Summary
Epidermal stem cells have become an object of intensive research. The epidermis constitutes one of the main sources of stem cells and is a tissue of choice for use in exploring their biology.
Stratified squamous epithelium (epidermis) possesses the capacity for self-renewal and repair due to the presence of epidermal stem cells (ESC). They have been identified within basal layer of the interfollicular epidermis (IFE), in the “bulge” of the hair follicles of rodents, and also in the human follicular bulge. Melanocyte stem cells (MSC) from hair follicles (precisely from the bulge region, which also contains epidermal stem cells) provide an attractive model for the study of stem cells and their regulation at the niche.
This review summarizes the rapidly developing field of epidermal stem cell research and their application in regenerative medicine, paying particular attention to melanocyte stem cells, their biology and some of the processes that occur during hair graying and regeneration of the pigmentary system, as well as discussing how aged-associated changes in the melanocyte stem cells compartment impact hair graying. This review also includes differentiation of human skin stem cells into functional epidermal melanocytes.
Keywords: melanocyte stem cells, regenerative medicine, epidermis
Background
Epidermal stem cells, because of their relatively easy availability and practical functions, have become an object of intensive research [1]. The epidermis is characterized by a high propensity to repair and constant renewal, and therefore constitutes a tissue of choice for use in exploring stem cells biology. The stem cells can be divided according to their ability to differentiate – totipotent, pluripotent, multipotent and unipotent stem cells. The totipotent nature of the cell is linked with its ability to development into any cell type of the organism. The pluripotent cells can give rise to any type of cell, differentiating into each of the 3 germ layers: mesoderm, ectoderm and endoderm, but they cannot convert back into totipotent cells. Embryonal stem cells (ESC) in the morula stage are totipotent, and during the blastocyst stage obtain pluripotent capacity. Multipotent cells can develop into all cell types within only 1 germ layer (for example, within the mesoderm they can differentiate into bone marrow or blood cells). The unipotent stem cells give rise to 1 type of mature cell and have the ability to divide. Adult stem cells (ASC) occur in mature organisms and have multipotent or unipotent capability. The capacity of the ASC to differentiate into other tissues is therefore limited in comparison with the ESC.
Adult stem cells constantly play key roles in regenerating and maintaining adult tissues. What are their characteristics? Adult stem cells must constantly renew their population and must have the potential and ability to engage in multilineage differentiation. Stem cells have varying degrees of differentiation potential, which can explain their plasticity. Epidermal stem cells are unipotential, so they are able, in theory, to generate only epidermal cells [2,3]. The ability to manipulate individual melanocyte stem cells from hair follicle (precisely from the bulge region, which also contains epidermal stem cells) provides an attractive model for the study of stem cells and their regulation at the niche (the 3-dimensional environment in which they reside) [4,5].
This review summarizes the rapidly developing field of epidermal stem cell research, paying particular attention to melanocyte stem cells, their biology and some of the processes that occur during hair graying and regeneration of the pigmentary system.
The Epidermis and Adult Epidermal Stem Cells
Skin, the biggest organ in the body, has important functions of temperature regulation, absorption, control of evaporation, maintenance of fluid balance and protection, and it is an anatomical barrier against pathogens and environmental assaults [6]. Stratified squamous epithelium (epidermis) possesses the capacity for self-renewal and repair due to the presence of epidermal stem cells (ESC), which have been identified within the basal layer of the interfollicular epidermis (IFE) and in the “bulge” of the hair follicle in rodents. Recent studies have shown that stem cells reside in the human follicular bulge and serve as a reservoir for the proliferation of new cells in the skin organ system. Scattered ESCs can also reside in the spinous and granular layers between the stratum corneum and basal layer [4,7,8]. A separate stem cell population in the hair follicle bulge maintains the epidermis in adult skin. The nerve-derived Sonic Hedgehog (Shh) model defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. This perineural niche is necessary to maintain bulge cells capable of becoming epidermal stem cells. Nerves cultivate a microenvironment where Shh creates a molecularly and phenotypically distinct population of hair follicle stem cells [9]. Epidermal stem cell biology has been well studied in the past several decades. If the conditions are appropriate, adult epidermal stem cells can be activated in vitro, making it possible to apply these cells in regenerative medicine and successfully implant them as a biological dressing in chronic wounds, acute burn injuries or other skin deformations. In normal epidermis, ESCs are usually slow cycling in vivo, can renew themselves, are responsible for regeneration of the tissue, and are defined by their great proliferative potential. It is believed that epidermal stem cells divide asymmetrically, giving rise to 1 transit-amplifying cell (which undergoes further differentiation) and 1 parent stem cell. This division provides a way to sustain a constant number of stem cells in the tissue, and thus its continuing renewal [10]. The plasticity of epidermal stem cells is still not noted. Thus far, there is a scant evidence of the ability of these cells to differentiate in directions other than products of the epidermis [6,11].
