Abstract
The present study showed that incorporation of CpG adjuvant into plasmid DNA coding for NcGRA7 antigen resulted in a twofold increase in the level of protection against congenital transfer of Neospora caninum. The level of protection was considerably higher than that observed in pups born from dams immunized with nonrecombinant plasmid.
Neospora caninum is recognized as an important cause of reproductive failure in dairy cattle worldwide (5). The fetus is infected by N. caninum tachyzoites that have arisen from a latent tissue cyst infection in the dam or from oocysts that are ingested by the cow during gestation. While a commercial vaccine is being marketed to prevent congenital neosporosis (1, 2), it relies on immunization of cows with a crude N. caninum protein extract emulsified in adjuvant. The ultimate goal of vaccine development against neosporosis is to utilize specific N. caninum antigens that elicit protective immunity (7, 15). A number of N. caninum antigens, in particular NcSRS2 (3, 17), NcMIC3 (4), and NcGRA7 (12), have shown efficacy against neosporosis. Direct immunization with plasmid DNA coding for NcSRS2 or NcGRA7 has elicited partial protection against N. caninum tachyzoite challenge (3, 12). The purpose of the present study was to determine if the combination of CpG adjuvant and plasmid DNA coding for NcGRA7 would improve the level of protection afforded by pNcGRA7 alone. CpGs (oligodinucleotides) are known to enhance the immune response to vaccination by activating Th1 cells and release of associated cytokines (for review, see references 9, 14, and 19). Our hypothesis was that coinjection of pNcGRA7 and CpG may increase the level of immune-mediated resistance by stimulating Th1 responses that are believed to play a role in protective immunity against N. caninum (8, 16, 18).
N. caninum tachyzoites (NC-1 strain) were maintained in human foreskin fibroblast cells and harvested as described previously (10). Plasmid DNA containing the NcGRA7 coding sequence downstream of a cytomegalovirus (CMV) promoter (12) was prepared with the PerfectPrep plasmid Maxi kit according to the instructions provided by the manufacturer (Eppendorf, Westbury, N.Y.). The integrity of the plasmid DNA was verified by agarose gel electrophoresis; the majority of each preparation was in supercoiled form. Prior to injection into mice, plasmid DNA concentrations were estimated by measuring A260. Plasmid DNA in sufficient quantity for each vaccination was ethanol precipitated and suspended in 0.15 M NaCl just prior to injection into mice. Adult female BALB/c mice (10 to 20 per group; National Cancer Institute, Frederick, Md.) were injected in the tibialis anterior muscles of both hind limbs with a total dose of 100 μg of pCMVi-NcGRA7 or nonrecombinant pCMVi plasmid DNA. An equal number of female mice were injected with recombinant or nonrecombinant plasmid DNA containing CpG adjuvant (ImmunoEasy; Qiagen) according to the manufacturer's directions. The mice were given a booster injection 3 weeks later with the same preparation used in the primary immunization. As a positive control for vaccine efficacy, a separate group of female mice were immunized subcutaneously with 5 μg of a crude extract of N. caninum tachyzoites by established procedures (10). One week after booster injections, the mice were date mated as described previously (11). All female mice were challenged on the same day with 105 N. caninum tachyzoites at days 10 to 12 of gestation. A control group of female mice remained uninfected. The results are a compilation of two independent vaccination experiments.
Pups born from experimental or control vaccinated dams were necropsied for brain and lungs at 7 days of age according to previously described procedures (10). DNA extracted from these tissues was subjected to the Nc5 Neospora-specific PCR assay (13, 20) according to previously described procedures (10). Amplification reactions were analyzed by polyacrylamide gel electrophoresis, ethidium bromide staining, and image capture with a charge-coupled device camera. An internal standard of an Nc5 competitor molecule was included in each amplification reaction to control for a false-negative PCR (11). Appropriate positive (N. caninum tachyzoite genomic DNA) and negative (H2O) controls were included in each set of reactions. A minimum of three independent PCRs were performed for each sample.
