Abstract
Objective
Adiponectin, an anti-inflammatory and anti-diabetogenic adipokine, has an important regulatory effect on both the innate and adaptive limbs of the immune response. The objective of this study was to determine whether adiponectin is present in amniotic fluid (AF) and if its concentration changes with gestational age, in the presence of labor and in the presence of intra-amniotic infection (IAI) in patients with spontaneous preterm labor (PTL) and intact membranes.
Study design
This cross-sectional study included 468 patients in the following groups: 1) women in the mid-trimester of pregnancy (14–18 weeks) who underwent amniocentesis for genetic indications and delivered a normal neonate at term (n=52); 2) normal pregnant women at term with (n=49) and without (n=41) spontaneous labor; 3) patients with an episode of PTL and intact membranes who were classified into: a) PTL who delivered at term (n=149); b) PTL who delivered preterm (<37 weeks gestation) without IAI (n=108); and c) PTL with IAI (n=69) Adiponectin concentration in AF was determined by ELISA.
Results
1) The median AF adiponectin concentration at term was significantly higher than in the mid-trimester (35.6 ng/mL, interquartile range [IQR] 26.4–52.7 vs. 29.9 ng/mL, IQR 19.9–35.2; p=0.01); 2) among women with PTL and intact membranes, the median amniotic fluid adiponectin concentration was significantly higher in patients with IAI than in those without IAI who delivered either at term (54.3 ng/mL, 39.0–91.8 vs. 50.1 ng/mL, 33.2–72.8; p = 0.02) or preterm (47.6 ng/mL, 32.6–74.6; p = 0.01); and 3) among women at term, there was no significant difference in the median amniotic fluid adiponectin concentration between those with and without labor (33.7 ng/mL, IQR 21.7–53.9 vs. 35.6 ng/mL IQR 26.4–52.7; respectively p=0.5).
Conclusions
1) Adiponectin is a physiologic constituent of AF; and 2) adiponectin concentrations in AF are increased significantly with advancing gestation and in the presence of IAI. Collectively, these findings suggest that adiponectin plays a dynamic role in normal gestation and in the presence of IAI.
Keywords: Adiponectin, Adipokines, Pregnancy, Preterm labor, Intra-amniotic infection, Inflammation, Chorioamnionitis, Preterm delivery, Preterm Birth
Introduction
Spontaneous preterm parturition is syndromic in nature.1;2 Consistent with this view, several mechanisms of disease have been implicated in the pathogenesis of this condition, including intra-amniotic infection/inflammation (IAI),3–9 uteroplacental ischemia,10–12 uterine overdistention,13;14 allergic reactions,15;16 cervical insufficiency,2;17–19 hormonal disorders20 and others. Despite the strong experimental and epidemiologic evidence suggesting an association between the aforementioned etiological factors and spontaneous preterm parturition, intra-amniotic infection/inflammation is the only pathological process for which a solid body of evidence supports a cause and effect relationship with preterm parturition.9;21
The mechanism by which microbial invasion of the amniotic cavity (MIAC) and intra-amniotic inflammation induce preterm parturition involves the production and secretion of a wide range of pro-inflammatory cytokines2;22–29 These cytokines and chemokines include: interleukin (IL)-1,30–36 IL-6,35;37–41 tumor necrosis factor (TNF)-α,34;35;42–47 IL-18,48 1L-16,49 IL-8,50;51 colony-stimulating factors,52 macrophage migration inhibitory factor,53 monocyte chemotactic protein-1 (MCP-1),54 macrophage inflammatory protein-1α (MIP-1 α),55 RANTES,56 epithelial cell-derived neutrophil-activating peptide-78,57 CXCL6,58 CXCL1359 and CCL20.60 Several lines of evidence support the causal link between cytokines and prematurity: 1) exposure of human decidua to bacterial products results in increased production of IL-1, IL-6 and TNF-α;47;61;62 2) TNF-α,63;64 IL-163;65 and IL-1β66;67 can stimulate uterine contractility by induction of prostaglandin production;65;68 3) matrix metalloproteinases (MMPs) have been implicated in membrane rupture69–71 and cervical ripening.72 TNF-α can stimulate MMPs production;72 4) administration of IL-1 can induce preterm labor and preterm birth in pregnant mice.36 Moreover, treatment of these mice with the natural antagonist of IL-1 (IL-1 receptor antagonist) abrogates preterm parturition;32 5) amniotic fluid concentrations of immunoreactive IL-1,30;33 TNF-α,42;43;45;61 IL-6,37;38;41;73;74 IL-18,48 IL-16,49 IL-8,50;51 MCP-1,54 MIP-1α,55 RANTES,56 are elevated in women with preterm labor in the presence of infection and/or inflammation.
Adiponectin is a member of a growing group of peptides and proteins secreted by adipose tissue, termed adipocytokine.75 Some of these active molecules are produced mainly by adipose tissue (e.g. leptin,76 adiponectin,77), while others are shared with other systems (e.g. TNF-α,78 IL-679). Adiponectin, identified independently by four groups,80–83 took position as an important adipocytokines with a wide range of biological actions including insulin-sensitizing,84 anti-atherogenic85 and angiogenic86 properties. In addition to its well-established role in glucose metabolism and regulation of vasculature, adiponectin also has a potent anti-inflammatory effect.87
Despite its pleiotropic effects on metabolism, inflammatory and immune responses, only one report concerning amniotic fluid concentrations of this adipocytokine has been published.88 Moreover, no data exist regarding amniotic fluid concentrations of adiponectin in patients at term, or in labor (either term or preterm). Thus, the objective of this study was to determine whether adiponectin is present in amniotic fluid (AF) and if its concentration changes with gestational age, in the presence of labor and in the presence of intra-amniotic infection (IAI) in patients with spontaneous preterm labor (PTL) and intact membranes.
Materials and Methods
Study design and population
A cross-sectional study was conducted by searching our clinical database and bank of biological samples and included 468 patients in the following groups: 1) women in the mid-trimester of pregnancy (14–18 weeks) who underwent amniocentesis for genetic indications and delivered a normal neonate at term (n=52); 2) normal pregnant women at term with (n=49) and without (n=41) spontaneous labor; 3) patients with an episode of PTL and intact membranes who were classified into: a) PTL who delivered at term (n=149); b) PTL who delivered preterm (<37 weeks gestation) without IAI (n=108); and c) PTL with IAI (n=69).
