Summary
Background
The internal mammary artery and vein is often used as a site of anastomoses in microvascular breast reconstruction. This area supports lymphatic drainage of the breast and its role in breast cancer metastasis remains unclear. We hypothesize that sampling of internal mammary lymph nodes at the time of microvascular anastomoses preparation may identify persistent or recurrent local disease and mandate the need for additional treatment in this area.
Material/Methods
A retrospective chart review from 519 patients in the time between January 2006 and September 2009 was performed on all patients who underwent internal mammary lymph node sampling at the time of microvascular breast reconstruction.
Results
Microvascular breast reconstruction was performed in 519 patients. Enlarged internal mammary lymph nodes were found and harvested in 195 patients for histological review. Six of 195 (3.08%) were found positive for metastatic disease requiring additional oncologic treatment.
Conclusions
The internal mammary lymphatic drainage system is an important and often underappreciated pathway for breast metastasis. Routine sampling of these lymph nodes at the time of microvascular breast reconstruction is easy to perform and is a useful tool to identify women, who might require additional treatment and increase cancer-free survival.
Keywords: autologous breast reconstruction, internal mammary lymph nodes, tumour staging, DIEP flap, breast cancer
Background
Breast cancer is a common health problem and affects approximately 1 in 8 women. Accurate diagnosis and staging is important in the planning and treatment of this disease [1–3]. The status of the axillary lymph nodes is one of the most important prognostic factors in early stage breast cancer and is typically staged using sentinel lymph node biopsy (SLNB) or axillary lymph node dissection. The sentinel node is usually identified by means of a scanner after injection of a small amount of radiolabeled colloid into the area of the tumour before operation. Additional injection of blue dye in close proximity to the cancer during operation can further increase the gain of relevant sentinel lymph nodes [4].
However, there are limitations to the SLNB technique for the detection of internal mammary (IM) nodes as there is often interference from radioactivity at the primary tumour site resulting in a rather low specificity [4–6]. Additional methods for internal mammary assessment include MRI or PET scanning, but these techniques are not suitable to definitively identify positive nodes [5].
Since primary and secondary autologous breast reconstruction offers the ability to easily excise exposed IM lymphatic tissue in women who suffered from breast cancer, the present study wants to reveal the relevance of IM lymph node sampling in this specific situation.
Today, individualized reconstructive breast surgery should be a standard, integral part of the treatment options offered by breast centres. In many centres, autologous tissue is preferred for breast reconstruction as it provides a natural look and a natural look and sense to the to the reconstructed breast.
Sophisticated techniques are being adopted to ensure viability of grafted tissue, to reduce post-operative complications and to enhance long-term outcomes. In particular, the use of perforator flaps can minimize donor-site morbidity and optimise flap durability [7–9].
Koshima and Soeda first described a technique harvesting only lower abdominal skin and fat for reconstructive surgery [10]. Through the dissection of one or more perforators, who branch from the deep inferior epigastric vessels and traverse the muscle, rectus abdominis resection was avoided. Later Allen and Treece [11] published the first breast reconstruction using a deep inferior epigastric perforator (DIEP) flap. Today, many centres routinely use free DIEP flaps as they most closely resemble the look and texture of normal breast tissue and have less abdominal donor site morbidity when compared to other traditional autologous reconstructive techniques [7,8,12,13].
The internal mammary artery and vein are the primary recipient vessels in microvascular breast reconstruction. Associated with these vessels is the internal mammary lymphatic system which provides regional drainage for the anterior chest and breast and provides a means of possible breast cancer metastasis [14]. There have been reports, that sentinel node imaging studies identify internal mammary drainage in as many as 26–53% of subjects [15,16]. However, even if a positive hot spot is identified in this area, the IM lymph nodes are difficult to sample surgically especially in patients undergoing breast conserving surgery [16]. Thereby, the surgical management of IM nodes remains controversial today.
In the present study we investigated the sampling of IM lymph nodes at the time of microvascular breast reconstruction to identify the rate of previously undetected metastatic diseases. Once identified these patients would be candidates for adjuvant oncologic therapy. We hypothesized that this in return may increase survival in a small subgroup of patients who are at risk for a poor prognosis for cancer-free survival.
Material and Methods
Study design
The study design was a retrospective review performed at the Sana Hospital Duesseldorf (Germany). All patients who underwent microvascular breast reconstruction between January 2006 and September 2009 were included in the study if internal mammary lymph node sampling was performed. Demographic data obtained on each patient included age, diagnosis, staging, cancer treatment regimes, operative reports, histopathology, follow-up notes and complications.
