood 82:1300-1307. [PubMed] [Google Scholar]
- 38.Leal, I. S., B. Smedegard, P. Andersen, and R. Appelberg. 2001. Failure to induce enhanced protection against tuberculosis by increasing T-cell-dependent interferon-gamma generation. Immunology 104:157-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lima, V. M., V. L. Bonato, K. M. Lima, S. A. Dos Santos, R. R. Dos Santos, E. D. Goncalves, L. H. Faccioli, I. T. Brandao, J. M. Rodrigues-Junior, and C. L. Silva. 2001. Role of trehalose dimycolate in recruitment of cells and modulation of production of cytokines and NO in tuberculosis. Infect. Immun. 69:5305-5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindblad, E. B., M. J. Elhay, R. Silva, R. Appelberg, and P. Andersen. 1997. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect. Immun. 65:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorentzen, J. C. 1999. Identification of arthritogenic adjuvants of self and foreign origin. Scand. J. Immunol. 49:45-50. [DOI] [PubMed] [Google Scholar]
- 42.Mackaness, G. B. 1968. The immunology of antituberculous immunity. Am. Rev. Respir. Dis. 97:337-344. [DOI] [PubMed] [Google Scholar]
- 43.McBride, B. W., A. Mogg, J. L. Telfer, M. S. Lever, J. Miller, P. C. Turnbull, and L. Baillie. 1998. Protective efficacy of a recombinant protective antigen against Bacillus anthracis challenge and assessment of immunological markers. Vaccine 16:810-817. [DOI] [PubMed] [Google Scholar]
- 44.Murata, J., T. Kitamoto, Y. Ohya, and T. Ouchi. 1997. Effect of dimerization of the d-glucose analogue of muramyl dipeptide on stimulation of macrophage-like cells. Carbohydr. Res. 297:127-133. [DOI] [PubMed] [Google Scholar]
- 45.O'Hagan, D. T., M. L. MacKichan, and M. Singh. 2001. Recent developments in adjuvants for vaccines against infectious diseases. Biomol. Eng. 18:69-85. [DOI] [PubMed] [Google Scholar]
- 46.Olds, G. R., L. Chedid, E. Lederer, and A. A. Mahmoud. 1980. Induction of resistance to Schistosoma mansoni by natural cord factor and synthetic lower homologues. J. Infect. Dis. 141:473-478. [DOI] [PubMed] [Google Scholar]
- 47.Olsen, A. W., L. A. van Pinxteren, L. Meng Okkels, P. Birk Rasmussen, and P. Andersen. 2001. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Infect. Immun. 69:2773-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orme, I. M., D. N. McMurray, and J. T. Belisle. 2001. Tuberculosis vaccine development: recent progress. Trends Microbiol. 9:115-118. [DOI] [PubMed] [Google Scholar]
- 49.Pimm, M. V., R. W. Baldwin, J. Polonsky, and E. Lederer. 1979. Immunotherapy of an ascitic rat hepatoma with cord factor (trehalose-6,6′-dimycolate) and synthetic analogues. Int. J. Cancer 24:780-785. [DOI] [PubMed] [Google Scholar]
- 50.Rockett, K. A., R. Brookes, I. Udalova, V. Vidal, A. V. Hill, and D. Kwiatkowski. 1998. 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect. Immun. 66:5314-5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ross, G. D., J. A. Cain, B. L. Myones, S. L. Newman, and P. J. Lachmann. 1987. Specificity of membrane complement receptor type three (CR3) for beta-glucans. Complement 4:61-74. [DOI] [PubMed] [Google Scholar]
- 52.Schwarz, L. A., J. L. Johnson, M. Black, S. H. Cheng, M. E. Hogan, and J. C. Waldrep. 1996. Delivery of DNA-cationic liposome complexes by small-particle aerosol. Hum. Gene Ther. 7:731-741. [DOI] [PubMed] [Google Scholar]
- 53.Serbina, N. V., and J. L. Flynn. 2001. CD8(+) T cells participate in the memory immune response to Mycobacterium tuberculosis. Infect. Immun. 69:4320-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva, R. A., T. F. Pais, and R. Appelberg. 2000. Effects of interleukin-12 in the long-term protection conferred by a Mycobacterium avium subunit vaccine. Scand. J. Immunol. 52:531-533. [DOI] [PubMed] [Google Scholar]
- 55.Singh, M., and D. O'Hagan. 1999. Advances in vaccine adjuvants. Nat. Biotechnol. 17:1075-1081. [DOI] [PubMed] [Google Scholar]
- 56.Soltysik, S., J. Y. Wu, J. Recchia, D. A. Wheeler, M. J. Newman, R. T. Coughlin, and C. R. Kensil. 1995. Structure/function studies of QS-21 adjuvant: assessment of triterpene aldehyde and glucuronic acid roles in adjuvant function. Vaccine 13:1403-1410. [DOI] [PubMed] [Google Scholar]
- 57.Sterne, J. A., L. C. Rodrigues, and I. N. Guedes. 1998. Does the efficacy of BCG decline with time since vaccination? Int. J. Tuberc. Lung Dis. 2:200-207. [PubMed] [Google Scholar]
- 58.Ulrich, J. T., and K. R. Myers. 1995. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. Pharm. Biotechnol. 6:495-524. [PubMed] [Google Scholar]
- 59.World Health Organization. 2001. Global tuberculosis control. W.H.O. Report 2001. W.H.O./CDS/TB/2001.287. World Health Organization, Geneva, Switzerland.
- 60.Yamagami, H., T. Matsumoto, N. Fujiwara, T. Arakawa, K. Kaneda, I. Yano, and K. Kobayashi. 2001. Trehalose 6,6′-dimycolate (cord factor) of Mycobacterium tuberculosis induces foreign-body- and hypersensitivity-type granulomas in mice. Infect. Immun. 69:810-815. [DOI] [PMC free article] [PubMed] [Google Scholar]