All participating women provided written informed consent prior to the collection of amniotic fluid. The collection and utilization of amniotic fluid for research purposes was approved by the Institutional Review Boards of the participant institutions and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. Many of these samples have been previously used to study the biology of inflammation, hemostasis, and growth factor concentrations in normal pregnant women and those with pregnancy complications.
Definitions
Patients were considered to have a normal pregnancy outcome if they did not have any medical, obstetrical, or surgical complication, and delivered a term neonate (≥37 weeks) of appropriate birth weight for gestational age89;90 without complications. Spontaneous preterm labor was defined by the presence of regular uterine contractions occurring at a frequency of at least two every 10 minutes associated with cervical change before 37 completed weeks of gestation that required hospitalization. Intra-amniotic infection was defined as a positive amniotic fluid culture for micro-organisms. Intra-amniotic inflammation was diagnosed by an amniotic fluid IL-6 concentration ≥2.6 ng/mL.91
Sample collection
Amniotic fluid samples were obtained by transabdominal amniocentesis performed for genetic indication, evaluation of microbial status of the amniotic cavity and/or assessment of fetal lung maturity in patients approaching term. Women at term in labor consisted of women who were admitted for suspected preterm labor because of uncertain dates and had an amniocentesis for the assessment of fetal lung maturity. The criteria for considering that these patients were at term in labor was derived retrospectively if the following criteria were met: 1) spontaneous labor; 2) delivery within 24 hours from amniocentesis; 3) analysis of amniotic fluid consistent with maturity; 4) birthweight >2500 grams; 5) absence of respiratory distress syndrome or other complications of prematurity; and 6) physical examination of the newborn by pediatricians consistent with a term neonate. Samples of amniotic fluid were transported to the laboratory in a sterile capped syringe and cultured for aerobic/anaerobic bacteria and genital mycoplasmas. White blood cell (WBC) count, glucose concentration and Gram-stain were also performed shortly after collection as previously described92–94. The results of these tests were used for clinical management. Amniotic fluid IL-6 concentrations were used only for research purposes. Amniotic fluid not required for clinical assessment was centrifuged for 10 minutes at 4°C and the supernatant was aliquoted and stored at −70°C until analysis.
The correlation between amniotic fluid adiponectin concentrations and maternal age, BMI, amniotic fluid WBC count, amniotic fluid concentrations of glucose and IL-6 as well as with birthweight was determined among patients with spontaneous preterm labor with intact membranes who delivered within 48 hours. The 48 hours interval was chosen to preserve a meaningful temporal relationship between amniotic fluid adiponectin concentration and amniotic fluid concentrations of glucose, IL-6 as well as with birthweight
Determination of human adiponectin concentration in amniotic fluid
Specific and sensitive enzyme-linked immunoassays were used to determine concentrations of adiponectin in human amniotic fluid. Immunoassays for human adiponectin were purchased from Linco Research (Human Adiponectin ELISA, LINCO Research Inc, St Charles, MO, USA). Adiponectin assays were validated for use in human amniotic fluid in our laboratory prior to their use in this study. The calculated inter-assay and intra-assay coefficients of variation for resistin in our laboratory were 1.6% and 3.4% respectively. The sensitivity was 0.47 ng/ml.
Statistical analysis
The normality of the data was tested using the Shapiro-Wilk and Kolmogorov-Smirnov tests. Because amniotic fluid adiponectin concentrations were not normally distributed, non-parametric tests were used for analyses. Comparisons between proportions were performed with the Chi-square test. Kruskal-Wallis with post-hoc analysis and Mann-Whitney U tests were used for continuous variables. Adjustment for multiple comparisons was performed using the Bonferroni method. Spearman rank correlation was utilized to assess correlations between amniotic fluid concentration of adiponectin, WBC count and IL-6. A p-value of 0.05 was considered statistically significant. The statistical package used was SPSS v.14.0 (SPSS Inc., Chicago, IL, USA).
Results
Demographic and clinical characteristics of the study population
Table I presents the demographic and clinical characteristics of patients in the mid-trimester, term not in labor and term in labor groups. Table II displays the demographic and clinical characteristics of patients with spontaneous preterm labor and intact membranes. Among patients with PTL, those with IAI had a significantly lower median gestational age at amniocentesis than those without IAI who delivered preterm and those who delivered at term (Table II).
Table I.
Demographic and clinical characteristics of patients in the midtrimester and those at term with and without spontaneous labor
| Mid-trimester (n=52) | pa | Term No labor (n=41) | Term In labor (n=49) | pb | |
|---|---|---|---|---|---|
| Maternal age (years) | 36 (35 – 38) | <0.001 | 27 (21 – 32) | 22 (19 – 26) | 0.007 |
| GA at amniocentesis (weeks) | 16 (16 – 17) | <0.001 | 38 (38 – 39) | 38 (37.7 – 39.3) | NS |
| GA at delivery (weeks) | 39 (38 – 40) | NS | 38 (38 – 39) | 38.5 (38 – 39) | NS |
| Birth weight (grams) | 3344 (3144–3589) | NS | 3260 (3070–3668) | 3360 (3085–3550) | NS |
Values are expressed as median (interquartile range).
NS: not significant.
pa: comparison between patients in the mid-trimester and those at term not in labor
pb: comparison between patients at term not in labor and those at term in labor
Table II.
