Summary
Background
Adequate persistence of oral antidiabetic treatment is highly important to achieve proper glycemic control in patients with type 2 diabetes. The aim of this study was to evaluate the persistence of initial treatment with metformin and/or sulphonylureas in patients with type 2 diabetes.
Material/Methods
The study was performed among diabetic patients (n=256,384) who were with newly prescribed oral antidiabetic drugs (metformin and/or sulphonylureas) between 2007 and 2009. For making comparison, patients with newly prescribed statin or clopidogrel therapy (with and without percutaneous coronary intervention) were investigated. The database of the Hungarian National Health Insurance Fund Administration was used.
Results
The 1-year persistence of initial treatment with metformin, sulphonylureas or metformin/sulphonylurea combination was 47.7%, 45.4% and 55.8%, respectively, which was significantly better than the persistence of statin therapy (26.3%) but worse than that of clopidogrel therapy in patients undergoing coronary intervention (73.2%). Within the sulphonylurea group there was a tendency of better persistence of treatment with the “modified-release” tablets at 12 months compared to the conventional sulphonylureas (47.8 vs. 42.2%). The persistence of therapy using metformin 1000 mg – 60 tablets was significantly better (60.4%) at 12 months than that of other forms of metformin therapy with lower doses and smaller boxes (with fewer tablets) analyzed together (47.7%).
Conclusions
The persistence of initial treatment with metformin and/or sulphonylureas is far from optimal. Better diabetic care and continuous patient education should be encouraged to achieve higher persistence of oral antidiabetic treatment in patients with type 2 diabetes.
Keywords: type 2 diabetes mellitus, oral antidiabetic treatment, persistence of treatment, metformin, sulphonylureas, statin, clopidogrel
Background
Successful treatment of chronic diseases depends on a number of factors. It is understood that in order to achieve the treatment goals not only physicians and medical personnel but also the patients have to make adequate efforts.
The term “compliance”, according to the traditional approach, focuses on the activities of the physician. This concept is somewhat out-of-date now, as the work overload of doctors is a limiting factor and patients are able to obtain information about their illness from a number of other sources. It is also clear that besides external motivation (medical advice), developing internal motivation of the patient is of fundamental importance. The term “adherence” reflects the more modern, patient-oriented concept of patient cooperation that is often used in relation to a particular therapy. Adherence reflects the proportion of medications effectively taken. “Persistence”, the duration of continuous therapy from initiation to discontinuation of a particular treatment [1], is another easily measurable indicator.
Diabetes mellitus is a lifelong disease that requires appropriate lifestyle modification and drug treatment in order to achieve efficient metabolic control and to prevent late complications. Most diabetic patients have type 2 disease. This type of diabetes usually manifests in adults and in the elderly, but has recently been diagnosed in an increasing number of younger patients, too. If signs of acute metabolic deterioration cannot be detected, the treatment of patients – besides complying with lifestyle and dietary recommendations – means the use of oral antidiabetic agents. Among the oral antidiabetic drugs, according to the international and national guidelines, metformin is recommended as first-line treatment. If metformin intolerance is present or the drug is contraindicated, a sulphonylurea may be prescribed [2,3]. Because of the progressive nature of type 2 diabetes mellitus, diabetic patients might later receive combination therapy [4–6]. According to the results of a national survey conducted in Hungary (MULTI GAP), the most commonly used combination is metformin + sulphonylurea, with only a minority of patients receiving other, newer forms of combination therapy [7].
Adequate persistence of oral antidiabetic treatment of patients with type 2 diabetes mellitus is essential to achieve efficient glycemic control. Data on the persistence of oral antidiabetic treatment are not available in Hungary. In our study, using the database of the National Health Insurance Fund Administration, the persistence of the most common initial oral antidiabetic therapy – metformin and/or sulphonylureas – between 2007 and 2009 was analyzed. Our data were compared to the persistence of other widely used therapies for cardiovascular prevention (statins and clopidogrel).