Eligibility and inclusion criteria
To fulfil eligibility criteria, all procedures were performed using either the free DIEP flap or the fms-TRAM flap (free muscle sparing, transverse rectus abdominis muscle). Flap selection was determined by availability of sufficient abdominal tissue for flap harvesting, the patency of the inferior epigastric arteries (assessed by means of Doppler-ultrasound), and the presence of suitable perforator vessels in the target graft zone.
Surgical procedures
Surgical procedures were performed by two teams simultaneously. The used techniques are described in detail elsewhere [17].
Lymph node sampling
Microvascular breast reconstruction was performed following skin sparing mastectomy or as a secondary intervention. In either case, the pectoralis muscle overlying the third or fourth rib is opened or partially resected exposing the perichondrium and the intercostal space. The perichondrium is cut along the length of the anterior rib and the cartilaginous rib is removed. The posterior perichondrium is then opened exposing the internal mammary vessels. Typically two veins accompany one artery, although this pattern may vary as depicted in Figure 1.
Figure 1.
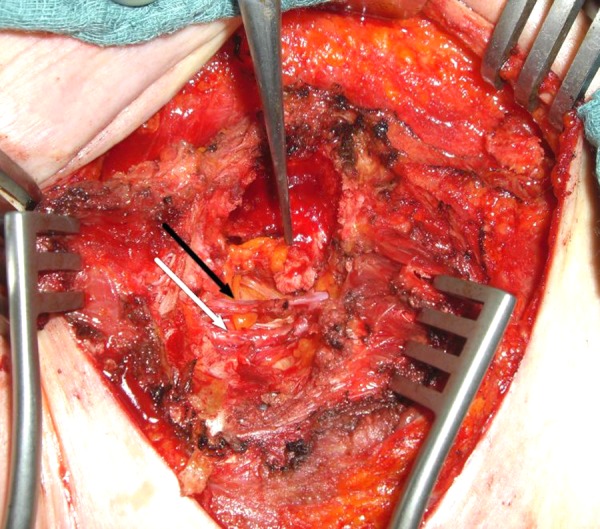
Internal mammary artery (white arrow) with concomitant vein (black arrow) after preparation.
By means of an operating microscope, visible lymphatic tissue is excised from the area surrounding the internal mammary vessels prior to preparation of the recipient vessels. Lymphatic sampling is only performed if there is evidence of enlarged lymphatic tissue. Specific imaging techniques like blue dye were not used for lymph node identification.
All dissected lymph nodes were sent to pathology for histological evaluation.
In patients who underwent primary reconstruction, axillary sentinel node biopsy was performed 2 weeks ahead of reconstructive surgery, using a standardized protocol for detection of sentinel nodes [18].
Patient demographics
Unilateral internal mammary dissection was performed in 166 patients (85.1%) and 29 patients (14.9%) had a bilateral procedure. The majority of women underwent delayed breast reconstruction (n=163, 83%) while 32 patients (16.4%) underwent primary reconstruction at the time of their mastectomy. 38 patients had in-situ disease (DCIS – ductal carcinoma in situ) (19.4%), 59 patients had early stage tumours (30.2%) and 76 patients had advanced tumour stages (38.9%). 14 patients had recurrence cancer (7.1%) and 8 patients had a prophylactic mastectomy with immediate reconstruction (4.1%). The number of lymph nodes harvested from the internal mammary system ranged from 1 to 4 (median: 2, diameter: 0.3–1.5 cm). A total of 92 patients received an adjuvant external-beam radiation therapy (EBRT) in the area of mastectomy prior to reconstruction.
Results
Between January 2006 and September 2009, a total of 519 patients underwent autologous breast reconstruction with either an unilateral DIEP or fms-TRAM flap (Table 1). Of these, 195 patients (37.6%) were found to have an enlarged or suspicious lymph node at the time vessel preparation (Figure 2).
Table 1.
Characteristics of 195 patients who received internal mammary lymph node sampling during reconstructive breast surgery.
| Number of patients (lymph node +) | 195 |
|
| |
| Mean age in years (range) | 49.8 (24–73) |
|
| |
| Type of operation | |
| Unilateral | 166 (85.1%) |
| Bilateral | 29 (14.9%) |
|
| |
| Used flap type | |
| DIEP | 115 (59.0%) |
| fms-TRAM | 80 (41.0%) |
|
| |
| Type of reconstruction | |
| Primary | 32 (16.4%) |
| Secondary | 163 (83.6%) |
Figure 2.
