Summary
Background
Central serous chorioretinopathy (CSC) is a condition that originates from alterations of the choroidal circulation. The aim of this paper was to evaluate the use of indocyanine green angiography (ICGA) in patients with chronic CSC.
Material/Methods
The analysis included 17 patients (34 eyes) with chronic CSC in at least 1 eye. The eye examination included: distance and near visual acuity, biomicroscopy, applanation tonometry, fundus examination, colored and red-free fundus photography, evaluation of autofluorescence, optical coherence tomography, and fluorescein and indocyanine green angiography.
Results
In 34 eyes (100%) involved in the ICGA study the results revealed zones of transient increased choroidal vessels permeability. In 18 eyes (52.9%) choroidal changes were accompanied by a focal serous pigment epithelial detachment. In 4 eyes (11.8%) of 3 patients’ the ICGA examination confirmed the presence of occult choroidal neovascularization (CNV). In the patient with bilateral diffuse retinal pigment epitheliopathy, CNV was present in 1 eye, in the patient with unilateral chronic CSC it was also present in 1 eye, and in the third patient with bilateral chronic CSC it was detected in both eyes.
Conclusions
ICGA is a very useful examination that enables ophthalmologists to visualize choroidal changes due to chronic CSC, as well as to diagnose occult CNV in chronic CSC.
Keywords: central serous chorioretinopathy, occult choroidal neovascularization, indocyanine green angiography, optical coherence tomography, pigment epithelium detachment
Background
Central serous chorioretinopathy (CSC) is an idiopathic disease of the retina and choroid, affecting 1 or both eyes. It appears as a well-limited serous detachment of neurosensitive retina in a posterior pole. It is an effect of focal or diffused liquid leakage from choroidal vessels located on the level of retinal pigment epithelium (RPE).
This disease was first described by Albrecht von Graefe in 1866 as recurrent central retinitis (1). Discovery of fluorescein and indocyanine green angiography in the second half of the 20th century enabled doctors to understand that the pathological process is extended not only to the retina, but also to the choroid [1–5] which is why the disease is currently known as central serous chorioretinopathy.
CSC usually affects men at the age of 30–50 years with type A personality [1]. It is believed that high stress level contributes to the development of the disease [1,6]. A wide variety of systemic factors are associated with CSC. CSC is more common among patients with collagenosis, especially systemic lupus erythematosus (SLE) [7–9], accelerated hypertension [10,11] and hypercorticoidism [12]. Other risk factors include antibiotic treatment [11], alcohol overdose [11], and corticosteroid therapy [11,13–16]. CSC after organ grafts [17] and within the period of pregnancy were also studied and described [18,19].
CSC is accompanied by decreasing visual acuity, which can be partially improved with a small hyperopic correction. Some patients report distortion of straight lines, reduced contrast sensitivity, darkened areas in the visual field of the affected eye, metamorphopsia and micropsia. In most patients CSC is a self-limiting condition, which resolves spontaneously within few months [1], but in 40–50% of cases [20,21] it is recurring or chronic. The characteristic features of chronic CSC also known as diffuse retinal pigment epitheliopathy (DRPE) are usually bilateral multifocal or diffused alteration of RPE, with the serous detachment of the retina and different degrees of leakage [22–24]. Chronic CSC persists for years, with periods of aggravation and remission; it affects rather elderly people and is connected with serious decrease of visual acuity [1].
Although rare, the most serious complication of CSC is development of choroidal neovascularization (CNV) [25–30]. CNV usually affects patients in their 50s and older [25,29], and is a serious threat to vision. Indocyanine green angiography (ICGA) is an examination that completely exposes pathological changes in CSC and helps differentiate it from CNV, especially in DRPE [23,24].
Indocyanine green (ICG) is a water-soluble tricarbocyanine dye. ICG absorbs and emits light waves in a near-infrared portion, with maximum emission of 835 μm. This optical property of ICG enables observation of the choroid through normal eye pigment in the RPE and even through pathologies such as hemorrhages and lipid exudates. ICG is both lipophilic and hydrophilic, and ICG in 98% is bound by proteins, in 80% of cases especially by globulins such as A1-lipoproteins and phospholipids [1,31]. IGC bonded with proteins leaks slowly through fenestrated choroidal vasculature, showing choroidal circulation and pathology within. Fluorescein angiography is a diagnostic method used to assess circulation, mostly retinal. As opposed to indocyanine green, fluorescein used in FA leaks very quickly through choriocapillaries, blurring the anatomy of choroid, and additionally 59–75% of fluorescence of choroid is absorbed by RPE, so fluorescein angiography is not useful for diagnosing choroidal pathology.