Material and Methods
Patients (n=256,384) starting oral antidiabetic therapy (metformin and/or sulphonylureas) between January 1, 2007 and March 31, 2009 according to the database of the National Health Insurance Fund Administration (study license number: 44-p-82/2010) were enrolled in this study. The database was analyzed anonymously. No dispensing of oral antidiabetic drugs or insulin to the patients occurred since January 1, 2006, therefore they can be considered as receiving starting antidiabetic therapy for the first time. We excluded patients whose initial oral antidiabetic medication was other than metformin and/or sulphonylureas. More women (55%) than men (45%) were involved but this corresponds to the total population of Hungary (56% women, 44% men, total population 10,066,158 by Jan 1, 2007). The highest number of patients was in the 50–59 years age group, followed 60–69 years and ≥70 years (Table 1.) In the patients receiving sulphonylureas, the persistence of conventional and “modified-release” preparations were also evaluated. At that time, gliclazide MR was the only available “modified-release” sulphonylurea in Hungary. In the metformin group, the persistence of a given preparation with higher dose and larger box (Metformin® 1000 mg – 60 tablets) was determined separately. As more than 20 sulphonylurea or metformin generic drugs with different doses were available during the study period in Hungary, no other particular statistical analysis than “modified-release” sulphonylurea preparation (ie, gliclazide MR) versus all other conventional sulphonylureas or metformin with higher dose and larger box (ie, Metformin® 1000 mg – 60 tablets) versus all other metformin preparations could be carried out. For each patient, dispensing of the prescribed drugs was followed until March 31, 2010.
Table 1.
Distribution of diabetic patients (n=256,384) according to sex and age-groups.
| Men n (%) | Women n (%) | Total n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Metformin | 51.898 (45) | 63.528 (55) | 115.426 (100) | |||||
| Metformin + sulphonylurea | 7.824 (50) | 7.772 (50) | 15.596 (100) | |||||
| Sulphonylurea | 55.438 (44) | 69.924 (56) | 125.362 (100) | |||||
| Total | 115.160 (45) | 141.224 (55) | 256.384 (100) | |||||
| <20 ys n (%) | 20–29 ys n (%) | 30–39 ys n (%) | 40–49 ys n (%) | 50–59 ys n (%) | 60–69 ys n (%) | ≥70 ys n (%) | Total n (%) | |
| Metformin | 1.196 (1) | 3.740 (3) | 7.872 (7) | 16.826 (15) | 36.534 (32) | 29.902 (25) | 19.356 (17) | 115.426 (100) |
| Metformin + sulphonylurea | 166 (1) | 294 (2) | 984 (6) | 2.342 (15) | 5.192 (33) | 4.194 (27) | 2.424 (16) | 15.596 (100) |
| Sulphonylurea | 1.156 (1) | 1.850 (1) | 5.552 (4) | 14.706 (12) | 35.776 (29) | 34.190 (27) | 32.132 (26) | 125.362 (100) |
| Total | 2.518 (1) | 5.884 (2) | 14.408 (6) | 33.874 (13) | 77.502 (30) | 68.286 (27) | 53.912 (21) | 256.384 (100) |
The persistence curves were obtained by using time-to-event analysis and the numerical value of persistence in each case was calculated by Kaplan-Meier method [8]. Patients were considered persistent if: 1) the medicine was taken (dispensed) throughout the test period (continuous monotherapy), 2) a different oral antidiabetic drug or insulin was started while initial oral therapy was maintained (add-on combination therapy), or 3) if a replacement therapy had been initiated by any antidiabetic drug inclusive of insulin (discontinuation with initial oral therapy and switching to any other antidiabetic drug). Overall, this means that patients were considered to be persistent if they started their oral antidiabetic therapy with metformin and/or sulphonylureas and later continued to use (drugs were dispensed) any hypoglycemic agents (insulin included). Consequently, patients were classified as non-persistent if they started the antidiabetic therapy and then later – including the grace period – did not receive any antidiabetic medication. The grace period is the permissible time gap for the patient to resume taking the medication following a discontinuation and still be considered as persistent.
It was determined in advance for how many days the defined dosage forms of the medication would last for a patient. Based on these results it was calculated for how long the dispensed quantities would be enough for the patients. A grace period of 180 days was applied according to the ISPOR (International Society for Pharmacoeconomics and Outcomes Research) criteria often used in international literature [9]. The therapy was considered continuous if the patient still had medication (either an oral antidiabetic drug or insulin) 180 days before the end of the study period. A patient was considered non-persistent if the last dispensed amount of medication had been used up, based on the earlier dosing, and within a further 180 days no repeated dispensing occurred.
We excluded those patients who had a “negative” drug dispensing event (technical operation, correction or cancellation of a prescription), because consumption of medication could not be properly estimated in these cases. The length of insulin therapy was determined as follows: when administered once a day, our previously defined, estimated value was used. In case of multiple daily administrations, we estimated the average daily amount of insulin the patient used, and based on this value, we determined the duration that the actual dispensed amount was sufficient for.