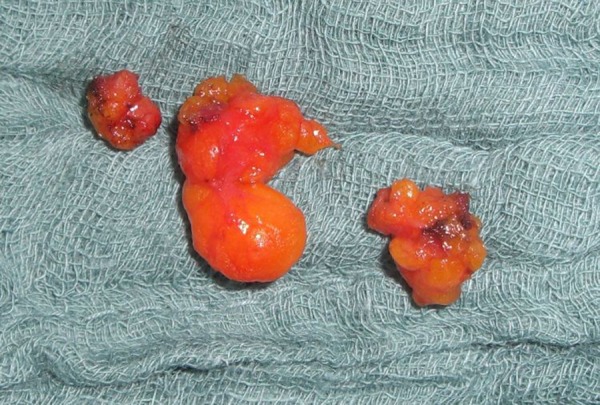
Macroscopically enlarged mediastinal tissue with suspected mediastinal lymph nodes.
Six of the 195 patients (3.08%) who underwent breast reconstruction and lymph node harvest tested positive for breast cancer metastasis (node positive group). The remaining 189 patients were found to be absent of metastatic lymphatic disease (node negative group). Micrometastases were present in all six node positive patients and three of these patients also demonstrated clinical suspicious tissue (macrometastases) and died during the observation period from May 2006 until November 2009. Lymph node sampling from the contralateral side was performed in 3 patients (all primary breast reconstructions). In these cases no metastases were detected within the obtained tissue. All patients belonging to the node positive group were tumour staged before and after surgery as depicted in Table 2.
Table 2.
Diagnostic stage migration due to internal mammary lymph node sampling including initial clinic stage in patients who received neo-adjuvant chemo-therapy.
| Initial clinical Stage | Date | Preoperative Staging (after MRM/SNB) | Date | Staging after Lymph node sampling (Stage migration) | Date |
|---|---|---|---|---|---|
| cT2 cN0 | 05/06 | ypT2 pN0 (0/8) G2 | 10/06 | pN1b | 07/07 |
| cT2 cN0 | 03/06 | ypT3 pN0 (0/23) G3 | 08/06 | pN1b | 03/08 |
| cT2 cN0 | 06/07 | ypT2 pN0 (0/3sn)G3 | 12/07 | pN1b | 02/08 |
| pT1a pTis N2 (4/13) G3 | 12/94 | rpT1b G2 | 08/04 | pN3b | 05/08 |
| pT2 pN1a (1/14) G2 | 08/06 | pN3b | 09/09 | ||
| cT2 cN2a | 12/05 | ypT3m ypN3a (16/17) G3 R0 | 03/06 | yrpTx R1 | 01/09 |
Within the node positive group, 4 patients received previous radiation therapy to the axillary region as well as to the thoracic wall, 5 patients had received previous chemotherapy, and 4 patients were treated with hormones (i.e. Tamoxifen). In the node negative group, 87 patients had undergone previous radiation therapy, 114 patients had received prior chemotherapy and 91 patients were treated with hormones.
The preoperative tumour localisation within the node positive group is represented in Table 3.
Table 3.
Initial tumour localisation of patients tested positive for internal mammary lymph node metastasis.
| Initial tumour localisation within the breast | Patients (n) sampled positively |
|---|---|
| Upper outer quadrant | 3 |
| Upper inner quadrant | 1 |
| Lower outer quadrant | 1 |
| Lower inner quadrant | 0 |
| Center of the breast | 1 |
Patients in the lymph node positive group were referred to a multi-disciplinary oncologic conference to determine the adjuvant therapy. Additional therapy included chemotherapy, radiation of the parasternal area and hormonal substitution with an aromatase inhibitor. Median survival in the node positive group was 15 months. One patient with a history of Hodgkin’s disease and irradiation to the mediastinum prior to breast cancer treatment was initially diagnosed with metastatic disease but was found to be node negative on a secondary pathologic review. There were no complications related to the lymph node harvest of recipient vessel dissection. A total flap loss occurred in one case of all 195 included patients (flap loss rate <1%).