Observed counts of N. caninum-positive and -negative pups were subjected to exact binary logistic regressions (6) by using LogXact4 for Windows (Cytel Software Corporation, Cambridge, Mass.). In this analysis, the groups receiving nonrecombinant pCMVi plasmid DNA (pCMViNR) with or without CpG adjuvant, the positive control N. caninum tachyzoite vaccine (NcTZ), phosphate-buffered saline (PBS) followed by N. caninum tachyzoite challenge, or PBS with no subsequent challenge, were specified as the reference treatment in separate logistic regression analyses. Odds ratios and associated confidence intervals were calculated for each of the treatments versus the reference treatment.
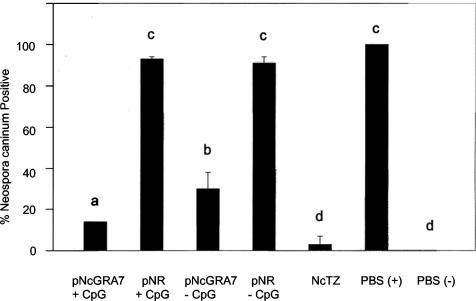
Consistent with earlier studies, the percentage of N. caninum-positive pups was lower in groups receiving pNcGRA7 than in those receiving nonrecombinant plasmid (P < 0.001, Fig. 1). Only 30% of pups born from pNcGRA7-immunized mice were positive for N. caninum DNA, compared to pups from the pNR-immunized mice, 91% of which were positive. The combination of CpG adjuvant and pNcGRA7 improved the level of protection over twofold (from 30 to 14%) compared to the result with pNcGRA7 alone (Fig. 1, P < 0.05). While this resistance to congenital transfer was not as high as that achieved with native N. caninum tachyzoite extract, it was over fivefold greater than the percentage of N. caninum-positive pups born from unimmunized dams or from mothers immunized with nonrecombinant plasmid containing CpG adjuvant (Fig. 1). Similar to our previous findings (12), there was a higher degree of variability among groups immunized with pNcGRA7 alone (Table 1). This finding may reflect differences in delivery of plasmid DNA and/or response to pNcGRA7 injection between adult mice. The addition of CpG adjuvant to the pNcGRA7 may decrease the variation in immune response elicited by stimulating a uniform Th1 environment at immunization site.
FIG. 1.
Protection against vertical transmission of N. caninum tachyzoites in pups born from mothers immunized directly with pNcGRA7 or pNR plasmid DNA with or without CpG adjuvant. Control groups that were challenged with N. caninum tachyzoites included pups born from dams immunized with NcTZ vaccine (positive vaccine) or PBS [PBS(+), negative vaccine]) PBS(−) controls were not challenged with N. caninum tachyzoites. Treatments labeled with different letters are statistically different (P < 0.05) by exact binary logistic regression analysis.
TABLE 1.
Protection against vertical transmission of N. caninum in pups born to BALB/c mice immunized with plasmid DNA encoding NcGRA7 antigen or with nonrecombinant plasmid DNA with or without CpG adjuvanta
| Vaccination | NcTZ vaccine challengeb | No. of positive pups/total
|
Vaccination | NcTZ vaccine challengeb | No. of positive pups/total
|
|||
|---|---|---|---|---|---|---|---|---|
| Vaccine trial 1 | Vaccine trial 2 | Vaccine trial 1 | Vaccine trial 2 | |||||
| pCMV-NcGRA7 + CpG | Yes | 0/5 | 0/5 | |||||
| 0/8 | 2/5 | |||||||
| 0/3 | 0/5 | |||||||
| 1/7 | 0/1 | |||||||
| 0/5 | 1/4 | |||||||
| 3/8 | 1/5 | |||||||
| 0/5 | ||||||||
| 4/8 | ||||||||
| 1/5 | ||||||||
| pCMV-NcGRA7 | Yes | 5/6 | 1/2 | |||||
| 3/5 | 0/5 | |||||||
| 0/6 | 3/5 | |||||||
| 0/4 | 3/5 | |||||||
| 5/10 | 0/6 | |||||||
| 1/6 | 0/5 | |||||||
| 0/5 | 0/5 | |||||||
| 3/6 | 2/3 | |||||||
| 4/6 | 0/4 | |||||||
| 0/2 | ||||||||
| pCMV-NRd + CpG | Yes | 8/8 | 3/4 | |||||
| 9/9 | 5/6 | |||||||
| 8/8 | 5/5 | |||||||
