Summary
Background
The purpose of the study is an analysis of intrascleral drainage vessels formed in rabbits’ eyes after non-penetrating deep sclerectomy (NPDS) with absorbable and non-absorbable implants, and comparison to eyes in which surgery was performed without implanted material.
Material/Methods
NPDS was carried out in 12 rabbits, with implantation of non-absorbable methacrylic hydrogel (N=10 eyes) or absorbable cross-linked sodium hyaluronate (N=6 eyes), or without any implant (N=8 eyes). All the animals were euthanized 1 year after surgery. Twenty-one eyeballs were prepared for light microscopy and 3 were prepared for transmission electron microscope (TEM) analysis. Aqueous humour pathways were stained with ferritin in 6 eyeballs.
Results
By light microscopy, small vessels adjacent to the areas of scarring were the most common abnormality. Vessel density was significantly higher in operated sclera compared to normal, healthy tissue, regardless of the type of implant used. The average vessel densities were 2.18±1.48 vessels/mm2 in non-implanted sclera, 2.34±1.69 vessels/mm2 in eyes with absorbable implants, and 3.64±1.78 vessels/mm2 in eyes with non-absorbable implants. Analysis of iron distribution in ferritin-injected eyes showed a positive reaction inside new aqueous draining vessels in all groups. TEM analysis showed that the ultrastructure of new vessels matched the features of the small veins.
Conclusions
Aqueous outflow after NPDS can be achieved through the newly formed network of small intrascleral veins. Use of non-absorbable implants significantly increases vessel density in the sclera adjacent to implanted material compared to eyes in which absorbable implants or no implants were used.
Keywords: glaucoma, non-penetrating deep sclerectomy, aqueous outflow, intrascleral drainage vessels, intrascleral implant
Background
Non-penetrating deep sclerectomy (NPDS) is a surgical procedure for lowering intraocular pressure in patients with uncontrolled open-angle glaucoma. As a result of the surgery an intrascleral space is created, separated from the anterior chamber by a thin membrane built of trabeculum, and Descemet’s membrane [1,2]. The aqueous humour, which easily enters the intrascleral space, has 4 possible means of filtration: subconjunctival, suprachoroidal, intrascleral, and episcleral vein outflow via Schlemm’s canal [3]. Delarive et al described the formation of new drainage vessels in the operated sclera of an animal model, proving the presence of intrascleral outflow after surgery [4].
Intrascleral implants were introduced to enhance the success rate of NPDS [5]. The clinical studies confirmed better surgical results with absorbable collagen implant than without implant [6–9]. Dahan et al reported that a non-absorbable acrylic implant had a beneficial effect on long-term NPDS results [10]. In spite of the relatively high number of clinical trials, there are very few experimental studies exploring the influence of absorbable and non-absorbable implants on aqueous outflow after NPDS in animal models [4,11–14]. A recent study shows significant increase in number of drainage vessels in eyes with non-absorbable polymethylmetacrylate implant, compared to absorbable collagen, 6 months after surgery [15].
This study is a long-term analysis of intrascleral drainage vessels formed in rabbits’ eyes after NPDS with absorbable and non-absorbable implants, compared to eyes in which surgery was performed without any implanted material.
Material and Methods
Implants and experimental subjects
NPDS was performed in 12 healthy, adult, pigmented rabbits (N=24 eyes total) weighing between 3.5 and 4.5 kg. Ten eyes were implanted with non-absorbable material (group 1), 6 eyes were implanted with absorbable material (group 2), and no implant was used in 8 eyes (group 3). A copolymer of methyl methacrylate and vinylpyrrolidone (MMA/VP), which is available as contact lens (Omniflex Softblue, Hydron Ltd., Farnborough, Hampshire, UK), was used as the non-absorbable implant. This is a methacrylic hydrogel with a 70% water content and non-ionic surface. The absorbable material used in this study was commercially available cross-linked sodium hyaluronate (SKGEL 3.5; Corneal, Paris, France). Methacrylic hydrogel implants were prepared in aseptic operating room conditions immediately before surgery. The equilateral triangles (approximately 4 mm long, 300 μm thick) were cut from a new sterile contact lens by the surgeon under an operation microscope. Cross-linked sodium hyaluronate was commercially available as equilateral triangles (3.5 mm long, approximately 500 μm thick); therefore no additional preparation was necessary.