However, it has been shown that after appropriate stimulation of epidermal stem cells, they may show the expression of antigens characteristic for embryonic stem cells and hemopoietic stem cell lines. Recent studies proved that it is possible to reconstruct the cornea of the eye using autologous transplant epidermal stem cells [12,13]. The epidermal stem cell population exhibits a capacity to generate human epithelium, providing great opportunities in the production of skin substitutes and wound-healing products [1,14]. Takahashi et al discovered that exogenous addition of only 4 transcription factors (Oct4, Sox2, Myc and Klf4) made it possible to reprogram mature differentiated fibroblast cells into pluripotent embryonic-like stem cells, with the ability to differentiate into all types of body cells. It was also suggested that bulge cells or other keratinocyte stem cells might constitute the ideal source of cells for nuclear reprogramming into pluripotent embryonic stem cells and the re-differentiation of these cells toward cell types such as cardiac cells, neurons, hepatocytes, or pancreas cells, and could be used in defective human diseases [15,16].
Therefore, skin, with it all specific advantages, constitutes a new source of adult stem cells for regenerative medicine.
Melanocytes Biology, Localization and Development
Melanocytes are specialized neural crest-derived cells. They are responsible for hair, skin and eye pigmentation, so they are specialized pigment-producing cells. The melanins are pigments that are synthesized in melanosomes. Melanocytes transfer melanins to neighboring keratinocytes by using the dendrites, giving the skin color, and protecting the genetic material of keratinocytes from damage caused by UV radiation [17].
Human melanocytes occur throughout the skin (mostly in the basal layer of the epidermis and sometimes in the dermis), in mucous membranes, hair follicles, hair matrix, as well as in other organ systems including the heart, the uvea of the eye, the inner ear, and the central nervous system. In mouse skin, melanocytes are only located in hair follicles and in hairless regions of the epidermis or dermis, such as the tail, ear and ventral paw [18,19]. Melanoblasts are the melanocyte precursor cells and arise during gastrulation of embryogenesis at the dorsal edge of the neural crest [20]. The formation of bipotential glial-melanocyte cells depends on the action of Pax3, Sox10 and Wnt proteins [21]. Melanocyte precursor cells migrate through the dermis and epidermis into newly developing hair follicles. This migration and the survival of melanoblasts are dependent on c-kit receptor and its ligand stem cell factor (SCF), as neonatal deletion of either SCF or c-kit results in an irreversible loss of rodent coat pigmentation and piebaldism in humans [8,22,23]. In the bulge region of the hair follicle, melanoblasts can differentiate into melanocytes or can remain as MSCs. The microenvironment of MSCs niche protects from differentiation of MSCs into melanocytes. Low concentration of Pax10, high levels of TGFβ and increased activity of Notch pathway protect MSCs from the differentiation process [24].