The duration of antidiabetic therapies was compared to the persistence of clopidogrel and statin therapies; in both cases a 180-day grace period was used. In the case of statins only those patients whose calculated daily dose was 1±0.25 tablet were included in the analysis. A dose of 1 tablet/day was assumed for each patient at the first dispensing; the daily dose was later estimated using the dispensed quantities and the interval between repeated dispensing. If the calculated daily dose appeared to fall below or above the range of 1±0.25 tablet per day, the patient was excluded from the study. In the case of clopidogrel we separately analyzed patients in whom clopidogrel therapy was initiated following (or up to 30 days before) percutaneous coronary intervention (PCI) from those who did not undergo such intervention during the treatment period. When determining the persistence of use of statins and clopidogrel, all dosage forms available in Hungary were taken into account. Those patients who discontinued any statin or clopidogrel treatment (no more drugs dispensed) were considered non-persistent. The persistence curves of statins and clopidogrel were analyzed similarly to those of oral antidiabetic drugs, for a 27-month monitoring period.
Persistence was determined using Kaplan-Meier survival analysis method and length-of-therapy data were plotted using Kaplan-Meier curves. The National Health Insurance Fund Administration provided the data only in an aggregate and individually unidentifiable manner in order to protect the patients’ privacy. In case of less than 10 patients being included in the database in a given month, the “no data” notation appeared, as with fewer than 10 patients the protection of privacy would not have been fulfilled. Because of this the Kaplan-Meier curves could not cover the entire duration of the study. The numerical value (with 95% confidence interval [95% CI]) of persistence of the actual drug therapy (the proportion of persistent patients in the given cohort) was determined 12 months after the initiation of the medication. The statistical significance of persistence values at 12 months were compared by using the Peto-Wilcoxon test. The p<0.05 value was considered statistically significant.
Results
During the study period 115,426 patients started metformin monotherapy; 125,362 patients initiated sulphonylurea monotherapy, whereas metformin + sulphonylurea combination therapy was begun in 15,596 patients. The persistence of drug therapy fell after 2 months to 57.4% (95% CI 57.2–57.7) in the group with sulphonylurea monotherapy, to 64.8% (95% CI 64.6–65.1) in the group with metformin monotherapy and to 68.0% (95% CI 67.3–68.7) in the group with sulphonylurea + metformin combination therapy. At the 12th month of follow-up the persistence values were as follows: sulphonylurea treatment 45.4% (95% CI 45.1–45.7), metformin therapy 47.7% (95% CI 47.4–48.0) and metformin + sulphonylurea combination therapy 55.8% (95% CI 55.1–56.6) (p<0.0001 in all comparisons).
Within the sulphonylurea group there was a significantly better persistence of treatment with the “modified-release” tablets at 12 months compared to the other conventional sulphonylureas (47.8% [95% CI 47.4–48.2] vs. 42.2% [95% CI 41.8–42.6], p<0.001).
The persistence of therapy using metformin 1000 mg – 60 tablets (Meforal®) was significantly (p<0.001) better (60.4%; 95% CI 59.9–60.9) at 12 months than that of other forms of metformin therapy with lower dose and smaller box analyzed together (40.4%; 95% CI 40.1–40.8). There was a considerable difference between men and women in the persistence of therapy using Meforal® 1000 mg – 60 tablets (men: 62.2%; 95% CI 61.5–62.8; women: 58.8%; 95% CI 58.2–59.5; p<0.001). The persistence of therapy using Meforal® 1000 mg – 60 tablets was the best in patients aged between 40 and 70 years (60–66%), the persistence was reduced both in patients over 70 years of age (58%) and less than 40 years of age (20–50%, mean values in respective age-groups by decades).