Discussion
Today, breast cancer is the most common malignancy in women worldwide. It is the leading cause of cancer related to mortality, affecting 10–12% of the female population [19]. Detection of lymph node metastasis is an integral part of the staging process and an important component of the treatment regime. It is also a major prognostic factor of long-term survival. Although the axilla is the most common pathway for cancer metastasis, the breast exhibits several pathways for the lymphatic drainage. The internal mammary lymphatic system receives drainage from all four breast quadrants with a preference for the lower inner quadrant [20]. Spillane and his co-workers have shown in a sentinel node imaging study that internal mammary drainage occurred in 53% of medial tumours and 24% of lateral tumours [15]. Due to its complex anatomic location and coverage by the ribcage and anterior chest wall musculature, accessibility for elective lymph node sampling and ultrasound diagnosis is difficult in this region. Further, the technique of SLNB is limited for the identification of IM nodes due to a low rate of specificity [4,5,21,22]. An elective surgical node dissection has failed to improve survival rates [23]. Former studies questioning the value of elective internal mammary lymph node sampling revealed metastasis rates from 1.3% to 8% [14,24]. Of concern was that a positive metastatic sampling correlated with a poor prognosis and reduced survival. Additionally, positive internal mammary lymph nodes often coincide with axillary metastases. When found in isolation, the prognosis of ipsilateral parasternal lymph metastases is comparable to axillary metastases.
However, a standardized treatment algorithm has not been established for patients with localized internal mammary metastases [25]. Data concerning the beneficial effect of additional treatment options in case of internal mammary lymph node metastasis is limited and further investigation is warranted. Despite this void of knowledge, most oncologic surgeons would agree that additional therapy like increasing chemotherapy or adding radiotherapy to the parasternal region should be considered in patients with parasternal metastasis. In the present study, all patients from the node positive group received an additional radiation of the parasternal area and a re-staging including physical examination, blood sample, chest x-ray and abdominal ultrasound after presentation to a multidisciplinary tumour board. Four patients were recommended for an additional endocrine therapy. The results of our study point out that the correlation between “clinical suspicious or enlarged” internal mammary lymph nodes and detected metastasis is very poor. Hence one cannot rely on clinical judgement, a routine dissection of these nodes would be beneficial as it would change the stage of these patients and alter adjuvant therapy. A patient consent for this procedure might be necessary.
The predominance of secondary breast reconstructions in the present study is most likely caused by the limited number of centres who provide perforator based flaps (e.g. DIEP-flap) reconstruction. At this stage internal mammary sentinel node visualization via high-resolution lymphoscintigraphy is not feasible [15,26].
However, autologous breast reconstructions using free flaps can be easily combined with an internal mammary lymph node sampling. The internal mammary artery and vein are most commonly used donor vessels for microvascular anastomoses [27] secondary to their ease of access and the suitability for flap positioning. Usually a partial removal of the third and fourth rib is used for the access to the internal mammary vessels [28]. Fortunately, this area exhibits the highest metastasis rate when the internal mammary lymph nodes are affected (77%) [20]. During vessel preparation, the lymphatic chain can be easily removed. Hence, internal mammary lymph node sampling does not increase the operation time significantly. Newly propagated approaches for breast reconstruction with the aim to avoid an operative tissue transfer for reconstruction include techniques of tissue engineering and fat cell injection that have been claimed to mimic stem cell regenerative properties. However, to date, these methods have not reached a level that would allow for safe clinical application yet [29–32].
Intraoperative evidence of enlarged lymph nodes was found in 195 of the 519 patients. Of the 195 patients, only six were identified with internal mammary lymph node metastasis (3.08%). In a comparable study based on a small number of patients (n=11) who received internal lymph node sampling during breast reconstruction, one patient was found to have metastatic disease.
An explanation for the relatively low amount of positive lymph nodes that have been found in our study is that evaluation of internal mammary metastasis was limited to patients with suspicious or enlarged lymph nodes at the time of vessel preparation and no sentinel node imaging of the mammary internal lymph nodes had been performed prior surgery. This had been mainly, previously performed by others and might explain that the prevalence of positive internal mammary lymph nodes is lower than reported elsewhere [15,16,26]. Nevertheless, a prevalence of >3% within this present collective and the ease of sampling emphasize the relevance of internal mammary lymph node sampling during autologous breast reconstruction.
Conclusions
The internal mammary lymphatic drainage system is a relevant and often underappreciated pathway for breast metastasis. In our study we found the prevalence for internal mammary lymph node metastasis at primary and delayed reconstruction surgery. Interestingly, correlation between “clinical suspicious or enlarged” internal mammary lymph nodes and detected metastasis was rather poor. This might be due to the fact that only enlarged lymph nodes were taken into consideration and no sentinel node imaging prior surgery had been performed. However, we think that routine sampling of the internal mammary lymph nodes during microvascular breast reconstruction is important for two reasons. First, accessibility and removal is simple to perform at the time of internal mammary vessel preparation. Secondly, a notable rate of positive tested internal mammary lymph nodes was found in this current study at the time of breast reconstruction. Therefore we conclude that internal mammary lymph node sampling during autologous breast reconstruction is a straightforward tool to improve the staging of patients with breast cancer and to identify women, who might require additional treatment.