The aim of our study was to evaluate the use of ICGA in patients with chronic CSC.
Material and Methods
We examined 17 patients: 12 men and 5 women (34 eyes), ages 48–67 years, with chronic CSC in at least 1 eye (Table 1). Chronic CSC was defined as a serous retinal detachment (SRD) lasting longer than 6 months, with multifocal or diffused alteration of RPE. Patients were seen in the Department of Ophthalmology, University Hospital No. 5, Medical University of Silesia in Katowice, Poland, from January to December 2010. All patients consented to their examination. Medical research was in full compliance with the Declaration of Helsinki revised by the World Medical Organization. The examinations were performed in both eyes. All patients had undergone an ophthalmic examination including: best distance and near corrected visual acuity on Snellen’s chart (BDCVA, BNCVA), refractometry, biomicroscopy, applanation tonometry, fundus examination, colored and red-free fundus photography, evaluation of autofluorescence, optical coherence tomography (OCT), FA and ICGA. In 1 of female patients AF was not performed due to her allergy to fluorescein.
Table 1.
Demographic analysis of study group with chronic CSC.
| Patients’ characteristic | ||||
|---|---|---|---|---|
| Number of patients | Number of eyes | Age | Mean age | |
| Patients | 17 | 34 | 48–67 | 55.4 (±5.8) |
| Males | 12 | 24 | 51–67 | 57.17 (±5.9) |
| Females | 5 | 10 | 48–54 | 51.0 (±2.5) |
Indocyanine green was injected intravenously in the dose of 25mg. During the first minute of ICGA the photographs were taken continuously, then every 60 seconds in a 5-minute interval of ICGA, and then at 5- to 10-minute intervals in the remaining time of the examination, which was 40 to 60 minutes. In patients with bilateral chronic CSC, the early stages of angiogram were assessed in the eye with the worse visual acuity. All examinations were performed by the same physician with extensive experience in retinal disorder diagnosis.
Exclusion criteria were: age-related macular degeneration, ocular inflammation, intraocular tumor, angioid streaks, some congenital malformations of the optic disc such as congenital optic disc pit, and those patients who had eyes with previous retinal photocoagulation, intraocular surgery, or trauma of the eye.
Results
Thirty-four eyes of 17 patients with chronic CSC were examined. Seven patients had chronic bilateral CSC (14 eyes) and 10 patients had unilateral CSC (10 eyes). Within patients with unilateral chronic CSC, in 6 fellow eyes focal changes of RPE were observed and 4 fellow eyes were normal during ophthalmoscopy. The average period of chronic CSC ranged from 17 months to 18 years (average=3.27 years). None of the patients were previously treated with laser photocoagulation, photodynamic therapy or intravitreal injections of anti-VEGF. BDCDA was 1.0 to 0.1 and BNCVA was 0.5 to 2.25 (Table 2). General risk factors included hypertension, systemic lupus erythematosus and costeroids intake. Six patients (4 men and 2 women) were diagnosed with hypertension; all of them were treated with hypotension drugs. Systolic blood pressure (SBP) ranged from 150 mmHg to 110 mmHg and diastolic blood pressure (DBP) ranged from 100 mmHg to 60 mmHg during ophthalmological examination. One woman was treated with Encorton, daily dose 10 mg, because of lupus.
Table 2.
Mean best corrected distance and near visual acuity and intraocular pressure in study group with chronic CSC.
| Parameters | Mean | Standard deviation | Min. | Max. |
|---|---|---|---|---|
| BDCVA | 0.60 | ±0.28 | 0.10 | 1.00 |
| BNCVA | 0.93 | ±0.53 | 0.50 | 2.25 |
| Intraocular Pressure | 16.03 | ±2.69 | 12.00 | 22.00 |
ICGA results of the examinations in patients with chronic CSC are shown in Table 3.