Demographic and clinical characteristics of patients presenting with spontaneous preterm labor with intact membranes
| PTL without IAI Term delivery (n=149) | p | PTL without IAI Preterm delivery (n=108) | pa | PTL with IAI Preterm delivery (n=69) | pb | |
|---|---|---|---|---|---|---|
| Maternal age (years) | 22 (19 – 30) | NS | 22 (19 – 30) | NS | 23 (20 – 27) | NS |
| GA at amniocentesis (weeks) | 31.8 (29.4 – 33.3) | NS | 31.9 (29.8 – 33.0) | <0.001 | 28.5 (24.9 – 32.7) | <0.001 |
| GA at delivery (weeks) | 38.7 (37.9 – 39.7) | <0.001 | 34.6.5 (33.1 – 35.5) | <0.001 | 29.4 (25.2 – 32.9) | <0.001 |
| Birth weight (grams) | 3165 (2899–3532) | <0.001 | 2335 (1940–2677) | <0.001 | 1140 (699–1995) | <0.001 |
Values expressed as median (interquartile range)
p: comparison between PTL who delivered at term and PTL without IAI
pa: comparison between PTL who delivered preterm without IAI and PTL with IAI
pb: comparison between PTL who delivered at term and PTL with IAI
PTL: preterm labor; GA: gestational age; IAI: intra-amniotic infection/inflammation
NS: not significant
Amniotic fluid adiponectin concentrations in the mid-trimester and at term
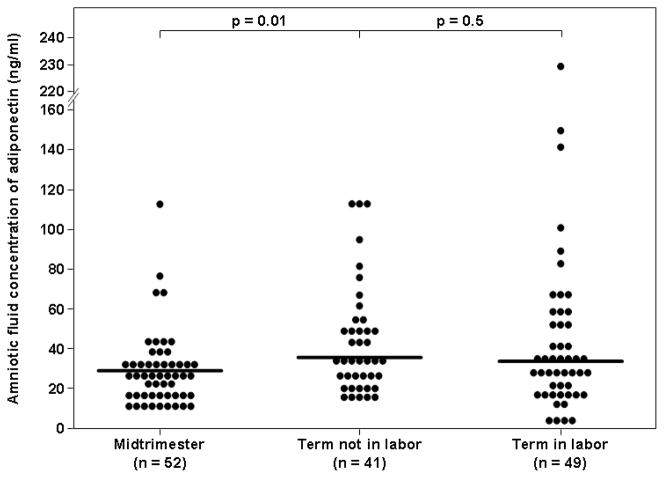
The median amniotic fluid concentration of adiponectin was significantly higher in patients at term not in labor than in those in the mid-trimester of pregnancy (35.6 ng/mL, interquartile range [IQR] 26.4–52.7 vs. 29.9 ng/mL, IQR 19.9–35.2; p=0.01, Figure 1). Among women at term, there was no significant difference in the median amniotic fluid adiponectin concentration between those with and without labor (33.7 ng/mL, IQR 21.7–53.9 vs. 35.6 ng/mL IQR 26.4–52.7 respectively; p=0.5, Figure 1).
Figure 1. Amniotic fluid adiponectin concentrations in women with a normal pregnancy in the mid-trimester and in those at term not in labor.
The median amniotic fluid concentration of adiponectin was significantly lower in the mid-trimester than at term. Among women at term, there was no significant difference in the median amniotic fluid concentration of adiponectin between patient in labor and those not in labor.
Amniotic fluid adiponectin concentrations in spontaneous preterm labor and intact membranes
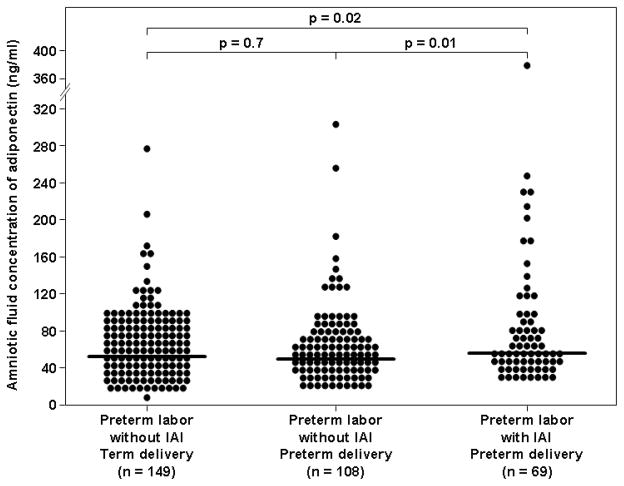
Among women with PTL and intact membranes, the median amniotic fluid adiponectin concentration was significantly higher in patients with IAI than in those without IAI who delivered either at term (54.3 ng/mL, 39.0–91.8 vs. 50.1 ng/mL, 33.2–72.8; p = 0.02, Figure 2) or preterm (54.3 ng/mL, 39.0–91.8 vs. 47.6 ng/mL, 32.6–74.6; p = 0.01, Figure 2). Among women with preterm labor without IAI, no significant difference was found in the median amniotic fluid concentration of adiponectin between women who delivered at term and those who delivered preterm (p=0.7, Figure 2).
Figure 2. Amniotic fluid concentration of adiponectin in women with spontaneous preterm labor and intact membranes.
Among women with preterm labor and intact membranes, the median amniotic fluid adiponectin concentration was significantly higher in patients with IAI than in those without IAI who delivered either at term or preterm.
Amniotic fluid adiponectin concentrations and intra-amniotic infection/inflammation
Amniotic fluid adiponectin concentrations correlated with amniotic fluid WBC count (Spearman rho coefficient: 0.26, p=0.01), amniotic fluid concentrations of glucose concentration (r=−0.37, p=0.002), and IL-6 (r=0.34, p<0.001).
Discussion
Principal findings of the study
1) adiponectin is a constituent of the amniotic fluid; 2) the median amniotic fluid concentration of adiponectin is higher at term that in the mid-trimester; 3) among women with spontaneous PTL and intact membranes, those with IAI had a significantly higher median amniotic fluid adiponectin concentration than those without IAI who delivered either at term or preterm; 4) amniotic fluid adiponectin concentrations were positively correlated with amniotic fluid WBC count and IL-6 concentrations; and 5) amniotic fluid adiponectin concentrations were negatively correlated with amniotic fluid glucose concentrations.
Adiponectin is a potent anti-inflammatory adipocytokine
In addition to its well-established role in metabolic regulation, adiponectin has emerged as a potent anti-inflammatory adipocytokine. The evidence in support of this view includes: 1) adiponectin induces production of anti-inflammatory cytokines (IL-10, IL-1 receptor antagonist95) by human monocytes, macrophages and dendritic cells; 2) adiponectin suppresses macrophage production of pro-inflammatory cytokines (TNF-α,96 interferon-gamma,95 IL-6) and mitigates their phagocytic activity in response to stimulation with lipopolysaccharide (LPS);97 3) adiponectin abates T-cell ability to evoke an allogenic T-cell response;95 4) adiponectin inhibits activation of the nuclear transcription factor NF-κB in endothelial cells;98 and 5) alterations in adiponectin concentrations characterize systemic inflammatory conditions such as overweight/obesity 99;100 insulin resistance,101 and systemic lupus erythematosus.102 The importance of adiponectin in major metabolic pathways, as well as in the innate and adaptive limbs of the immune response suggests that this adipocytokine plays a role in the regulation of the intricate interface between inflammation and metabolism.