Hair Follicle – Localization of Melanocyte Stem Cells (MSCs)
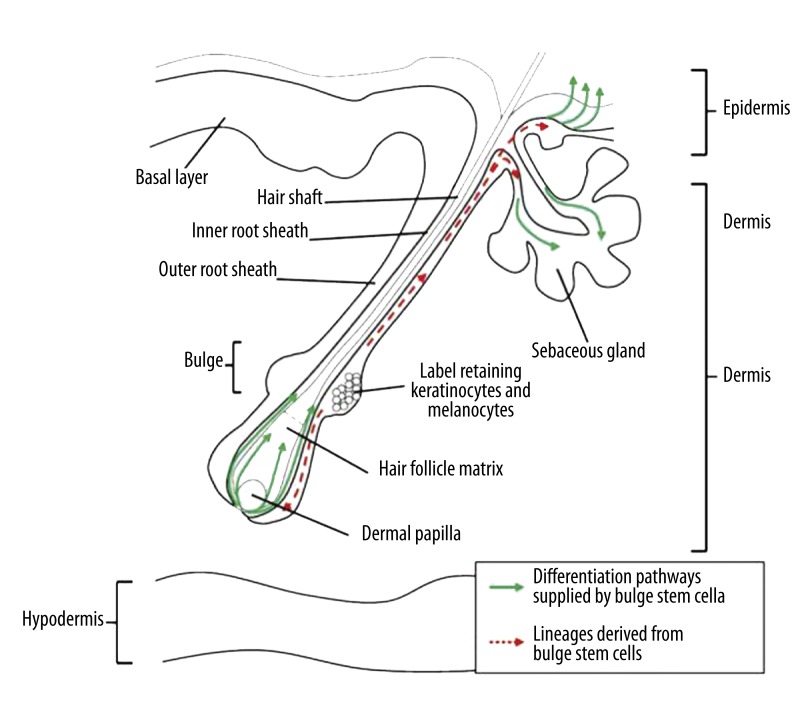
The hair follicle is a satisfactory model system by which the process of tissue regeneration may be analyzed under physiological conditions [25]. It is a “mini-organ” and its transit portion (lower follicle localization) regenerate completely during the hair cycle [26]. In addition, in the mature hair follicle among the melanocyte lineage, there are 3 anatomically and functionally discrete compartments: melanocyte stem cells, melanocyte progenitor cells and terminally differentiated melanocytes. Melanocyte stem cells were first identified in the hair follicle and are located in the bulge region (Figure 1), which is their niche [8]. Hair follicle melanocytes are formed in the hair bulge at the beginning of the hair cycle (anagen phase) and die by apoptosis at the end (catagen phase) [5]. Melanoblasts localized in the bulge region of the hair follicle can transform into melanocyte stem cells or differentiated melanocytes, which produce melanin pigment. Packets of melanin are transferred to adjacent keratinocytes, which confers color to the hair shaft. Melanocyte stem cells and progenitor stem cells localized in the bulge region express Dct and Trp1, but lack Tyr (a rate-limiting enzyme for pigment synthesis), so they do not produce melanin. At the anagen phase of the hair cycle, progenitors differentiate into mature melanocytes that show ability to express all enzymes necessary for the synthesis of melanin enzymes (Trp1, Dct and Tyr) [8,24]. Differentiated pigment cells migrate to anagen hair bulbs and are responsible for newly forming hair pigmentation [8,25].
Figure 1.
Hair follicle and the localization of melanocyte and keratinocyte stem cells. As depicted in this picture, melanocyte stem cells reside in the lower part of the hair bulge (a niche, where also occur epidermal stem cells) among of the matrix keratinocytes. Dividing keratinocytes give rise to the keratinized hair shaft. During the transition from telogen to anagen, activation of a melanocyte stem cell leads to development of melanocyte progenitors, after all to differentiated melanocytes which produce melanin pigment. Packets of melanin are transferred to adjacent keratinocytes, which confers color to the hair shaft [39].
Skin scars are very often hypopigmented; this is due to hair follicle destruction. This is a topic of interest to bioengineers. Innovative transformation therapies must lead not only to incorporation and induction of differentiated structures, but also the insertion pigmentation system in skin substitutes. Melanocytes were incorporated for this purpose, and were also used as therapeutic products for treatment of vitiligo. Development of technology using application of mixed cell slurries containing keratinocytes and fibroblasts may offer a functional and satisfactory model system for treating structural and cosmetic aspects of skin conditions [3,8].