Table 3.
ICGA symptoms in study group with chronic CSC.
| ICGA symptoms of chronic CSC | Number of eyes |
|---|---|
| Multifocal zones of increased choroidal hyperpermeability | 34 (100.0%) |
| Window defect of atrophy of RPE | 5 (14.7%) |
| PED | 18 (52.9%) |
| CNV | 4 (11.8%) |
In 34 eyes (100%) the ICGA examination revealed transient hyperpermeability of choroidal vessels, visible as a multifocal hyperfluorescence, increasing in the middle phase and fading in the late phase of the study. This hyperfluorescence appeared in the macula, around the optic disc and behind the vascular arcade. Localization of hyperpermeability spots did not coincide with visible changes in AF or with liquid in OCT. In addition, they were also present in the healthy fellow eyes (n=4). In most cases, the hyperfluorescence areas were preceded by hypo-perfusion and dilatation of venous vessels. In 5 eyes (14.7%) ICGA examination revealed a huge area of atrophy RPE as the window defect in the early phase of ICGA, and in the late phase as a hypofluorescence, which corresponded with early hyperfluorescence type window defect in AF examination.
In 18 eyes (52.9%) choroidal changes were accompanied by pigment epithelial detachments (PED), recognized as the foci of early hyperfluorescence, and in the late phase of ICGA as hypofluorescence surrounded by a bright margin. These PEDs were usually oval, circular or irregular in shape, and smaller than 1dd. In 14 eyes we observed several PEDs and in 4 eyes solitary PED. All PEDs were also revealed in OCT.
In 4 eyes (11.8%) of 3 patients, ICGA examination pointed to the presence of CNV. In the patient with bilateral diffuse retinal pigment epitheliopathy, CNV was present in 1 eye (Figure 1); in the patient with unilateral chronic CSC, CNV was also present in 1 eye (Figure 2); and in the third patient with bilateral chronic CSC, CNV was detected in both eyes (Figures 3,4). In these eyes the hyperfluorescence was increasing from the middle to the late phase of the ICGA examination, showing an ill-defined, late-staining plaque which confirmed occult CNV. CNV was found in the macula in 3 eyes, and above the macula in 1 eye. AF located in the plaque showed an area of early hyperfluorescence with focal leakage points and late diffusion. OCT located in the plaque revealed irregular thickness between Bruch’s membrane and RPE in 3 eyes, and the thickness below RPE within its elevation (PED) in 1 eye. In these 4 eyes with CNV, OCT also showed SRD in each eye, cystoid macular edema in 1 eye, and multifocal detachment of retinal pigment epithelium in 1 eye. In eyes with CNV there were also RPE changes such as multifocal mottling in 2 cases and a large area of RPE atrophy extending downwards (known as “gravitational tract”) in the other 2 eyes, best seen in autofluorescence images and AF. Ophthalmoscopic examination revealed lipid exudates in 2 eyes with CNV, and blood was not observed in any of the examined eyes.
Figure 1.