Acknowledgments
We thank Jennifer Covi (native speaker) for proof reading.
Footnotes
Conflict of interest statement
There are no commercial associations or financial relationships that might pose or create a conflict of interest with information presented in this manuscript.
Source of support: Departmental sources
References
- 1.Dragu A, Linke R, Kuwert T, et al. Interesting image. Tc-99m sestamibi SPECT/CT as a new tool for monitoring perfusion and viability of buried perforator based free flaps in breast reconstruction after breast cancer. Clin Nucl Med. 2010;35(1):36–37. doi: 10.1097/RLU.0b013e3181c3614d. [DOI] [PubMed] [Google Scholar]
- 2.Rybarova S, Vecanova J, Hodorova I, et al. Association between polymorphisms of XRCC1, p53 and MDR1 genes, the expression of their protein products and prognostic significance in human breast cancer. Med Sci Monit. 2011;17(12):BR354–63. doi: 10.12659/MSM.882121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franceschini G, Terribile D, Scafetta I, et al. Conservative treatment of a rare case of multifocal adenoid cystic carcinoma of the breast: case report and literature review. Med Sci Monit. 2010;16(3):CS33–39. [PubMed] [Google Scholar]
- 4.Albertini JJ, Lyman GH, Cox C, et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA. 1996;276(22):1818–22. [PubMed] [Google Scholar]
- 5.Harlow S, Krag D, Weaver D, Ashikaga T. Extra-Axillary Sentinel Lymph Nodes in Breast Cancer. Breast Cancer. 1999;6(2):159–65. doi: 10.1007/BF02966925. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi M, Tsugawa K, Miwa K, Taniya T. Sentinel lymph node biopsy and axillary lymph node dissection. Gan To Kagaku Ryoho. 2000;27(7):961–66. [PubMed] [Google Scholar]
- 7.Granzow JW, Levine JL, Chiu ES, Allen RJ. Breast reconstruction with the deep inferior epigastric perforator flap: history and an update on current technique. J Plast Reconstr Aesthet Surg. 2006;59(6):571–79. doi: 10.1016/j.bjps.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Busic V, Das-Gupta R, Mesic H, Begic A. The deep inferior epigastric perforator flap for breast reconstruction, the learning curve explored. J Plast Reconstr Aesthet Surg. 2006;59(6):580–84. doi: 10.1016/j.bjps.2005.04.061. [DOI] [PubMed] [Google Scholar]
- 9.Dragu A, Unglaub F, Wolf MB, et al. Scars and perforator-based flaps in the abdominal region: a contraindication? Can J Surg. 2010;53(2):137–42. [PMC free article] [PubMed] [Google Scholar]
- 10.Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg. 1989;42(6):645–48. doi: 10.1016/0007-1226(89)90075-1. [DOI] [PubMed] [Google Scholar]
- 11.Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. 1994;32(1):32–38. doi: 10.1097/00000637-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Blondeel PN. The sensate free superior gluteal artery perforator (S-GAP) flap: a valuable alternative in autologous breast reconstruction. Br J Plast Surg. 1999;52(3):185–93. doi: 10.1054/bjps.1998.3032. [DOI] [PubMed] [Google Scholar]
- 13.Blondeel PN, Boeckx WD. Refinements in free flap breast reconstruction: the free bilateral deep inferior epigastric perforator flap anastomosed to the internal mammary artery. Br J Plast Surg. 1994;47(7):495–501. doi: 10.1016/0007-1226(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 14.Galimberti V, Veronesi P, Arnone P, et al. Stage migration after biopsy of internal mammary chain lymph nodes in breast cancer patients. Ann Surg Oncol. 2002;9(9):924–28. doi: 10.1007/BF02557532. [DOI] [PubMed] [Google Scholar]
- 15.Spillane AJ, Noushi F, Cooper RA, et al. High-resolution lymphoscintigraphy is essential for recognition of the significance of internal mammary nodes in breast cancer. Ann Oncol. 2009;20(6):977–84. doi: 10.1093/annonc/mdn725. [DOI] [PubMed] [Google Scholar]
- 16.Chen RC, Lin NU, Golshan M, et al. Internal mammary nodes in breast cancer: diagnosis and implications for patient management – a systematic review. J Clin Oncol. 2008;26(30):4981–89. doi: 10.1200/JCO.2008.17.4862. [DOI] [PubMed] [Google Scholar]