Adiponectin and human pregnancy
Congruent with studies in non-pregnant individuals, adiponectin has been implicated in the metabolic adaptations to gestation 103–106, as well as in complications of pregnancy such as GDM107;108 and preeclampsia.109–114 Indeed, the importance of adiponectin in human pregnancy has been corroborated in the following reports: 1) normal pregnancy is associated with alterations in circulating adiponectin;104;115–119 2) circulating maternal adiponectin correlates with insulin resistance indices during pregnancy;103;120;121 3) gestational diabetes mellitus (GDM) is associated with decreased maternal concentrations of adiponectin as compared to normal pregnant women;121–124 4) overweight pregnant women have a lower plasma concentration of adiponectin125;126 than non-obese pregnant women; and 5) preeclampsia is associated with altered maternal circulating adiponectin.127–131
Adiponectin is a physiological component of the amniotic fluid
Adiponectin was detected in the amniotic fluid of all patients included in this study, from the early second trimester until 42 weeks of gestation, suggesting that adiponectin is a physiologic component of human amniotic fluid. This is a novel finding. Amniotic fluid adiponectin concentrations were determined in only one study.88 Baviera et al.88 reported the presence of adiponectin in 50 normal pregnant patients between 15–18 weeks of gestation. The results of the present study are in agreement with the latter study. In both studies adiponectin was detected in all samples. Moreover the mean and IQR of patients in mid-trimester are remarkably similar: 29.0 ng/mL, 19.9–35.2 in the present study and 26.8 13.9–37.3 ng/mL in the study conducted by Baviera et al.88 The results reported herein extend our knowledge by demonstrating that adiponectin is present in amniotic fluid in a wide range of gestational ages, at term and during preterm and term labor. Moreover, we were able to report higher amniotic fluid adiponectin concentrations with advancing gestation and in the presence of IAI.
Our findings characterize adiponectin as a novel physiologic constituent of amniotic fluid. Adiponectin is argued to be produced exclusively by adipocytes. Thus its presence in amniotic fluid deserves comment. A possible explanation could be a contamination from the maternal and fetal circulation. However, this possibility is highly unlikely from the following reasons: 1) contamination at the time of amniocentesis is rare, with maternal origin ranging from 0.3–10.8%, whereas fetal injury rates range between 0.6–2%.132 Of note, these data were obtained from a review that was published before the widespread use of ultrasound. Adiponectin was detected in all samples in the present report as well as in the study by Baviera et al;88 2) adiponectin concentrations are higher in the presence of IAI and correlate with amniotic fluid WBC count IL-6 and glucose concentrations; and 3) the mean and IQR of amniotic fluid adiponectin concentration of patients in mid-trimester are remarkably similar in the present study and the one conducted by Baviera et al.88 Collectively these data strongly suggest that adiponectin is a genuine component of amniotic fluid and its presence in that compartment cannot be attributed to contamination by maternal or fetal blood.
High amniotic fluid concentrations of adiponectin at term and in the presence of intra-amniotic infection: possible ontology and etiology
The presence of adiponectin in amniotic fluid is an intriguing finding since it has been argued that this adipokine is produced exclusively by adipocytes. Nevertheless, adiponectin have been detected in other body fluids including synovial,133 cerebrospinal,134;135 peritoneal,136 saliva137 and urine.102;138 Thus, although, the source for amniotic fluid adiponectin remains unknown, putative origins include fetal urine and fetal membranes.
Several lines of evidence support fetal urine as a source for amniotic fluid adiponectin. Circulating fetal adiponectin concentrations are very high.115;116;119;139;140 Indeed, they are two-to-four fold higher than the adults population.104;116;119 Since adiponectin is secreted in the urine102;138 (in its native conformation141) it is conceivable that amniotic fluid adiponectin originate from fetal urine. Moreover, term newborns have higher circulating adiponectin than preterm neonates.142;143 Thus, the higher median amniotic fluid concentrations of adiponectin at term than in mid trimester reported herein, can be explained by higher urine adiponectin in mature fetuses. Finally, fetal urine origin of adiponectin can also account for the higher amniotic fluid adiponectin in the presence of IAI. Renal dysfunction is associated with increased urinary adiponectin secretion.102;138 Fetal renal insult has been associated with intra-amniotic infection inflammation.144;145 Taken together, it is possible that renal dysfunction in fetuses affected by the inflammatory process results in increased fetal urine adiponectin concentration which in turn leads to high amniotic fluid adiponectin.
An additional explanation for the presence of adiponectin in the amniotic fluid could be the production and secretion by the fetal membranes. Lappas et al.146 have demonstrated an ex-vivo secretion of adiponectin by human amnion. Consistent with the findings of the latter report, we have recently found mRNA expression of adiponectin in human amnion.147 In additional, adiponectin was detected in the amniotic fluid as early as the 15th week of gestation, at which period the major contribution for amniotic fluid is not the fetal urine. Collectively, these data strongly suggest that the amnion is a source for amniotic fluid adiponectin. Secretion of adiponectin by the amnion can account for the higher concentrations at term since the surface area of the amnion is significantly larger at term than in the mid trimester. Finally, pathway analysis of human amnion gene expression identified adipocytokine signaling pathway as one of the signaling pathways associated with labor, which is an inflammatory process.148 Thus, it is tempting to suggest that the increased of amniotic fluid adiponectin in the presence of IAI is regulated by the amnion. Further studies will be needed in order to establish the intriguing relationship between the amnion and adiponectin in the context of IAI. These putative sources of adiponectin, i.e. fetal urine and amnion and the suggested explanations for the increased adiponectin at term and in the presence of IAI are not necessarily mutually exclusive and it is possible that both are involved in the regulation of adiponectin.