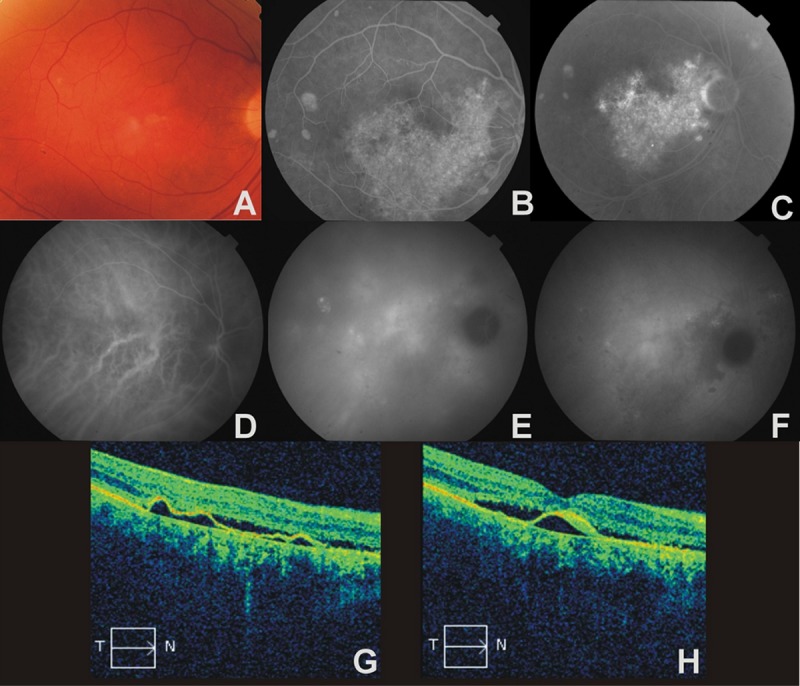
The right eye of a 67-year-old man with diffuse retinal pigment epitheliophaty complicated by choroidal neovascularization. (A) – color photograph, multiple yellow and white lesions, (B) – early phase AF, large area of window defect of RPE, gravitational tract, (C) – late phase AF, hyperfluorescence due to focal leakage of dye and late diffusion, (D) – early phase ICGA, zones of hypoperfusion and dilatation of venous vessels, (E) – midphase ICGA, multiple patchy areas of transient increased choroidal hyperfluorescence, (F) – late phase ICGA, broad area of hypofluorescence corresponding to hyperfluorescence seen in AF examination; late hyperfluorescence in the macula confirms CNV, (G) – OCT, shallow, irregular PED, RPE atrophy, (H) – OCT, subfoveal moderate hyperreflectivity band below RPE sugests CNV.
Figure 2.
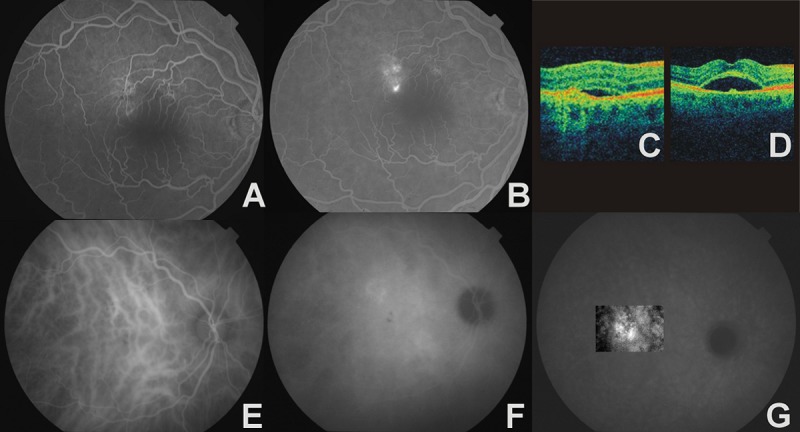
The right eye of a 65-year-old man with chronic CSC and CNV. (A) – early phase AF, window defect of RPE, (B) – late phase AF, focal leakage points, (C) –4OCT, thickening of RPE/choriocapillaris complex with serous retinal detachment, (D) – OCT, neurosensory retina detachment with bulge protruding from the RPE, (E) – early phase ICGA, venous dilatation, (F) – midphase ICGA, zones of choroidal hyperpermeability, (G) – late phase ICGA, ill-defined, late-staining area that confirms occult CNV.
Figure 3.
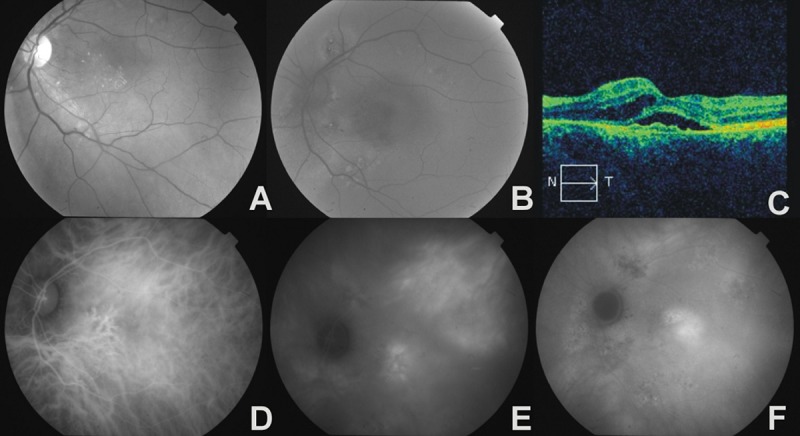
The left eye of a 54-year-old female with bilateral chronic CSC and CNV. (A) – red-free photograph, hard exudates in macula region, (B) – fundus autofluorescence image, multifocal areas of hyper- and hypoautofluorescence showing RPE abnormality, (C) – OCT, irregular thickening of RPE/choriocapillaris complex with serous retinal detachment, intraretinal edema, (D) – early phase ICGA, areas of hypoperfusion and venous vessels dilatation, (E) – midphase ICGA, multiple zones of choroidal hyperpermeability, (F) – late phase ICGA, ill-defined area of late hyperfluorescence in place of occult CNV.