| 5/7 | 4/4 | |||||||
| 3/3 | 4/4 | |||||||
| 6/8 | ||||||||
| 5/5 | ||||||||
| 8/8 | ||||||||
| 7/7 | ||||||||
| pCMV-NR | Yes | 4/4 | 6/6 | |||||
| 5/5 | 4/4 | |||||||
| 6/6 | 5/5 | |||||||
| 6/6 | 5/6 | |||||||
| 4/6 | 2/7 | |||||||
| 6/6 | 6/8 | |||||||
| 3/3 | 8/8 | |||||||
| 5/6 | 3/3 | |||||||
| 3/3 | 6/6 | |||||||
| 9/9 | ||||||||
| NcTZc | Yes | 0/1 | 0/7 | |||||
| 1/10 | 0/5 | |||||||
| 0/6 | ||||||||
| 0/4 | ||||||||
| 0/6 | ||||||||
| PBS | Yes | 8/8 | 6/6 | |||||
| 6/8 | 5/5 | |||||||
| 5/6 | 7/7 | |||||||
| 6/6 | 2/2 | |||||||
| 9/9 | ||||||||
| 4/6 | ||||||||
| PBS | No | 0/5 | 0/7 | |||||
| 0/7 | 0/4 | |||||||
| 0/5 | 0/6 | |||||||
| 0/3 | ||||||||
Data are expressed as the number of pups positive for N. caninum DNA b, PCR/total number of pups in the litter.
N. caninum tachyzoite challenge infection.
PBS-insoluble whole N. caninum tachyzoite antigen.
NR, nonrecombinant plasmid.
While CpGs are known to activate nonspecific innate immunity, the presence of NcGRA7 appears to be critical for establishing immune resistance specifically against N. caninum tachyzoite infection in utero. Mice immunized with nonrecombinant pCMV-INT plasmid containing CpG adjuvant exhibited negligible protection against congenital neosporosis. Development of an effective vaccine against neosporosis may be feasible because, unlike studies of other protozoan parasites, immunization with N. caninum tachyzoite extract confers nearly 100% protection against tachyzoite invasion of the fetus in mice. NcGRA7 and NcSRS2 are probably two components of N. caninum tachyzoites that elicit this protective response. Future studies will test the efficacy of plasmids for both antigens in combination with CpG adjuvant at inducing levels of protection equal to native tachyzoite antigen. Whether immunization with plasmid DNA coding for specific N. caninum tachyzoite antigens prevents abortion in dairy cattle remains to be determined.
Acknowledgments
We acknowledge the excellent technical support of Oliver Kwok and Samuel Shen.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Andrianarivo, A. G., L. Choromanski, S. P. McDonough, A. E. Packham, and P. Conrad. 1999. Immunogenicity of a killed whole Neospora caninum tachyzoite preparation formulated with different adjuvants. Int. J. Parasitol. 29:1613-1625. [DOI] [PubMed] [Google Scholar]
- 2.Andrianarivo, A. G., J. D. Rowe, B. C. Barr, M. L. Anderson, A. E. Packham, K. W. Sverlow, L. Choromanski, C. Loui, A. Grace, and P. A. Conrad. 2000. A POLYGEN-adjuvanted killed Neospora caninum tachyzoite preparation failed to prevent foetal infection in pregnant cattle following i.v./i.m. experimental tachyzoite challenge. Int. J. Parasitol. 30:985-990. [DOI] [PubMed] [Google Scholar]
- 3.Cannas, A., A. Naguleswaran, N. Muller, S. Eperon, B. Gottstein, and A. Hemphill. 2003. Vaccination of mice against experimental Neospora caninum infection using NcSAG1- and NcSRS2-based recombinant antigens and DNA vaccines. Parasitology 126:303-312. [DOI] [PubMed] [Google Scholar]
- 4.Cannas, A., A. Naguleswaran, N. Muller, B. Gottstein, and A. Hemphill. 2003. Reduced cerebral infection of Neospora caninum-infected mice after vaccination with recombinant microneme protein NcMIC3 and ribi adjuvant. J. Parasitol. 89:44-50. [DOI] [PubMed] [Google Scholar]
- 5.Dubey, J. P. 2003. Neosporosis in cattle. J. Parasitol. 89:S42-S56. [Google Scholar]
- 6.Hosmer, D. W., and S. Lemeshow. 1989. Applied logistic regression. John Wiley & Sons, Inc., New York, N.Y.