In conclusion, this is the first study describing the presence of adiponectin in amniotic fluid of term and preterm women and its association with IAI. These observations are in line with, and lend credence to, our previous studies that suggest an association between amniotic fluid adipokines and IAI. 149–151 Collectively, these novel findings suggest that adipokines play a dynamic, and hitherto unrecognized role in both normal gestation and in intra-amniotic infection. Concomitantly, the source of amniotic fluid adiponectin is unknown. Identification of amniotic fluid origin is of major importance in order to further elucidate the interaction between the IAI, adiponectin and fetal-placental compartments.
Acknowledgments
Supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–84. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113 (Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–84. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–28. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–07. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 6.Ledger WJ. Infection and premature labor. Am J Perinatol. 1989;6:234–36. doi: 10.1055/s-2007-999583. [DOI] [PubMed] [Google Scholar]
- 7.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–79. [PubMed] [Google Scholar]
- 8.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig. 2005;12:145–55. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–91. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 11.Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187:1137–42. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- 12.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–69. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 13.Hill LM, Breckle R, Thomas ML, Fries JK. Polyhydramnios: ultrasonically detected prevalence and neonatal outcome. Obstet Gynecol. 1987;69:21–25. [PubMed] [Google Scholar]
- 14.Ludmir J, Samuels P, Brooks S, Mennuti MT. Pregnancy outcome of patients with uncorrected uterine anomalies managed in a high-risk obstetric setting. Obstet Gynecol. 1990;75:906–10. [PubMed] [Google Scholar]
- 15.Bytautiene E, Romero R, Vedernikov YP, El-Zeky F, Saade GR, Garfield RE. Induction of premature labor and delivery by allergic reaction and prevention by histamine H1 receptor antagonist. Am J Obstet Gynecol. 2004;191:1356–61. doi: 10.1016/j.ajog.2004.06.092. [DOI] [PubMed] [Google Scholar]
- 16.Romero R, Mazor M, Avila C, et al. Uterine “allergy”: A novel mechanism for preterm labor. Am J Obstet Gynecol. 1991;164:375. Ref Type: Abstract. [Google Scholar]
- 17.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Pineles BL, Gotsch F, et al. Recurrent preterm birth. Semin Perinatol. 2007;31:142–58. doi: 10.1053/j.semperi.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633–38. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 20.Check JH, Lee G, Epstein R, Vetter B. Increased rate of preterm deliveries in untreated women with luteal phase deficiencies. Preliminary report Gynecol Obstet Invest. 1992;33:183–84. doi: 10.1159/000294877. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Gomez R, Mazor M, Ghezzi F, Yoon BH. The preterm labor syndrome. In: Elder MG, Romero R, Lamont RF, editors. Preterm labor. New York, NY: Churchill Livingstone; 1997. pp. 29–49. [Google Scholar]
- 22.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–83. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 23.Challis JR, Lye SJ, Gibb W, Whittle W, Patel F, Alfaidy N. Understanding preterm labor. Ann NY Acad Sci. 2001;943:225–34. doi: 10.1111/j.1749-6632.2001.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–28. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 25.Goldenberg RL, Andrews WW, Hauth JC. Choriodecidual infection and preterm birth. Nutr Rev. 2002;60:S19–S25. doi: 10.1301/00296640260130696. [DOI] [PubMed] [Google Scholar]
- 26.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22:281–342. [PubMed] [Google Scholar]
- 27.Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. In: Critchely H, Bennett P, Thornton S, editors. Preterm Birth. London: RCOG Press; 2004. pp. 28–60. [Google Scholar]
- 28.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–79. [PubMed] [Google Scholar]
- 29.Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil. Steril. 2004;82:799–804. doi: 10.1016/j.fertnstert.2004.05.076. [DOI] [PubMed] [Google Scholar]
- 30.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–23. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 31.Romero R, Sepulveda W, Mazor M, Brandt F, Cotton DB, Dinarello CA, et al. The natural interleukin-1 receptor antagonist in term and preterm parturition. Am J Obstet Gynecol. 1992;167:863–72. doi: 10.1016/s0002-9378(12)80003-2. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041–45. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- 33.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–23. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 34.Bry K, Hallman M. Transforming growth factor-beta 2 prevents preterm delivery induced by interleukin-1 alpha and tumor necrosis factor-alpha in the rabbit. Am J Obstet Gynecol. 1993;168:1318–22. doi: 10.1016/0002-9378(93)90388-y. [DOI] [PubMed] [Google Scholar]
- 35.Fidel PL, Jr, Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–75. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165:969–71. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- 37.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp. 1992;167:205–20. doi: 10.1002/9780470514269.ch13. [DOI] [PubMed] [Google Scholar]
- 38.Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol. 1993;30:167–83. doi: 10.1111/j.1600-0897.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 39.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81:941–48. [PubMed] [Google Scholar]
- 40.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32:200–10. doi: 10.1111/j.1600-0897.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 41.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336–41. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 43.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166:1576–87. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 44.Baumann P, Romero R, Berry S, Gomez R, McFarlin B, Araneda H, et al. Evidence of participation of the soluble tumor necrosis factor receptor I in the host response to intrauterine infection in preterm labor. Am J Reprod Immunol. 1993;30:184–93. doi: 10.1111/j.1600-0897.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 45.Maymon E, Ghezzi F, Edwin SS, Mazor M, Yoon BH, Gomez R, et al. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol. 1999;181:1142–48. doi: 10.1016/s0002-9378(99)70097-9. [DOI] [PubMed] [Google Scholar]
- 46.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–79. [PubMed] [Google Scholar]