Figure 4.
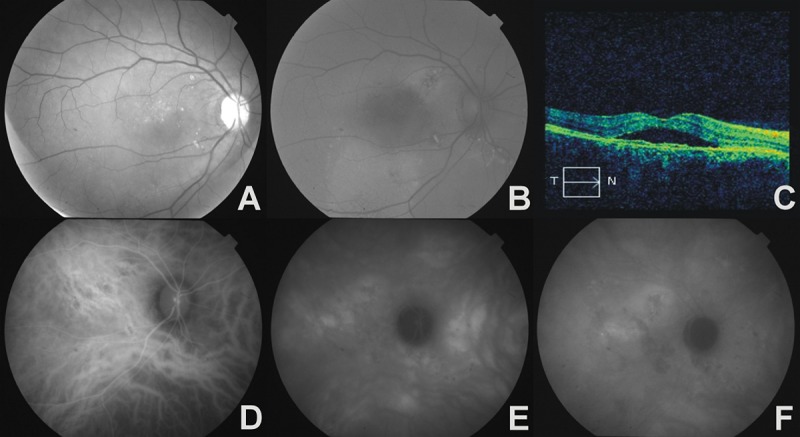
The right eye of a 54-year-old female with bilateral chronic CSC and CNV. (A) – red-free photograph, hard exudates above fovea, (B) – fundus autofluorescence image, multifocal areas of hyper- and hypoautofluorescence in place of RPE abnormalities, and large area of hyperautofluorescence of gravitational tract, (C) – OCT, irregular thickening of RPE/choriocapillaris complex with serous retinal detachment, (D) – early phase ICGA, zones of hypoperfusion and venous vessels dilatation, (E) – midphase ICGA, multiple areas of increased choroidal diffusion, (F) – late phase ICGA, late-staining plaque – occult CNV.
AF and OCT results in the examined group of all patients are shown in Tables 4 and 5.
Table 4.
Fluorescein angiography symptoms in study group with chronic CSC.
| Symptoms of chronic CSC in AF | Number of eyes |
|---|---|
| Window defect | 30 (88.2%) |
| Leakage of dye | 14 (41.2%) |
| Late diffusion | 7 (20.6%) |
| Pulling of dye | 18 (52.9%) |
| Mottled hyperfluorescence | 25 (73.5%) |
Table 5.
OCT symptoms in study group with chronic CSC.
| Symptoms of chronic CSC in OCT | Number of eyes |
|---|---|
| Serous retinal detachment | 24 (70.6%) |
| RPE alterations | 29 (85.3%) |
| PED | 18 (52.9%) |
| Irregularities of the photoreceptors | 12 (35.3%) |
| RPE atrophy | 28 (82.4%) |
Discussion
The pathophysiology of CSC has not yet been fully elucidated. Guyer et al. [32] suggested a pathomechanism of choroidal changes based on transient choroidal hyperpermeability causing an increase of hydrostatic choroid pressure, and subsequent mechanical damage of the RPE barrier and fluid leakage under the neurosensory retina.
Some authors [5,33–35] have found delayed arterial filling in some zones of the choroid in the early phase of ICGA, and dilatation of venous vessels. Due to this fact, ischemia is thought to be the cause of choroidal hyperpermeability [1,22].