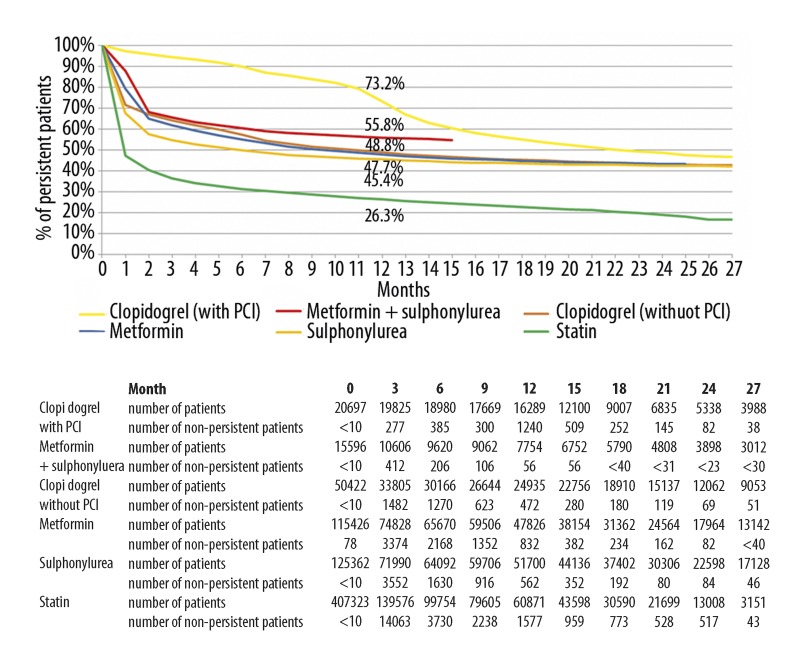
During the study period 20,697 patients received clopidogrel therapy after PCI, while 50,422 patients receiving clopidogrel did not undergo PCI. Statin therapy was initiated in 607,422 patients, but 158,849 (26.1%) patients were excluded from the analysis because of suspected insufficient or dual therapy. Since 41,250 (6.8%) patients were excluded due to other reasons (fully subsidized statin therapy, ezetimibe monotherapy as initial treatment), data of 407,323 patients were included in the analysis. The persistence of antidiabetic therapy was better than that of statin therapy (26.3%; 95% CI 26.2–26.5) at 12 months, while non-PCI patients receiving clopidogrel therapy had a similar persistence value (48.8%; 95% CI 48.4–49.2). The persistence of clopidogrel therapy in patients who had PCI exceeded (73.2%; 95% CI 72.6–73.8) only that of the oral antidiabetic agents (Figure 1).
Figure 1.
Persistence curves of oral antidiabetic drugs (metformin and/or sulphonylureas), clopidogrel and statins (number of patients: metformin 115,426, sulphonylurea 125,362, metformin + sulphonylurea 15,596, statin 407,323, clopidogrel with PCI 20,697, clopidogrel without PCI 50,422). Persistence data (mean values) at 12 months: clopidogrel with PCI 73.2%, metformin + sulphonylurea 55.8%, clopidogrel without PCI 48.8%, metformin 47.7%, sulphonylurea 45.4%, statin 26.3%.
Discussion
Our data show that the 1-year persistence of initial treatment with metformin, sulphonylureas or with metformin/sulphonylurea combination was 47.7%, 45.4% and 55.8%, respectively, which was significantly better than the persistence of statin therapy (26.3%) but worse than that of clopidogrel therapy in patients undergoing coronary intervention (73.2%).
The 1-year persistence of oral antidiabetic therapy (metformin and/or sulphonylureas) (45.4–55.8%) proved to be surprisingly low. In addition, the rapid drop of persistence values within 2 months after initiation warrants further notice. Due to the inherent limitation of a database study, we can only speculate about the premature discontinuation of medication. Clearly, some medical conditions should be taken into account. Accordingly, uncertainties surrounding the indication for metformin prescription (polycystic ovary syndrome or type 2 diabetes) and other likely explanations for medication withdrawal (exercise, weight loss, renal impairment, adverse effects) might be present in some cases [10–12]. More likely, disregarding the importance of type 2 diabetes mellitus and the need for continuous medical treatment (lack of sufficient information, possibility of negligence) is the explanation in the majority of patients. Clearly, more effort should be made to improve persistence immediately after initiation of antidiabetic drugs in patients with type 2 diabetes. The particular impact of early and continuous intensive antihyperglycemic treatment on micro- and macrovascular complications even in the long run was documented in newly diagnosed type 2 diabetic patients by the UKPDS [13,14].
One specific dosage form of metformin (Meforal® 1000 mg – 60 tablets) was separately tested and the 1-year persistence was found to be significantly better than that of the other metformin preparations with lower doses and smaller boxes. Changes of subsidy costs cannot be ruled out as an explanation since this dosage form was supported throughout the study period, while the support of other metformin preparations has changed or ceased in Hungary. Persistence curves analyzed by age group showed that patients younger than 40 years and over 70 years were less persistent than the 40–70-year-old group. Adherence has always been a major problem in younger age groups, and possible contraindications may result in discontinuation of the drug in the elderly.