Hair Graying: Loss of Melanocyte Stem Cells
One of the most common phenotypes of human aging is hair graying, which is caused by gradual loss of pigment cells from hair follicles. Melanocytes synthesize pigment for growing hair and their presence is responsible for maintenance of hair pigmentation. In addition, melanocyte stem cells are giving the beginning of the mature pigment cells. This raises the question of how aged-associated changes in the melanocyte stem cells compartment impact hair graying. [8]
One of the answers to this question is the “free radical theory of graying”, which partially explains age-related hair graying. According to this hypothesis, during melanin biosynthesis certain reactions produce reactive radicals. It might originate as oxidative stress and the subsequent cytotoxic elimination of mature melanocytes in the hair matrix. In spite of this, a series of recent observations of premature hair graying in mutant animals has provided evidence that hair graying occurs primarily due to a progressive loss of melanocyte stem cells rather than the “free radical theory of hair graying” or rather than impaired melanocyte function [25,28,29]. Finally, hair graying entails lack of some specified functions of melanocytes, such as maintenance of normal homeostasis or replenishment of melanin. Nishimura et al combined hair graying with the gradual disappearance of stem cells, which provide a constant supply of new melanocytes. According to them, specialized cells not only “die”, but also transform into different pigment cells or get into the wrong place. There also might be a possibility that changes occur in survival, proliferation or differentiation signals in the MSCs, or their limited number of cell division (intrinsically determined lifespan) which are related with human aging [8,30,31]. Understanding the process of aging in other tissues and cell lineages will be possible thanks to exact genetic and molecular analysis of hair graying.
Melanocyte Stem Cells: Model for Study
The melanocyte stem cell niche is located mainly in the hair follicles of mouse and human skin [32]. Unfortunately, because of this localization they lose their self-renewal capacity with aging, which probably leads to hair graying. Perifollicular repigmentation after phototherapy of vitiligo patients results from melanocyte stem cells migration from hair follicles to the surrounding epidermis. Marginal and central repigmentation in vitiliginous patches localized in glabrous areas is also observed. These facts lead us to suspect that melanocyte stem cell niches are present not only in the bulge region, but also in extrafollicular localization. Ling Li et al showed that multipotent dermis-derived stem cells (DSCs), isolated from human foreskin lacking hair follicles, are able to home to the epidermis. DSCs are also able to differentiate into melanocytes, suggesting the existence of a reservoir for melanocytes in the more protective dermal layer of the skin [33].
Tsutomu Motohashi et al studied the ability to induce pigment cell differentiation from embryonic stem cells [34]. It holds promise for functional therapeutic potential and development for the future cell-based therapy of various diseases caused by defective pigment cells. Zheng et al. proved the multiple regenerative roles of bulge stem cells (or dissociated cells). Despite the fact that these cells contribute to formation of sebaceous glands and epidermis, they are key in the production of hair follicles, injecting a mixture of isolated neonatal dermal cells with epidermal aggregates into the dermis of nude mice. These aggregates were then able to interact and undergo relatively normal hair morphogenesis to give rise to cycling hair follicles within 8 to 12 days. Such projects hold great hope for the future use of differentiated structures in a new generation of skin substitutes [35,36].
Cultured human melanocytes are increasingly being used in the treatment of vitiligo. Rafal Czajkowski et al wanted to assess the safety of the transplantation of cultured autologous melanocytes. The purpose of their study was to verify the risk of the development of mutations in selected genes in the RAS/RAF/MEK/ERK signaling pathway during the culturing of melanocytes in various growth media. They concluded that TPA (Tetradecanoylphorbol Acetate) and high concentrations of other growth factors accelerate the proliferation of melanocytes, without the risk of carcinogenesis of transplanted cells [37].