In our analyses, ICGA revealed transient choroidal hyperpermeability in 34 eyes (100%). According to other authors, the frequency of hyperpermeability appearance in CSC varies from 63–100% [25,32,33–37]. Tsujikawa et al. [33] reported that focal increased diffusion areas were present in 93% of cases with active CSC, and in 78% of fellow asymptomatic eyes. Guyer et al. [32] revealed an increased choroidal permeability in all patients with acute and chronic CSC, in eyes with inactive CSC, and in normal fellow eyes. Similar results were presented by Lafaut et al. [25] and Piccolino et al. [36].
In our analyses, increased diffusion was located in the macula, behind the vascular arcade and around the optic disc, comparable with results of other studies [25,33]. Souied et al. [23] reported that in ICGA, transient hyperfluorescence caused by hyperpermeability in multiple choroidal areas is characteristic of chronic CSC.
In our analyses, the zones of the choroid showing increased diffusion in ICGA were preceded by hypoperfusion and venous dilatation. Similar results presented by many authors [5,28,33–35] revealed delayed filling of 1 or more choroidal lobules, followed by vessel dilatation, which was detected by scanning laser ophthalmoscopy (SLO) in up to 100% of examined eyes. The concept of Caccavale et al. [38] that increased plasminogen activator inhibitor-1 (PAI-1) affects choroid ischemia, allowed them to successfully treat patients with CSC with low-dose acetyl salicylic acid (aspirin).
In the present study we detected PEDs in 18 eyes (52.9%) with chronic CSC. Guyer et al. [32] reported that PEDs occurred in 84% of eyes with chronic CSC and in 75% of eyes with active CSC. Van Velthoven et al [39], using C-scan OCT, documented that PEDs were present in 52% of eyes with active CSC and in 100% of eyes with inactive CSC. Numerous small PEDs accompanying CSC were also detected in other studies [37,40].
CSC is an idiopathic disease; however, it is present in other systemic diseases [7–12]. Tittl et al. [10] claimed that CSC progression is directly connected with hypertension; however, according to the Venecia and Jampol [41] only in cases of malignant or uncontrolled hypertension, serous detachment of retina was noted. In our research 6 patients were treated for hypertension, but blood pressure level was not higher than 150/100 mmHg. It is assumed that vascular pathology in hypertension, especially vasospasm, might be the factor responsible for choroidal ischemia and induction of CSC [1].
In the present study, 1 patient was diagnosed with systemic lupus erythematosus (SLE) and was receiving steroids. SLE is a chronic systemic and autoimmune disease. Ophthalmological complications occur among about 1/3 of patients [42]. The more frequent appearance of CSC among patients with SLE can be explained by steroid intake, but also inflammatory and thrombotic processes. Steroid therapy impairs vascular autoregulation, mostly by interaction with β-adrenergic receptors [43]. In SLE ophthalmological complications caused by immune complex deposition and other antibody-related mechanisms can sometimes occur. The presence of immune complex deposition was revealed in eye basal membranes, peripheral nerves and also several blood vessels in the retina and choroid [44]. Apart from that, increased vascular resistance was observed in retrobulbar arterioles among patients with SLE [45].