It is striking that the number of patients treated with sulphonylureas exceeded the number of metformin-treated patients at the beginning of the study period. There is no doubt that medical practitioners in Hungary have more experience with sulphonylureas than with metformin. Nevertheless, adverse effects such as hypoglycaemia and weight gain caused by sulphonylureas may play a role in poor persistence [15]. Accordingly, we found 1-year persistence was worst in the case of sulphonylurea monotherapy (45.4%). Nevertheless, the better persistence of treatment with the “modified-release” tablets compared to the conventional sulphonylureas (47.8% vs. 42.2%) is in agreement with the improved safety profile of “modified release” gliclazide [16].
The 1-year persistence data of the metformin + sulphonylurea combination therapy (55.8%) might not be considered as a good result, because sulphonylurea therapy provides a relatively short glycemic durability [17], and patients on metformin + sulphonylurea combination therapy usually need an earlier modification of the treatment.
Overall, our data on the persistence of oral antidiabetic therapy are in agreement with those reported internationally, although it is difficult to compare the data directly due to the different populations and methodology. According to a meta-analysis of 25 studies published by Rubin in 2005 [18], the adherence to oral antidiabetic therapy ranges from 65% to 85%, and the rate of adherence was found to be only 36–54% in the case of some drug preparations or forms of treatment. More recently, the 12-month persistence of initiating therapy with metformin or sulphonylurea was found to be between 56% and 65% in a population-based cohort study from Quebec [19].
The low persistence of statin therapy in our study should not be considered unusual. From Poland, a very low rate (12%) of proper level of both compliance and persistence of patients treated with statins was recently reported [20]. The persistence of clopidogrel therapy was better in patients with than without PCI. Similar to our results, a negative impact on clopidogrel adherence in patients treated with PCI but without stenting (versus PCI with stenting) was recently reported [21].
Our study has some limitations. Due to the study being a database analysis, information on reason of non-persistence to medications could not be provided. In addition, clinical data about diabetic care (HbA1c testing, utilization of home blood glucose monitoring, frequency of office visits, and intensification of actual therapy [dose adjusting]) were not available. Bearing in mind all these limitations, our investigation with a relatively large number of patients and acceptable follow-up period – the first such study from Hungary – should be of interest.
Our data clearly indicate that the persistence of oral treatment for glycemic control should be improved in patients with type 2 diabetes. Importantly, there is still much to do in the field of education. Literature data suggest that regular control, perception of long-term treatment benefit, reduction of treatment complexity, preferred use of preparations with minimal adverse effects and appropriate reimbursement can greatly increase the persistence of oral antidiabetic therapy. These options should receive serious consideration in diabetes care [22–28] and could be cost-effective as well [29,30]. Nevertheless, the problem is significantly bigger and probably cannot be solved by a simple approach such as enhancing patient education. Continuous improvement of the entire diabetes health care delivery process and education to both health care providers and patients are the most likely needed solutions.
Conclusions
The persistence of initial treatment with metformin and/or sulphonylureas is far from optimal. Better diabetic care and continuous patient education should be encouraged to achieve higher persistence of oral antidiabetic treatment in patients with type 2 diabetes.