Surgical procedure
The same surgeon (JJK) performed all the operations with the same technique. After adequate general anaesthesia (ketamine hydrochloride 40 mg/kg and xylazine hydrochloride 5 mg/kg), the rabbit eyes were prepared and draped with sterile towels. Topical 0.5% proparacaine hydrochloride was instilled to prevent discomfort. The conjunctiva was then opened at the limbus in the upper part of the eye. Next, a superficial 5×5 mm scleral flap was dissected. A limbal-based triangle of deep sclera was removed, leaving a thin layer of sclera over the choroid. The dissection was performed anteriorly about 1 mm into clear cornea. The previously prepared implants were than put into place on the sclera. In eyes from group 3 (no material inserted) the intrascleral space was left empty. The superficial scleral flap was than repositioned over the implants or empty intrascleral space and secured with 2 10/0 nylon sutures placed at each posterior corner of the flap. In all eyes, the conjunctiva was closed with 3–4 single absorbable 8/0 vicryl sutures. After surgery, 0.3% gentamicin was applied topically.
Postoperative care
Postoperative care included instillation of a combination of dexamethasone and neomycin drops twice daily for 1 week. The intraocular pressure was measured with the Tono-Pen XL (Mentor) under local anaesthesia before NPDS, 2 weeks after surgery, and at the end of the 1st, 3rd, 6th, and 12th month of follow-up. All animals were euthanized with an overdose of anaesthetic 12 months after surgery. The rabbits were treated in accordance with guidelines, policies, and principles established by the Animal Welfare Act and the NIH Guide for Care and Use of Laboratory Animals. The study protocol was approved by the local ethics committee.
Anterior segment fluorescein angiography
Fluorescein angiography (FA) was performed to observe draining pathways of aqueous humour in rabbits’ eyes in vivo. In each eye the examination was carried out twice, first 2–4 months after NPDS, and then 10–12 months after surgery, always with general (ketamine hydrochloride 20 mg/kg and xylazine hydrochloride 5 mg/kg) and topical (0.5% proparacaine hydrochloride) anaesthesia. Fluorescein solution (0.1 mg/ml) was injected into the anterior chamber after limbal paracentesis. About 30 pictures of each eye were taken with a fundus camera (TRC-50XE, Topcon, Japan) within 40 minutes of dye injection. Eyes were treated with 1 drop of 0.3% gentamicin after examination.
Histological examination
The rabbits’ eyes were enucleated just after euthanasia and fixed in 10% buffered formalin. Within 4 weeks all the eyeballs were cut along the meridian going through the middle of the scleral flap on 2 hemispheres embedded in paraffin. In each hemisphere the area within 3 mm of the cutting line was divided into 20 evenly distributed planes marked from I to XX. Plane I was always located closest to the cutting line and plane XX the most distant from that line. From each of these planes, 2 4-μm sections were cut using a rotation microtome (HM 350, Ophton, Germany). Five sections – first from planes I–III, second from planes IV–VII, third from planes VIII–XI, fourth from planes XII–XVI, and fifth from planes XVII–XX — were selected from each hemisphere for photographic documentation and a computer image analysis based on the Nikon Eclipse E800 microscope with built-in digital camera, Nikon DS-U1, and Laboratory Universal Computer Image Analysis System (LUCIA DI v.5 2005).
In each of these sections 4–6 pictures were made covering the area from limbus to level of non-absorbable sutures. Each picture covered 650×870 μm of intrascleral tissue. From each eyeball, between 40 and 60 images of operated sclera and a similar number of healthy sclera located opposite to the operation site (control group) were collected.
The intrascleral vessels were counted in each image from both study and control groups. Vessels were defined as structures having lumen and identifiable endothelial layer in cross, longitudinal, or oblique section. Because the total area of examined sclera was measured, the density of vessels was calculated.