- 47.Romero R, Mazor M, Manogue K, Oyarzun E, Cerami A. Human decidua: a source of cachectin-tumor necrosis factor. Eur J Obstet Gynecol Reprod Biol. 1991;41:123–27. doi: 10.1016/0028-2243(91)90089-4. [DOI] [PubMed] [Google Scholar]
- 48.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, et al. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol. 2000;183:1138–43. doi: 10.1067/mob.2000.108881. [DOI] [PubMed] [Google Scholar]
- 49.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Yoon BH, et al. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2000;182:135–41. doi: 10.1016/s0002-9378(00)70502-3. [DOI] [PubMed] [Google Scholar]
- 50.Cherouny PH, Pankuch GA, Romero R, Botti JJ, Kuhn DC, Demers LM, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169:1299–303. doi: 10.1016/0002-9378(93)90297-v. [DOI] [PubMed] [Google Scholar]
- 51.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–20. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 52.Saito S, Kato Y, Ishihara Y, Ichijo M. Amniotic fluid granulocyte colony-stimulating factor in preterm and term labor. Clin Chim Acta. 1992;208:105–09. doi: 10.1016/0009-8981(92)90027-n. [DOI] [PubMed] [Google Scholar]
- 53.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, et al. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2005;18:405–16. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17:365–73. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 55.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, et al. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol. 1994;32:108–13. doi: 10.1111/j.1600-0897.1994.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 56.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Araneda H, et al. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol. 1999;181:989–94. doi: 10.1016/s0002-9378(99)70337-6. [DOI] [PubMed] [Google Scholar]
- 57.Keelan JA, Yang J, Romero RJ, Chaiworapongsa T, Marvin KW, Sato TA, et al. Epithelial cell-derived neutrophil-activating peptide-78 is present in fetal membranes and amniotic fluid at increased concentrations with intra-amniotic infection and preterm delivery. Biology of Reproduction. 2004;70:253–59. doi: 10.1095/biolreprod.103.016204. [DOI] [PubMed] [Google Scholar]
- 58.Mittal P, Romero R, Kusanovic JP, Edwin SS, Gotsch F, Mazaki-Tovi S, et al. CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol. 2008;60:246–57. doi: 10.1111/j.1600-0897.2008.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nhan-Chang CL, Romero R, Kusanovic JP, Gotsch F, Edwin SS, Erez O, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2008;21:763–75. doi: 10.1080/14767050802244946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamill N, Romero R, Gotsch F, Pedro KJ, Edwin S, Erez O, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med. 2008;36:217–27. doi: 10.1515/JPM.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Casey ML, Cox SM, Beutler B, Milewich L, MacDonald PC. Cachectin/tumor necrosis factor-alpha formation in human decidua. Potential role of cytokines in infection-induced preterm labor. J Clin Invest. 1989;83:430–36. doi: 10.1172/JCI113901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romero R, Wu YK, Brody DT, Oyarzun E, Duff GW, Durum SK. Human decidua: a source of interleukin-1. Obstet Gynecol. 1989;73:31–34. [PubMed] [Google Scholar]
- 63.Molnar M, Romero R, Hertelendy F. Interleukin-1 and Tumor-Necrosis-Factor Stimulate Arachidonic-Acid Release and Phospholipid-Metabolism in Human Myometrial Cells. American Journal of Obstetrics and Gynecology. 1993;169:825–29. doi: 10.1016/0002-9378(93)90011-7. [DOI] [PubMed] [Google Scholar]
- 64.Romero R, Mazor M, Wu YK, Avila C, Oyarzun E, Mitchell MD. Bacterial endotoxin and tumor necrosis factor stimulate prostaglandin production by human decidua. Prostaglandins Leukot Essent Fatty Acids. 1989;37:183–86. doi: 10.1016/0952-3278(89)90083-5. [DOI] [PubMed] [Google Scholar]
- 65.Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989;37:13–22. doi: 10.1016/0090-6980(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 66.Hertelendy F, Rastogi P, Molnar M, Romero R. Interleukin-1beta-induced prostaglandin E2 production in human myometrial cells: role of a pertussis toxin-sensitive component. Am J Reprod Immunol. 2001;45:142–47. doi: 10.1111/j.8755-8920.2001.450304.x. [DOI] [PubMed] [Google Scholar]
- 67.Hertelendy F, Molnar M, Romero R. Interferon gamma antagonizes interleukin-1beta-induced cyclooxygenase-2 expression and prostaglandin E(2) production in human myometrial cells. J Soc Gynecol Investig. 2002;9:215–19. [PubMed] [Google Scholar]
- 68.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–79. [PubMed] [Google Scholar]
- 69.Athayde N, Edwin SS, Romero R, Gomez R, Maymon E, Pacora P, et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol. 1998;179:1248–53. doi: 10.1016/s0002-9378(98)70141-3. [DOI] [PubMed] [Google Scholar]
- 70.Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS, et al. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol. 2000;183:887–94. doi: 10.1067/mob.2000.108878. [DOI] [PubMed] [Google Scholar]
- 71.Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, et al. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol. 2002;187:1125–30. doi: 10.1067/mob.2002.127312. [DOI] [PubMed] [Google Scholar]
- 72.Watari M, Watari H, DiSanto ME, Chacko S, Shi GP, Strauss JF., III Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. American Journal Of Pathology. 1999;154:1755–62. doi: 10.1016/S0002-9440(10)65431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169:805–16. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 74.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–70. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 75.Montague CT, O’Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–88. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- 76.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 77.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 78.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 79.Vidal H. Gene expression in visceral and subcutaneous adipose tissues. Ann Med. 2001;33:547–55. doi: 10.3109/07853890108995965. [DOI] [PubMed] [Google Scholar]
- 80.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 81.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–89. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 82.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem(Tokyo) 1996;120:803–12. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 83.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–49. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 84.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 85.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–63. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 86.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–74. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 87.Shimabukuro M, Higa N, Asahi T, Oshiro Y, Takasu N, Tagawa T, et al. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab. 2003;88:3236–40. doi: 10.1210/jc.2002-021883. [DOI] [PubMed] [Google Scholar]