Footnotes
Source of support: Departmental sources
References
- 1.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diagnosis of diabetes mellitus, treatment and care of adult patients with diabetes. National guideline of the Hungarian Diabetes Association 2011 (Hungarian) Jermendy G, editor. Diabetologia Hungarica. 2011;19(Suppl 1):5–72. [Google Scholar]
- 4.Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- 5.Tripathi BK, Srivastava AK. Diabetes mellitus: complications and therapeutics. Med Sci Monit. 2006;12(7):RA130–47. [PubMed] [Google Scholar]
- 6.Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain and hypoglycemia in Type 2 diabetes. JAMA. 2010;303:1410–18. doi: 10.1001/jama.2010.405. [DOI] [PubMed] [Google Scholar]
- 7.Jermendy Gy, Ádány R, Balogh S, et al. Glycaemic control in type 2 diabetes mellitus. Analysis of the patient cohort with diabetes of the Hungarian MULTI GAP Study (Hungarian) Metabolizmus. 2010;8:226–31. [Google Scholar]
- 8.Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 9.Barron TI, Bennett K, Feely J. A competing risks prescription refill model of compliance and persistence. Value Health. 2010;13:796–804. doi: 10.1111/j.1524-4733.2010.00741.x. [DOI] [PubMed] [Google Scholar]
- 10.Diamanti-Kandarakis E, Economou F, Palimeri S, Christakou C. Metformin in polycystic ovary syndrome. Ann N Y Acad Sci. 2010;1205:192–98. doi: 10.1111/j.1749-6632.2010.05679.x. [DOI] [PubMed] [Google Scholar]
- 11.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–56. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Haneda M, Morikawa A. Which hypoglycaemic agents to use in type 2 diabetic subjects with CKD and how? Nephrol Dial Transplant. 2009;24:338–41. doi: 10.1093/ndt/gfn616. [DOI] [PubMed] [Google Scholar]
- 13.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 14.Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 15.Burge MR, Sood V, Sobhy TA, et al. Sulphonylurea-induced hypoglycaemia in type 2 diabetes mellitus: a review. Diabetes Obes Metab. 1999;1:199–206. doi: 10.1046/j.1463-1326.1999.00031.x. [DOI] [PubMed] [Google Scholar]
- 16.Schernthaner G, Grimaldi A, Di Mario U, et al. GUIDE study: double-blind comparison of once-daily gliclazide MR and glimepiride in type 2 diabetic patients. Eur J Clin Invest. 2004;34:535–42. doi: 10.1111/j.1365-2362.2004.01381.x. [DOI] [PubMed] [Google Scholar]
- 17.Kahn SE, Haffner SM, Heise MA, et al. for the ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 18.Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med. 2005;118(Suppl 5A):27S–34S. doi: 10.1016/j.amjmed.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Grégoire JP, Sirois C, Blanc G, et al. Persistence patterns with oral antidiabetes drug treatment in newly treated patients – a population-based study. Value Health. 2010;13:820–28. doi: 10.1111/j.1524-4733.2010.00761.x. [DOI] [PubMed] [Google Scholar]
- 20.Wisniowska B, Skowron A. Evaluation of patients’ adherence to statins in Poland. Curr Med Res Opin. 2011;27:99–105. doi: 10.1185/03007995.2010.536745. [DOI] [PubMed] [Google Scholar]
- 21.Zhu B, Zhao Z, McCollam P, et al. Factors associated with clopidogrel use, adherence, and persistence in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Curr Med Res Opin. 2011;27:633–41. doi: 10.1185/03007995.2010.551657. [DOI] [PubMed] [Google Scholar]
- 22.Wens J, Vermeire E, Royen PV, et al. GPs’ perspectives of type 2 diabetes patients’ adherence to treatment: A qualitative analysis of barriers and solutions. BMC Fam Pract. 2005;6(1):20. doi: 10.1186/1471-2296-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vermeire E, Hearnshaw H, Rätsep A, et al. Obstacles to adherence in living with type-2 diabetes: an international qualitative study using meta-ethnography (EUROBSTACLE) Prim Care Diabetes. 2007;1:25–33. doi: 10.1016/j.pcd.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24:561–87. doi: 10.2337/diacare.24.3.561. [DOI] [PubMed] [Google Scholar]
- 25.Bissell P, May CR, Noyce PR. From compliance to concordance: barriers to accomplishing a re-framed model of health care interactions. Soc Sci Med. 2004;58:851–62. doi: 10.1016/s0277-9536(03)00259-4. [DOI] [PubMed] [Google Scholar]
- 26.Lawton J, Ahmad N, Hallowell N, et al. Perceptions and experiences of taking oral hypoglycaemic agents among people of Pakistani and Indian origin: qualitative study. BMJ. 2005;330:1247. doi: 10.1136/bmj.38460.642789.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Debussche X, Roddier M, Fianu A, et al. Health perceptions of diabetic patients in the REDIA study. Diabetes Metab. 2006;32:50–55. doi: 10.1016/s1262-3636(07)70246-x. [DOI] [PubMed] [Google Scholar]
- 28.Kosmala-Anderson J, Wallace ML, Turner A. Does the professional and working context of United Kingdom clinicians predict if they use practices to support patients with long term conditions to self manage? Arch Med Sci. 2010;6:CR815–21. doi: 10.5114/aoms.2010.17100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urbanski P, Wolf A, Herman WH. Cost-effectiveness of diabetes education. J Am Diet Assoc. 2008;108(4 Suppl 1):S6–11. doi: 10.1016/j.jada.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Gillett M, Dallosso HM, Dixon S, et al. Delivering the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cost effectiveness analysis. BMJ. 2010;341:c4093. doi: 10.1136/bmj.c4093. [DOI] [PMC free article] [PubMed] [Google Scholar]