Identification of aqueous outflow pathways with ferritin
In 6 eyeballs, 2 with non-absorbable implants, 2 with absorbable implants, and 2 without any implants, the identification of aqueous draining vessels using the iron storage protein ferritin (ferritin from horse spleen, Biochemika, Fluka Chemie, Buchs, Switzerland) was performed 12 months after NPDS. While animals were under general anaesthesia, 0.2 ml of 50 mg/ml ferritin solution was injected into the anterior chamber. The animals were euthanized 30 minutes after injection. All eyeballs were treated as described above. During histological preparation, 2 additional sections from each plane were cut and Perl’s iron staining was performed. The aqueous drainage vessels were identified by their blue colour due to presence of iron.
Transmission Electron Microscopy
Transmission Electron Microscopy was performed to evaluate the ultrastructure of newly formed vessels. Scleral and conjunctival samples of dimensions 1×1 mm were taken both from the operated and healthy side of 3 eyeballs, 1 from each examined group. The material was routinely prepared and analysed using a JEM-100CX transmission electron microscope (Jeol, Tokyo, Japan).
Results
Macroscopic evaluation
Mild conjunctival hyperemia and edema were observed 3 to 6 days postoperatively, and disappeared after topical anti-inflammatory drops. There was no occurrence of corneal edema, anterior chamber reaction, or endophtalmitis.
Shortly after surgery, the filtering bleb was visible in all eyes. However (due to intensive scarring 3 months after NPDS) thin, microcystic filtering blebs characteristic of successful trabeculectomy were not observed in any eye. Twelve months after surgery, the filtering bleb was flat and poorly vascularized, usually with significant scleral thinning along the margins of the flap.
Mean preoperative intraocular pressure (IOP) was 15.37±2.31 mmHg in group 1, 13.83±1.62 mmHg in group 2, and 14.17±2.10 mmHg in group 3. Two weeks after surgery a statistically significant drop of IOP was observed in all groups, to the respective values 8.47±2.05 mmHg, 7/22±1.11 mmHg, and 7/63±1/86 mmHg. Later, in follow-up, the mean IOP increased, gradually returning to preoperative values 3 months after surgery. Twelve months after NPDS, the mean IOP was 11.60±1.40 mmHg, 13.95±3.45 mmHg and 13.62±2.95 mmHg, respectively, and was statistically lower compared to preoperative values only in eyes with non-absorbable implants (group 1).
Fluorescein angiography
Three different patterns of hyperfluorescence within operated sclera were observed (Figure 1). Type 1 was diffuse dye leakage in the area of the filtering bleb, which started 2 to 5 minutes after beginning the examination. In type 2, single vessels filled with the dye appeared in the first minutes of angiography. Similarly, in type 3 the aqueous draining vessels were visible, but their number was high enough to form a network completely or partially covering the bleb area. Significant leakage from those vessels was observed in the late phase of angiography.
Figure 1.
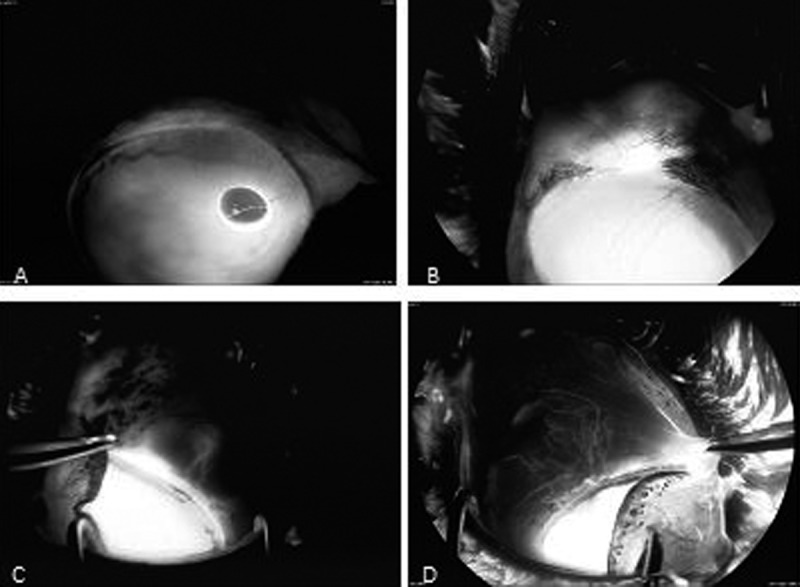
Types of hyperfluorescence observed after fluorescein injection to the anterior chamber: (A) Type 0: Lack of the dye within operated sclera. (B) Type 1: Diffuse leakage. (C) Type 2: Single vessels filled with dye. (D) Type 3: Vascular network partially covering bleb area.