The increasing number of studies revealing the use of stem cells in regenerative processes shows that they are revolutionizing regenerative medicine [38]. Skin hair follicle biology, because of the high individuality of this structure, is a topic that continues to present new problems. Biological models that allow easy access to MSCs open new research possibilities. To develop effective applications we must gather as much information about their structure, differentiation, self-renewal, hair graying, melanoma development and all its accompanying mechanisms.
Conclusions
The present data focusing on melanocyte stem cells provide an advantageous model for the study of stem cell biology. As described in this review, proper stimulation of epidermal stem cells reveals their new properties, which should help them become a new source of adult stem cells for regenerative medicine.
Elucidation of the process of hair graying is associated with a number of interrelated theories, and more analysis can help explore it accurately.
The high volume of stem cell research casts a bright light on the hope for stem cells and their role in regenerative medicine [38]. Suspicions that melanocyte stem cell niches are present not only in bulge region, but also in extrafollicular localization, strongly suggest the existence of a reservoir of melanocytes in the more protective dermal layer of the skin. Therefore, it is possible to induce differentiation of human skin stem cells into functional epidermal melanocytes and develop future cell-based therapy for defective pigmentary system diseases.
Footnotes
Source of support: Self financing
References
- 1.Pikuła M, Trzonkowski P. Biology of epidermal stem cells: Impact on Medicine. Postepy Hig Med Dosw. 2009;63:449–56. [PubMed] [Google Scholar]
- 2.Preston SL, Alison MR, Forbes SJ, et al. The new stem cell biology: something for everyone. J Clin Pathol. 2003;56:86–96. doi: 10.1136/mp.56.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–73. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aberdam D. Epidermal stem cell fate: what can we learn from embryonic stem cells? Cell Tissue Res. 2008;331:103–7. doi: 10.1007/s00441-007-0497-0. [DOI] [PubMed] [Google Scholar]
- 5.Robinson KC, Fisher DE. Specification and loss of melanocyte stem cells. Semin Cell Dev Biol. 2009;20:111–16. doi: 10.1016/j.semcdb.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Chunmeng S, Tianmin Ch. Skin: a promising reservoir for adult stem cell populations. Med Hypotheses. 2004;62:683–88. doi: 10.1016/j.mehy.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Ohta S, Imaizumi Y, Okada Y, et al. Generation of human melanocytes from induced pluripotent stem cells. PLoS ONE. 2011;6(1):e16182. doi: 10.1371/journal.pone.0016182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarin KY, Artandi SE. Aging, graying and loss of melanocyte stem cells. Stem Cell Rev. 2007;3:212–17. doi: 10.1007/s12015-007-0028-0. [DOI] [PubMed] [Google Scholar]
- 9.Brownell I, Guevara E, Bai CB, et al. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–65. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanpain C, Lowry WE, Geoghegan A, et al. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–38. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Shi Ch, Zhu Y, Su Y, Cheng T. Stem cells and their applications in skin-cell therapy. Trends Biotechnol. 2006;24:48–52. doi: 10.1016/j.tibtech.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Blazejewska EA, Schlötzer-Schrehardt U, Zenkel M, et al. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells. 2009;27:642–52. doi: 10.1634/stemcells.2008-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang K, Jiang Z, Wang D, Lian X, Yang T. Corneal epithelial-like transdifferentiation of hair follicle stem cells is mediated by pax6 and beta-catenin/Lef-1. Cell Biol Int. 2005;33:861–66. doi: 10.1016/j.cellbi.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Moldovan NI, Zhao Q, et al. Reconstruction of damaged cornea by autologous transplantation of epidermal adult stem cells. Mol Vis. 2008;14:1064–70. [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Greco V, Guasch G, Fuchs E, Mombaerts P. Mice cloned from skin cells. Proc Natl Acad Sci. 2007;104:2738–43. doi: 10.1073/pnas.0611358104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Słomiński A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 18.Metcalfe AD, Ferguson MWJ. Skin stem and progenitor cells: using regeneration as a tissue – engineering strategy. Cell Mol Life Sci. 2008;65:24–32. doi: 10.1007/s00018-007-7427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suder E, Brużewicz S. Melanocytes of fetal dermis – studies with anti-HMB-45 antibody. Med Sci Monit. 2004;10(7):BR229–32. [PubMed] [Google Scholar]