- 88.Baviera G, Corrado F, Dugo C, Cannata ML, Russo S, Rosario D. Midtrimester amniotic fluid adiponectin in normal pregnancy. Clin Chem. 2007;53:1723–24. doi: 10.1373/clinchem.2007.088542. [DOI] [PubMed] [Google Scholar]
- 89.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–68. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 90.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, et al. [A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000] Rev Med Chil. 2004;132:1155–65. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 91.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. American Journal of Obstetrics and Gynecology. 2001;185:1130–36. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 92.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–19. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 93.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–74. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 94.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821–30. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 95.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–35. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 96.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–29. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 97.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–32. [PubMed] [Google Scholar]
- 98.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 99.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 100.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, et al. Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J Perinat Med. 2007 doi: 10.1515/JPM.2007.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–46. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 102.Rovin BH, Song H, Hebert LA, Nadasdy T, Nadasdy G, Birmingham DJ, et al. Plasma, urine, and renal expression of adiponectin in human systemic lupus erythematosus. Kidney Int. 2005;68:1825–33. doi: 10.1111/j.1523-1755.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 103.Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, et al. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49:1677–85. doi: 10.1007/s00125-006-0264-x. [DOI] [PubMed] [Google Scholar]
- 104.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Wiser A, Schiff E, et al. Maternal serum adiponectin levels during human pregnancy. J Perinatol. 2007;27:77–81. doi: 10.1038/sj.jp.7211639. [DOI] [PubMed] [Google Scholar]
- 105.McLachlan KA, O’Neal D, Jenkins A, Alford FP. Do adiponectin, TNFalpha, leptin and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diabetes Metab Res Rev. 2006;22:131–38. doi: 10.1002/dmrr.591. [DOI] [PubMed] [Google Scholar]
- 106.McLachlan KA, O’Neal D, Jenkins A, Alford FP. Do adiponectin, TNFalpha, leptin and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diabetes Metab Res Rev. 2006;22:131–38. doi: 10.1002/dmrr.591. [DOI] [PubMed] [Google Scholar]
- 107.Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, Drevon CA. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004;83:341–47. doi: 10.1111/j.0001-6349.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 108.Worda C, Leipold H, Gruber C, Kautzky-Willer A, Knofler M, Bancher-Todesca D. Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2004;191:2120–24. doi: 10.1016/j.ajog.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 109.D’Anna R, Baviera G, Corrado F, Giordano D, De VA, Nicocia G, et al. Adiponectin and insulin resistance in early- and late-onset pre-eclampsia. BJOG. 2006;113:1264–69. doi: 10.1111/j.1471-0528.2006.01078.x. [DOI] [PubMed] [Google Scholar]
- 110.Haugen F, Ranheim T, Harsem NK, Lips E, Staff AC, Drevon CA. Increased plasma levels of adipokines in preeclampsia: relationship to placenta and adipose tissue gene expression. Am J Physiol Endocrinol Metab. 2006;290:E326–E333. doi: 10.1152/ajpendo.00020.2005. [DOI] [PubMed] [Google Scholar]
- 111.Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Erez O, Gotsch F, Chaiworapongsa T, Than NG, Kim SK, Nhan-Chang CL, et al. Maternal serum adiponectin multimers in preeclampsia. J Perinat Med. 2009;37(4):349–363. doi: 10.1515/JPM.2009.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Naruse K, Yamasaki M, Umekage H, Sado T, Sakamoto Y, Morikawa H. Peripheral blood concentrations of adiponectin, an adipocyte-specific plasma protein, in normal pregnancy and preeclampsia. J Reprod Immunol. 2005;65:65–75. doi: 10.1016/j.jri.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 113.Naruse K, Yamasaki M, Umekage H, Sado T, Sakamoto Y, Morikawa H. Peripheral blood concentrations of adiponectin, an adipocyte-specific plasma protein, in normal pregnancy and preeclampsia. J Reprod Immunol. 2005;65:65–75. doi: 10.1016/j.jri.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 114.Ramsay JE, Jamieson N, Greer IA, Sattar N. Paradoxical elevation in adiponectin concentrations in women with preeclampsia. Hypertension. 2003;42:891–94. doi: 10.1161/01.HYP.0000095981.92542.F6. [DOI] [PubMed] [Google Scholar]
- 115.Mazaki-Tovi S, Kanety H, Sivan E. Adiponectin and human pregnancy. Curr Diab Rep. 2005;5:278–81. doi: 10.1007/s11892-005-0023-2. [DOI] [PubMed] [Google Scholar]
- 116.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Efraty Y, Schiff E, et al. Determining the source of fetal adiponectin. J Reprod Med. 2007;52:774–78. [PubMed] [Google Scholar]
- 117.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Gotsch F, et al. Adiponectin multimers in maternal plasma. J Matern Fetal Neonatal Med. 2008;21:796–815. doi: 10.1080/14767050802266881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, et al. Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J Perinat Med. 2007;35:522–31. doi: 10.1515/JPM.2007.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sivan E, Mazaki-Tovi S, Pariente C, Efraty Y, Schiff E, Hemi R, et al. Adiponectin in human cord blood: relation to fetal birth weight and gender. J Clin Endocrinol Metab. 2003;88:5656–60. doi: 10.1210/jc.2003-031174. [DOI] [PubMed] [Google Scholar]
- 120.Lopez-Bermejo A, Fernandez-Real JM, Garrido E, Rovira R, Brichs R, Genaro P, et al. Maternal soluble tumour necrosis factor receptor type 2 (sTNFR2) and adiponectin are both related to blood pressure during gestation and infant’s birthweight. Clin Endocrinol(Oxf) 2004;61:544–52. doi: 10.1111/j.1365-2265.2004.02120.x. [DOI] [PubMed] [Google Scholar]
- 121.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. Reduced adiponectin concentration in women with gestational diabetes: a potential factor in progression to type 2 diabetes. Diabetes Care. 2004;27:799–800. doi: 10.2337/diacare.27.3.799. [DOI] [PubMed] [Google Scholar]