Types of hyperfluorescence observed in all examined groups are presented in Table 1. The lack of fluorescein dye within operated sclera is marked as type 0. In non-implanted eyes (group 3), type 1 of hyperfluorescence was the most common. Aqueous draining vessels were observed only in eyes where the implants were used. In group 2, those vessels were mostly visible 3 months after NPDS, and in some eyes vessels were also visible 12 months after surgery. Fluorescein angiography revealed draining vessels, the most distinctly in eyes with a non-absorbable implant (group 1). Type 3 hyperfluorescence was often represented here, both 3 and 12 months after surgery. Additionally, the effect of an increased number of draining vessels during the follow-up period was present only in group 1 (4 eyes) (Figure 2).
Table 1.
Distribution of different types of hyperfluorescence after fluorescein injection performed three and 12 months after surgery in study groups.
| Group 3 | Group 2 | Group 1 | ||||
|---|---|---|---|---|---|---|
| 3 months | 12 months | 3 months | 12 months | 3 months | 12 months | |
| Type 0 | 3 eyes | 5 eyes | 2 eyes | 4 eyes | 2 eyes | none |
| Type 1 | 5 eyes | 3 eyes | 2 eyes | none | 4 eyes | 4 eyes |
| Type 2 | none | none | 1 eye | 2 eyes | 1 eye | 3 eyes |
| Type 3 | none | none | 1 eye | none | 3 eyes | 3 eyes |
Figure 2.

Light microscopy analysis (H&E ×200). (A) Scar tissue (thin arrow) with single vessel (thick arrow) in operated sclera of eye without implant. (B) Adipose tissue (thin arrow) with vessels (thick arrows) filling space left after absorption of implant. (C) Posterior pole of non-absorbable implant with scar tissue (thin arrow), single macrophages, and vessels (thick arrows) within triangular space limited by implant (i), scleral flap (f) and bed (b).
Histological examination
The most common abnormality observed within operated sclera 12 months after NPDS without any implant (group 3) was characterized by the areas of scarring with a dense, irregular pattern of collagen fibers and a small number of fibrocytes (Figure 2A). These areas were located intrasclerally between the flap and the scleral bed. Within this pathology the small vessels with thin endothelial layer and single red blood cells could be found. In sclera of eyes from group 2, the remains of absorbable implant surrounded by thin fibrous capsule were observed. The space left after implant absorption was filled with adipose or scar tissue in which small vessels were often located (Figure 2B). In eyes with non-absorbable implant material (group 1) the whole implant was visible in all eyes. All methacrylic implants were surrounded by a thick fibrous capsule that consisted of bundles of collagen fibers running parallel to their surface. In proximity to both the anterior and posterior poles of the implant cavity, the triangular area limited by scleral flap, bed, and implant wall filled with irregular scar tissue was observed (Figure 2C). Single macrophages and vessels were commonly found within those triangular structures. The choroidal and conjunctival sides of the implant were similar in appearance, with fibrous capsule separating the implant from normal sclera. Small scleral vessels were also commonly located at the border between those 2 structures. Posterior to the implant, the cutting line between the scleral flap and the bed was filled with irregular scar tissue, with single vessels observed running toward the scleral surface.