- 20.Thomas AJ, Erickson CA. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008;21:598–610. doi: 10.1111/j.1755-148X.2008.00506.x. [DOI] [PubMed] [Google Scholar]
- 21.Hou L, Pavan WJ. Transcriptional and signaling regulation in neural crest stem cell-derived melanocyte development: do all roads lead to Mitf? Cell Res. 2008;18:1163–76. doi: 10.1038/cr.2008.303. [DOI] [PubMed] [Google Scholar]
- 22.Cable J, Jackson IJ, Steel KP. Mutations at the W locus affect survival of neural crest-derived melanocytes in the mouse. Mech Dev. 1995;50:139–50. doi: 10.1016/0925-4773(94)00331-g. [DOI] [PubMed] [Google Scholar]
- 23.Murphy M, Reid K, Williams DE, et al. Steel factor is required for maintenance, but not differentiation, of melanocyte precursors in the neural crest. Dev Biologicals. 1992;153:396–401. doi: 10.1016/0012-1606(92)90124-y. [DOI] [PubMed] [Google Scholar]
- 24.Kubic JD, Young KP, Plummer RS, et al. Pigmentation PAX-ways: the role of Pax3 in melanogenesis, melanocyte stem cell maintenance, and disease. Pigment Cell Melanoma Res. 2008;21:627–45. doi: 10.1111/j.1755-148X.2008.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osawa M. Melanocyte stem cells. Mol Vis. 2008;14:1064–70. [Google Scholar]
- 26.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–94. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 27.Słomiński A, Wortsman J, Tobin DJ, Shibahara S. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–24. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 29.Arck PC, Overall R, Spatz K, et al. Towards a “free radical theory of graying”: melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006;20:1567–69. doi: 10.1096/fj.05-4039fje. [DOI] [PubMed] [Google Scholar]
- 30.Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, and cancer. J Clin Invest. 2004;113:160–68. doi: 10.1172/JCI20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobin DJ, Paus R. Graying: gerontobiology of the hair follicle pigmentary unit. Exp Gerol. 2001;36:29–54. doi: 10.1016/s0531-5565(00)00210-2. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura EK, Jordan SA, Oshima H, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–60. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Fukunaga-Kalabis M, Yu H, et al. Human dermal stem cells differentiate into functional epidermal melanocytes. J Cell Sci. 2009;123:853–60. doi: 10.1242/jcs.061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motohashi T, Aoki H, Yoshimura N, Kunisada T. Induction of melanocytes from embryonic stem cells and their therapeutic potential. Pigment Cell Res. 2006;19:284–89. doi: 10.1111/j.1600-0749.2006.00317.x. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y, Du X, Wang W, et al. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J Invest Dermatol. 2005;124:867–76. doi: 10.1111/j.0022-202X.2005.23716.x. [DOI] [PubMed] [Google Scholar]
- 36.Fu X, Sun X. Can hematopoietic stem cells be an alternative source for skin regeneration? Ageing Research Reviews. 2009;8:244–49. doi: 10.1016/j.arr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Czajkowski R, Pokrywczynska M, Placek W, et al. Transplantation of Cultured Autologous Melanocytes: Hope or Danger? Cell Transplantation. 2010;19:639–43. doi: 10.3727/096368910X491798. [DOI] [PubMed] [Google Scholar]
- 38.Eve D, Fillmore R, Borlongan C, Sanberg P. Stem cells have the potential to rejuvenate regenerative medicine research. Med Sci Monit. 2010;16(10):RA197–217. [PubMed] [Google Scholar]
- 39.Hoffman RM. The hair follicle as a gene therapy target. Nature Biotechnology. 2000;18:20–21. doi: 10.1038/71866. [DOI] [PubMed] [Google Scholar]