- 122.Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, Drevon CA. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004;83:341–47. doi: 10.1111/j.0001-6349.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 123.Thyfault JP, Hedberg EM, Anchan RM, Thorne OP, Isler CM, Newton ER, et al. Gestational diabetes is associated with depressed adiponectin levels. J Soc Gynecol Investig. 2005;12:41–45. doi: 10.1016/j.jsgi.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 124.Worda C, Leipold H, Gruber C, Kautzky-Willer A, Knofler M, Bancher-Todesca D. Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2004;191:2120–24. doi: 10.1016/j.ajog.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 125.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, et al. Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J Perinat Med. 2007 doi: 10.1515/JPM.2007.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hendler I, Blackwell SC, Mehta SH, Whitty JE, Russell E, Sorokin Y, et al. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am J Obstet Gynecol. 2005;193:979–83. doi: 10.1016/j.ajog.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 127.Haugen F, Ranheim T, Harsem NK, Lips E, Staff AC, Drevon CA. Increased plasma levels of adipokines in preeclampsia: relationship to placenta and adipose tissue gene expression. Am J Physiol Endocrinol Metab. 2006;290:E326–E333. doi: 10.1152/ajpendo.00020.2005. [DOI] [PubMed] [Google Scholar]
- 128.Kajantie E, Kaaja R, Ylikorkala O, Andersson S, Laivuori H. Adiponectin concentrations in maternal serum: elevated in preeclampsia but unrelated to insulin sensitivity. J Soc Gynecol Investig. 2005;12:433–39. doi: 10.1016/j.jsgi.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 129.Naruse K, Yamasaki M, Umekage H, Sado T, Sakamoto Y, Morikawa H. Peripheral blood concentrations of adiponectin, an adipocyte-specific plasma protein, in normal pregnancy and preeclampsia. J Reprod Immunol. 2005;65:65–75. doi: 10.1016/j.jri.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 130.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, et al. Adiponectin in severe preeclampsia. J Perinat Med. 2007 doi: 10.1515/JPM.2007.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ramsay JE, Jamieson N, Greer IA, Sattar N. Paradoxical elevation in adiponectin concentrations in women with preeclampsia. Hypertension. 2003;42:891–94. doi: 10.1161/01.HYP.0000095981.92542.F6. [DOI] [PubMed] [Google Scholar]
- 132.Galle PC, Meis PJ. Complications of amniocentesis: a review. J Reprod Med. 1982;27:149–55. [PubMed] [Google Scholar]
- 133.Schaffler A, Ehling A, Neumann E, Herfarth H, Tarner I, Scholmerich J, et al. Adipocytokines in synovial fluid. JAMA. 2003;290:1709–10. doi: 10.1001/jama.290.13.1709-c. [DOI] [PubMed] [Google Scholar]
- 134.Kos K, Harte AL, da Silva NF, Tonchev A, Chaldakov G, James S, et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab. 2007;92:1129–36. doi: 10.1210/jc.2006-1841. [DOI] [PubMed] [Google Scholar]
- 135.Neumeier M, Weigert J, Buettner R, Wanninger J, Schaffler A, Muller AM, et al. Detection of adiponectin in cerebrospinal fluid in humans. Am J Physiol Endocrinol Metab. 2007;293:E965–E969. doi: 10.1152/ajpendo.00119.2007. [DOI] [PubMed] [Google Scholar]
- 136.Takemura Y, Osuga Y, Harada M, Hirata T, Koga K, Yoshino O, et al. Concentration of adiponectin in peritoneal fluid is decreased in women with endometriosis. Am J Reprod Immunol. 2005;54:217–21. doi: 10.1111/j.1600-0897.2005.00300.x. [DOI] [PubMed] [Google Scholar]
- 137.Toda M, Tsukinoki R, Morimoto K. Measurement of salivary adiponectin levels. Acta Diabetol. 2007;44:20–22. doi: 10.1007/s00592-007-0236-8. [DOI] [PubMed] [Google Scholar]
- 138.Koshimura J, Fujita H, Narita T, Shimotomai T, Hosoba M, Yoshioka N, et al. Urinary adiponectin excretion is increased in patients with overt diabetic nephropathy. Biochem Biophys Res Commun. 2004;316:165–69. doi: 10.1016/j.bbrc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 139.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Schiff E, Sivan E. Cord blood adiponectin in large-for-gestational age newborns. Am J Obstet Gynecol. 2005;193:1238–42. doi: 10.1016/j.ajog.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 140.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Yinon Y, Wiser A, et al. Adiponectin and leptin concentrations in dichorionic twins with discordant and concordant growth. J Clin Endocrinol Metab. 2008 doi: 10.1210/jc.2008-2118. [DOI] [PubMed] [Google Scholar]
- 141.Shen YY, Hughes JT, Charlesworth JA, Kelly JJ, Peake PW. Adiponectin is present in the urine in its native conformation, and specifically reduces the secretion of MCP-1 by proximal tubular cells. Nephrology (Carlton) 2008;13:405–10. doi: 10.1111/j.1440-1797.2008.00949.x. [DOI] [PubMed] [Google Scholar]
- 142.Kajantie E, Hytinantti T, Hovi P, Andersson S. Cord plasma adiponectin: a 20-fold rise between 24 weeks gestation and term. J Clin Endocrinol Metab. 2004;89:4031–36. doi: 10.1210/jc.2004-0018. [DOI] [PubMed] [Google Scholar]
- 143.Siahanidou T, Mandyla H, Papassotiriou GP, Papassotiriou I, Chrousos G. Circulating levels of adiponectin in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2007;92:F286–F290. doi: 10.1136/adc.2006.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 145.Yoon BH, Kim YA, Romero R, Kim JC, Park KH, Kim MH, et al. Association of oligohydramnios in women with preterm premature rupture of membranes with an inflammatory response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1999;181:784–88. doi: 10.1016/s0002-9378(99)70301-7. [DOI] [PubMed] [Google Scholar]
- 146.Lappas M, Yee K, Permezel M, Rice GE. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J Endocrinol. 2005;186:457–65. doi: 10.1677/joe.1.06227. [DOI] [PubMed] [Google Scholar]
- 147.Han YM, Romero R, Kim JS, Tarca AL, Kim SK, Draghici S, et al. Region-specific gene expression profiling: novel evidence for biological heterogeneity of the human amnion. Biol Reprod. 2008;79:954–61. doi: 10.1095/biolreprod.108.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Romero R. Spontaneous labor at term is characterized by a genomic signature of acute inflammation in the chorioamniotic membranes but not in the systemic circulation. Am J Obstet Gynecol. 2004;191:S138. [Google Scholar]
- 149.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, et al. Resistin in Amniotic Fluid and its Association with Intra-amniotic Infection and Inflammation. The Journal of Maternal-Fetal and Neonatal Medicine. 2008 doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med. 2008 doi: 10.1515/JPM.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Erez O, Than GN, Kim SK, et al. Retinol Binding Protein 4: An Adipokine Associated with Intra-amniotic Infection/Inflammation. J Matern Fetal Neonatal Med. 2009 doi: 10.3109/14767050902994739. [DOI] [PMC free article] [PubMed] [Google Scholar]