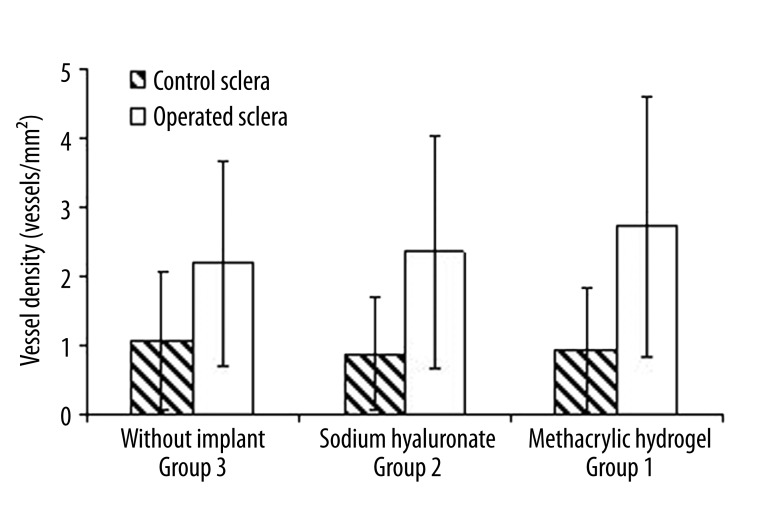
Comparison of vessel density between normal and operated sclera demonstrated statistically significant differences, regardless of the presence or type of implant (Figure 3). The average vessel density of operated sclera was 2.18±1.48 vessels/mm2 in group 3, and 2.34±1.69 vessels/mm2 in group 2. In group 1, average vessel density was 2.71±1.88 vessels/mm2. Although a small increase was observed, the difference was not statistically significant. Because the exact location of the non-absorbable implant under the scleral flap differs from eye to eye, the implant was not visible in all the evaluated planes taken from the operation site. Average vessel density in those planes where an implant was recognized was 3.64±1.78 vessels/mm2, compared to 2.01±1.58 vessels/mm2 in planes without an implant. This statistically significant difference (p<0.001) demonstrates that the non-absorbable material has a stimulating influence on vessel density, although that influence is limited to the sclera adjacent to the implant.
Figure 3.
Comparison of vessel density between non-operated healthy sclera, and sclera collected from operation site in subsequent groups. The difference was statistically significant (p<0.001) regardless of the type of implant used.
Analysis of sclera surrounding non-absorbable implants shows statistically significant variability (p<0.05) of vessel density according to location. The highest values were observed in sclera around the posterior and anterior poles of the implant cavity (5.78±2.81 and 4.53±2.18 vessels/mm2 respectively) and at the cutting line posterior to the implant (3.7±1.7 vessels/mm2). The number of vessels was significantly lower at the conjunctival and choroidal sides of the implant (2.97±1.82 and 2.64±1.06 vessels/mm2, respectively).
Identification of aqueous outflow pathways with ferritin
Blue staining was observed in all eyeballs, representing the presence of iron within Schlemms canal and aqueous draining channels in limbal sclera, as well as in suprachoroidal tissue of the uveo-scleral pathway of aqueous outflow. A similarly positive reaction was found in vessels adjacent to scar and adipose tissue in operated sclera distant from the limbus in eyes from groups 3 and 2 (Figure 4B, C). Significant blue staining inside vessels located in scar tissue close to the anterior pole of the non-absorbable methacrylic hydrogel (group 1) implants demonstrates their participation in aqueous outflow (Figure 4D). Blue staining was also observed in vessels from both the conjunctival and choroidal side of the implant cavity.
Figure 4.

Intrascleral pathways of aqueous outflow after injection of ferritine. Blue staining indicates positive reaction to the presence of iron. (A) Trabeculum, Schlemms canal, and aqueous draining channels in the control group. (B) Vessel (thick arrow) within the area of adipose tissue ingrowths in operated sclera from group 3. (C) Vessels (thick arrows) adjacent to scar tissue in eyes where absorbable implant was used (group 2). (D) Vessels (thick arrows) located in scar tissue in proximity to non-absorbable implant (group 1). t – trabeculum, Sc – Schlemms canal, dc – aqueous draining channels, i – implant.