- 7.Innes, E. A., A. G. Andrianarivo, C. Bjorkman, D. J. Williams, and P. A. Conrad. 2002. Immune responses to Neospora caninum and prospects for vaccination. Trends Parasitol. 18:497-504. [DOI] [PubMed] [Google Scholar]
- 8.Khan, I. A., J. D. Schwartzman, S. Fonseka, and L. H. Kasper. 1997. Neospora caninum: role for immune cytokines in host immunity. Exp. Parasitol. 85:24-34. [DOI] [PubMed] [Google Scholar]
- 9.Klinman, D. M. 2003. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2:305-315. [DOI] [PubMed] [Google Scholar]
- 10.Liddell, S., M. C. Jenkins, C. M. Collica, and J. P. Dubey. 1999. Prevention of vertical transfer of Neospora caninum in BALB/c mice by vaccination. J. Parasitol. 85:1072-1075. [PubMed] [Google Scholar]
- 11.Liddell, S., M. C. Jenkins, and J. P. Dubey. 1999. A competitive PCR assay for quantitative detection of Neospora caninum. Int. J. Parasitol. 29:1583-1587. [DOI] [PubMed] [Google Scholar]
- 12.Liddell, S., C. Parker, B. Vinyard, M. Jenkins, and J. P. Dubey. 2003. Immunization of mice with plasmid DNA coding for NcGRA7 or NcsHSP33 confers partial protection against vertical transmission of Neospora caninum. J. Parasitol. 89:496-500. [DOI] [PubMed] [Google Scholar]
- 13.Müller, N., V. Zimmermann, B. Hentrich, and B. Gottstein. 1996. Diagnosis of Neospora caninum and Toxoplasma gondii infection by PCR and DNA hybridization immunoassay. J. Clin. Microbiol. 34:2850-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutwiri, G., R. Pontarollo, S. Babiuk, P. Griebel, S. van Drunen Littel-van den Hurk, A. Mena, C. Tsang, V. Alcon, A. Nichani, X. Ioannou, S. Gomis, H. Townsend, R. Hecker, A. Potter, and L. A. Babiuk. 2003. Biological activity of immunostimulatory CpG DNA motifs in domestic animals. Vet. Immunol. Immunopathol. 91:89-103. [DOI] [PubMed] [Google Scholar]
- 15.Nishikawa, Y., T. Mikami, and H. Nagasawa. 2002. Vaccine development against Neospora caninum infection. J. Vet. Med. Sci. 64:1-5. [DOI] [PubMed] [Google Scholar]
- 16.Nishikawa, Y., K. Tragoolpua, N. Inoue, L. Makala, H. Nagasawa, H. Otsuka, and T. Mikami. 2001. In the absence of endogenous gamma interferon, mice acutely infected with Neospora caninum succumb to a lethal immune response characterized by inactivation of peritoneal macrophages. Clin. Diagn. Lab. Immunol. 8:811-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishikawa, Y., X. Xuan, H. Nagasawa, I. Igarashi, K. Fujisaki, H. Otsuka, and T. Mikami. 2001. Prevention of vertical transmission of Neospora caninum in BALB/c mice by recombinant vaccinia virus carrying NcSRS2 gene. Vaccine 19:1710-1716. [DOI] [PubMed] [Google Scholar]
- 18.Shibahara, T., T. Kokuho, M. Eto, M. Haritani, T. Hamaoka, K. Shimura, K. Nakamura, Y. Yokomizo, and I. Yamane. 1999. Pathological and immunological findings of athymic nude and congenic wild type BALB/c mice experimentally infected with Neospora caninum. Vet. Pathol. 36:321-327. [DOI] [PubMed] [Google Scholar]
- 19.Singh, M., and D. T. O'Hagan. 2003. Recent advances in veterinary vaccine adjuvants. Int. J. Parasitol. 33:469-478. [DOI] [PubMed] [Google Scholar]
- 20.Yamage, M., O. Flechtner, and B. Gottstein. 1996. Neospora caninum: specific oligonucleotide primers for the detection of brain “cyst” DNA of experimentally infected nude mice by the polymerase chain reaction (PCR). J. Parasitol. 82:272-279. [PubMed] [Google Scholar]