- 17.Langer S, Munder B, Seidenstuecker K, et al. Development of a surgical algorithm and optimized management of complications – based on a review of 706 abdominal free flaps for breast reconstruction. Med Sci Monit. 2010;16(11):CR518–22. [PubMed] [Google Scholar]
- 18.Sakorafas GH, Peros G. Sentinel lymph node biopsy in breast cancer: what a physician should know, a decade after its introduction in clinical practice. Eur J Cancer Care (Engl) 2007;16(4):318–21. doi: 10.1111/j.1365-2354.2006.00762.x. [DOI] [PubMed] [Google Scholar]
- 19.Hortobagyi GN, de la Garza Salazar J, Pritchard K, et al. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer. 2005;6(5):391–401. doi: 10.3816/cbc.2005.n.043. [DOI] [PubMed] [Google Scholar]
- 20.Estourgie SH, Nieweg OE, Olmos RA, et al. Lymphatic drainage patterns from the breast. Ann Surg. 2004;239(2):232–37. doi: 10.1097/01.sla.0000109156.26378.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanis PJ, Nieweg OE, Valdes Olmos RA, et al. Impact of non-axillary sentinel node biopsy on staging and treatment of breast cancer patients. Br J Cancer. 2002;87(7):705–10. doi: 10.1038/sj.bjc.6600359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Ent FW, Kengen RA, van der Pol HA, et al. Halsted revisited: internal mammary sentinel lymph node biopsy in breast cancer. Ann Surg. 2001;234(1):79–84. doi: 10.1097/00000658-200107000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veronesi U, Marubini E, Mariani L, et al. The dissection of internal mammary nodes does not improve the survival of breast cancer patients. 30-year results of a randomised trial. Eur J Cancer. 1999;35(9):1320–25. doi: 10.1016/s0959-8049(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 24.Estourgie SH, Tanis PJ, Nieweg OE, et al. Should the hunt for internal mammary chain sentinel nodes begin? An evaluation of 150 breast cancer patients. Ann Surg Oncol. 2003;10(8):935–41. doi: 10.1245/aso.2003.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Freedman GM, Fowble BL, Nicolaou N, et al. Should internal mammary lymph nodes in breast cancer be a target for the radiation oncologist? Int J Radiat Oncol Biol Phys. 2000;46(4):805–14. doi: 10.1016/s0360-3016(99)00481-2. [DOI] [PubMed] [Google Scholar]
- 26.Noushi F, Spillane AJ, Uren RF, Gebski V. Internal mammary lymph node metastasis in breast cancer: predictive models to assist with prognostic influence. Breast. 2011;20(3):278–83. doi: 10.1016/j.breast.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Beier JP, Horch RE, Bach AD. Breast reconstruction after breast-cancer surgery. N Engl J Med. 2009;360(4):418–19. doi: 10.1056/NEJMc082264. author reply 420–21. [DOI] [PubMed] [Google Scholar]
- 28.Dupin CL, Allen RJ, Glass CA, Bunch R. The internal mammary artery and vein as a recipient site for free-flap breast reconstruction: a report of 110 consecutive cases. Plast Reconstr Surg. 1996;98(4):685–89. doi: 10.1097/00006534-199609001-00013. discussion 690–92. [DOI] [PubMed] [Google Scholar]
- 29.Boos AM, Loew JS, Deschler G, et al. Directly auto-transplanted mesenchymal stem cells induce bone formation in a ceramic bone substitute in an ectopic sheep model. J Cell Mol Med. 2011;15(6):1364–78. doi: 10.1111/j.1582-4934.2010.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klumpp D, Horch RE, Kneser U, Beier JP. Engineering skeletal muscle tissue – new perspectives in vitro and in vivo. J Cell Mol Med. 2010;14(11):2622–29. doi: 10.1111/j.1582-4934.2010.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polykandriotis E, Popescu LM, Horch RE. Regenerative medicine: then and now – an update of recent history into future possibilities. J Cell Mol Med. 2010;14(10):2350–58. doi: 10.1111/j.1582-4934.2010.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beier JP, Horch RE, Hess A, et al. Axial vascularization of a large volume calcium phosphate ceramic bone substitute in the sheep AV loop model. J Tissue Eng Regen Med. 2010;4(3):216–23. doi: 10.1002/term.229. [DOI] [PubMed] [Google Scholar]