Transmission Electron Microscopy (TEM)
TEM analysis of conjunctival samples revealed the presence of blood and lymphatic vessels. The conjunctival veins had endothelium with continuous basement membrane, often with erythrocytes inside the lumen (Figure 5A). Lymphatic vessels were characterized by thin, waved, and sometimes overlapping endothelium and lack of continuous basement membrane (Figure 5B). In scleral samples obtained from all control and study groups, only blood vessels were detected. Samples usually had an ultrastructure of small veins with thick endothelium, continuous basement membrane, and pericytes covering the vessels (Figure 5C). In the smallest veins, the basement membrane was adjacent to collagen fibres of the sclera (Figure 5D).
Figure 5.
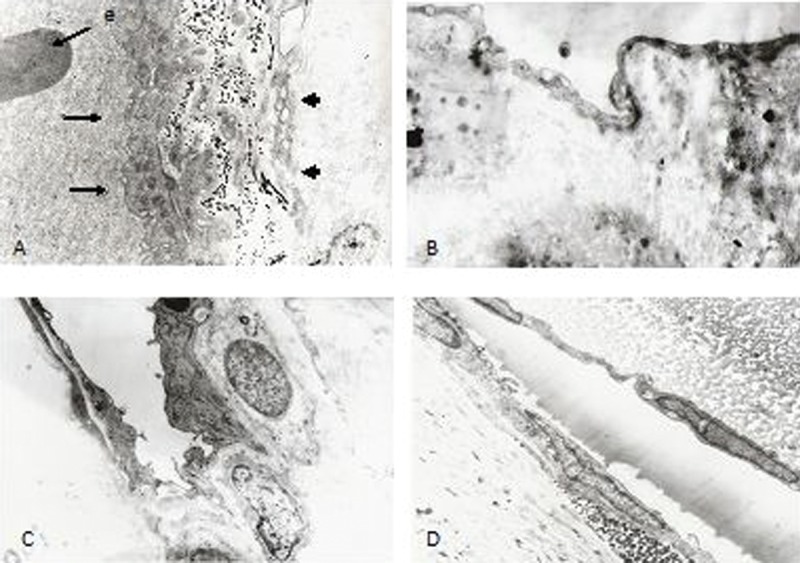
Ultrastructure of conjunctival and scleral vessels. (A) Conjunctival blood vessel (thin arrows) with erythrocyte and thick endothelium. The lymphatic vessel (thick arrow) had thin, partially overlapping endothelium without erythrocytes in the lumen (5000×). (B) Fragment of conjunctival lymphatic vessel wall with waved endothelium and absent continuous basement membrane (5000×). (C) Typical features of scleral vein with endothelium, basement membrane, and pericytes (5000×). (D) Small scleral venule with endothelium and basement membrane laying adjacent to the collagen fibers (5000×).
Discussion
Intrascleral filtration is a possible direction of aqueous outflow after NPDS [3]. Clinical observations confirm the presence of scleral direction of drainage. A flat filtering bleb with low IOP is a more common finding after NPDS compared to trabeculectomy. Features of subconjunctival filtration were not observed in 10% of eyes after successful NPDS in ultrasound biomicroscopy [16].
Our study demonstrates the significance of intrascleral filtration after NPDS in an animal model. A few experimental animal studies that analyzed the mechanism of filtration after NPDS have reported findings consistent with our results. Delarive et al compared histological findings after surgery between eyes without implant and with absorbable collagen [4]. In both groups, the number of intrascleral vessels was higher in implanted vs. unoperated sclera. Similar to our study, the difference in number of vessels between eyes with absorbable implant and without any implant was not statistically significant. A second study demonstrated statistically insignificant influence of absorbable collagen implant shape on scleral vascularity [13]. Basso et al observed an increased number of outflow vessels near non-absorbable x-shaped zirconium implants compared to native sclera, although 6 months after surgery these vessels were not longer visible [14]. The most recent study of Codreanu et al showed the presence of new draining vessels, which were more numerous around non-absorbable polymethylmethacrylate implant compared to absorbable collagen [15]. Because the collagen implant is absorbed within 6–9 months after surgery, the 6-month follow-up in this study seems insufficient to ensure full bioabsorption of collagen, and this difference would probably increase during further observation. This issue is especially important in the context of long-term efficacy of glaucoma surgery and possible clinical applications of non-absorbable implants. In the current study, the problem of long-term influence of non-absorbable methacrylic implant on draining vessels formation was analysed.
The mechanism of intrascleral vessel formation after NPDS can be explained by postoperative inflammation. Shortly after surgery, inflammatory infiltration with domination of granulocytes and macrophages appears in operated sclera [12]. These cells release angiogenic factors that are responsible for vascular proliferation. Seven days after surgery, the intensive inflammatory reaction, together with vessels, was observed within an irregular connective tissue-filled space between the implant, scleral flap, and bed [12]. In non-implanted eyes the surgical trauma inflammatory response gradually diminishes and the stimulus for vessel proliferation ceases.
Inflammation is an important element of foreign body reaction related to the presence of intrascleral implant. However, duration of absorbable implants is limited by the time required for material absorption [17,18]. Implantation of non-absorbable material induces chronic inflammatory reaction with domination of macrophages. This chronic inflammation develops as long as the implant is in the tissue, thus vascular proliferation stimulus limited to the area directly adjacent to the implant can persist for a long time in operated sclera [19]. This probably explains why the vessel density was highest in the sclera surrounding methacrylic hydrogel in our study. Because the inflammation has local range, the vessel density increases only in the area adjacent to the implant.
The lack of statistically significant IOP differences before and 12 months after NPDS in eyes without implant and with absorbable implant demonstrates that new vessels have a limited impact on IOP. Similar conclusions were reached by Delarive et al., who analyzed intrascleral filtration after absorbable collagen implant use [4]. IOP reduction was observed in the first weeks after NPDS in all our examined groups. Intensive scarring in rabbits’ eyes resulted in pressure increase during follow-up, which was probably related to the disappearance of the microcystic filtering bleb and loss of subconjunctival filtration. One year after surgery, IOP was statistically lower compared to baseline only in the non-absorbable implant group. Because intrascleral vessel density was highest in this group, one may conclude that this could be related to new draining vessel formation.
Our results warrant further studies directed towards increasing the number of aqueous draining vessels via implant shape and surface modifications in order to achieve more significant IOP reduction. This study has obvious limitations. The rabbit is not an ideal model for glaucoma filtering surgery, because scarring is much more rapid than in humans. We believe that changes in the scleral structure observed 12 months after surgery in rabbits correspond to long-term reaction in the human eye.
Conclusions
As a result of NPDS, new vessels appear in operated sclera regardless of what implant is used. These new vessels have the structure of small veins and are involved in aqueous outflow after surgery. The density of newly formed vessels was highest in eyes that received a non-absorbable implant, although the increase in density was limited to the sclera adjacent to the implanted material.
Footnotes
Source of support: Departmental sources
References
- 1.Fiodorow SN, Kozlow WI, Timoszkina NT. Nonpenetrating deep sclerectomy in open angle glaucoma. Ophthalmosurgery. 1989;3–4:52–55. [Google Scholar]
- 2.Kershner RM. Nonpenetrating trabeculectomy with placement of a collagen drainage device. J Cataract Refract Surg. 1995;21:608–11. doi: 10.1016/s0886-3350(13)80553-6. [DOI] [PubMed] [Google Scholar]
- 3.Mermoud A, Ravinet E. Mechanism of filtration in non-penetrating filtering surgeries. In: Mermoud A, Shaarawy T, editors. Non-penetrating Glaucoma Surgery. 1st ed. London: Martin Dunitz; 2001. pp. 57–65. [Google Scholar]
- 4.Delarive T, Rossier A, Rossier S, et al. Aqueous dynamic and histological findings after deep sclerectomy with collagen implant in an animal model. Br J Ophthalmol. 2003;87:1340–44. doi: 10.1136/bjo.87.11.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kozlow WI, Bagrow SN, Anisimowa SY, et al. Nonpenetrating deep sclerectomy with collagen. Ophthalmosurgery. 1990;3:44–46. [Google Scholar]
- 6.Demailly P, Lavat P, Kretz G, Jenteur-Lunel MN. Non-penetrating deep sclerectomy with or without collagen device in primary open-angle glaucoma: middle-term retrospective study. Int Ophthalmol. 1997;20:131–40. doi: 10.1007/BF00212959. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez E, Schnyder CC, Sickenberg M, et al. Deep sclerectomy: results with and without collagen implant. Int Ophthalmol. 1997;20:157–62. doi: 10.1007/BF00212963. [DOI] [PubMed] [Google Scholar]
- 8.Shaarawy T, Nguyen C, Schnyder C, Mermoud A. Comparative study between deep sclerectomy with and without collagen implant: long term follow up. Br J Ophthalmol. 2004;88:95–98. doi: 10.1136/bjo.88.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaarawy T, Mermoud A. Deep sclerectomy in one eye vs deep sclerectomy with collagen implant in the contralateral eye of the same patient: long-term follow up. Eye. 2005;19:298–302. doi: 10.1038/sj.eye.6701469. [DOI] [PubMed] [Google Scholar]
- 10.Dahan E, Ravinet E, Ben-Simon GJ, Mermoud A. Comparison of the efficacy and longevity of nonpenetrating glaucoma surgery with and without a new, nonabsorbable hydrophilic implant. Ophthalmic Surg Lasers Imaging. 2003;34:457–63. [PubMed] [Google Scholar]
- 11.Kuddusi E, Ozkiris A, Evereklioglu C, et al. Deep sclerectomy with various implants: an experimental and histopathologic study in rabbit model. Ophthalmologica. 2004;218:264–69. doi: 10.1159/000078618. [DOI] [PubMed] [Google Scholar]
- 12.Kaluzny JJ, Jozwicki W, Wisniewska H. Histological biocompatibility of new, non-absorbable glaucoma deep sclerectomy implant. J Biomed Mater Res B Appl Biomater. 2007;81:403–9. doi: 10.1002/jbm.b.30677. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen Ch, Boldea R, Roy S, et al. Outflow mechanisms after deep sclerectomy with two different designs of collagen implant in an animal model. Graefe’s Arch Clin Exp Ophthalmol. 2006;244:1659–67. doi: 10.1007/s00417-006-0275-9. [DOI] [PubMed] [Google Scholar]
- 14.Basso A, Roy S, Mermoud A. Biocompatibility of an x-shaped zirconium implant in deep sclerectomy in rabbits. Graefe’s Arch Clin Exp Ophthalmol. 2008;246:849–55. doi: 10.1007/s00417-008-0782-y. [DOI] [PubMed] [Google Scholar]
- 15.Codreanu A, Tran HV, Wiaux C, et al. In vivo study comparing an X-shape polymethylmetacrylate and a cylindrical collagen implant for deep sclerectomy. Clin Exp Ophthalmol. 2011;39:135–41. doi: 10.1111/j.1442-9071.2010.02436.x. [DOI] [PubMed] [Google Scholar]
- 16.Marchini G, Marraffa M, Brunelli C, Morbio R, Bonomi L. Ultrasound biomicroscopy and intraocular-pressure-lowering mechanisms of deep sclerectomy with reticulated hyaluronic acid implant. J Cataract Refract Surg. 2001;27:507–17. doi: 10.1016/s0886-3350(00)00857-9. [DOI] [PubMed] [Google Scholar]
- 17.Ratner BD. Reducing capsular thickness and enhancing angiogenesis around implant drug release systems. J Control Release. 2001;78:211–18. doi: 10.1016/s0168-3659(01)00502-8. [DOI] [PubMed] [Google Scholar]
- 18.Jacob JT, Assouline M, Byrd T, McDonald M. Synthetic scleral reinforcement materials: I. Development and in vivo tissue biocompatibility response. J Biomed Mater Res. 1994;28:699–12. doi: 10.1002/jbm.820280607. [DOI] [PubMed] [Google Scholar]
- 19.Brauker JH, Carr-Brendel VE, Martinson LA, et al. Neovascularization of synthetic membranes directed by membrane architecture. J Biomed Mater Res. 1995;29:1517–24. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]