CNV is a rare complication of CSC [1,22], but carries a seriously deteriorating prognosis for visual acuity and thus requires different treatment. The simultaneous presence of CNV and CSC was mentioned in many studies [25–30]. It is suspected that the cause of progression of CNV might be chronic decompensation or rupture of RPE and Bruch’s membrane. CNV might also develop due to argon laser photocoagulation in CSC treatment [27,29,46,47]. Lafaut et al. [25] reported that CNV disclosure among elderly patients with CSC is usually connected with classic CNV, which is confirmed by other authors [26,27]. The mechanism of developing classic CNV as a complication as a consequence of RPE and Bruch’s membrane rupture, seems to be clearly explained by Miller et al. [48]. They claimed that RPE and Bruch’s membrane rupture enables choriocapillaris vessels to extend into the subretinal space, which subsequently leads to neovascular membrane overgrowth. In our research, subretinal CNV was revealed in 4 eyes; in all of these cases it was found to be occult neovascularization. Diagnosing classic CNV with angiography does not create any problems. The “lacy” pattern and quick leakage of contrast are clearly visible in AF even with other coexisting changes on the fundus of the eye, whereas diagnosing occult CNV in eyes with chronic CSC may be difficult. Both in occult CNV and in chronic CSC, similar symptoms in ophthalmoscopic examination, AF and OCT may occur. These are: depigmentation and focal hyperpigmentation of RPE, detachment of neurosensory retina, PED, cystoid edema of retina, chorioretinal atrophy, and subretinal accumulation of fibrin and lipids. Presence of blood might be a factor supporting CNV diagnosis. In our research there was no blood, but all changes mentioned above were present with different degrees of intensification. In these cases the only examination that confirms or excludes occult CNV is ICGA [23,24]. Increased amount of lipoprotein binding receptors in CNV promotes accumulation of indocyanine bound with lipoproteins, which allows good disclosure of occult CNV in late stages of ICGA examination. This feature of CNV was used in photodynamic CNV therapy with application of Verteporfin [49]. Apart from that, modern methods of IGCA examination with scanning laser ophthalmoscope application allow revealing of the pathological network of vessels in early stage of examination. Constant development of ICGA techniques allows us to broaden its application [50,51].
Conclusions
Indocyanine green angiography is a relevant supplement diagnosis of chronic CSC. ICGA is a valuable examination, exposing changes in the choroid in the course of chronic CSC. It is also useful in revealing occult CNV, which requires different and urgent treatment.
Footnotes
Source of support: Departmental sources
References
- 1.Klais CM, Ober MD, Ciardella AP, Yannuzzi LA. Central serous chorioretinopathy. In: Ryan SJ, editor. Retina. 4. USA: Elsevier Mosby; 2006. pp. 1135–61. [Google Scholar]
- 2.Gass JDM. Patogenesis of disciform detachment of the neuro-epithelium. I. General concepts and classification. Am J Ophthalmol. 1967;63:573–85. [Google Scholar]
- 3.Gass JDM. Patogenesis of disciform detachment of the neuro-epithelium. II. Idiopathic central serous choroidopathy. Am J Ophthalmol. 1967;63:587–615. [Google Scholar]
- 4.Guyer DR, Yannuzzi LA, Slakter JS, et al. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112:1057–62. doi: 10.1001/archopht.1994.01090200063023. [DOI] [PubMed] [Google Scholar]
- 5.Prünte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121:26–345. doi: 10.1016/s0002-9394(14)70531-8. [DOI] [PubMed] [Google Scholar]
- 6.Jampol LM, Weinreb R, Yannuzzi L. Involvement of corticosteroids and catecholamines in the pathogenesis of central serous chorioretinopathy: a rationale for new treatment strategies. Ophthalmology. 2002;109(10):1765–66. doi: 10.1016/s0161-6420(02)01303-9. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham ET, Jr, Alfred ER, Irvine AR. Central serous chorioretinopathy in patients with systemic lupus erythematosus. Ophthalmology. 1996;103:2081–90. doi: 10.1016/s0161-6420(96)30385-0. [DOI] [PubMed] [Google Scholar]
- 8.Chan WM, Li EK, Chan AY, Lam DS. Bilateral retinal detachment in a young woman. Lancet. 2003;361:2044. doi: 10.1016/S0140-6736(03)13642-2. [DOI] [PubMed] [Google Scholar]
- 9.Khng CG, Yap EY, Au-Eong KG, et al. Central serous retinopathy complicating systemic lupus erythematosus: a case series. Clin Experiment Ophthalmol. 2000;28(4):309–13. doi: 10.1046/j.1442-9071.2000.00328.x. [DOI] [PubMed] [Google Scholar]
- 10.Tittl MK, Spaide RF, Wong D, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 1999;128(1):63–68. doi: 10.1016/s0002-9394(99)00075-6. [DOI] [PubMed] [Google Scholar]
- 11.Haimovici R, Koh S, Gagnon DR, et al. Central Serous Chorioretinopathy Case-Control Study Group. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111(2):244–49. doi: 10.1016/j.ophtha.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Bouzas EA, Scott MH, Mastorakos G, et al. Central serous chorioretinopathy in endogenous hypercortisolism. Arch Ophthalmol. 1993;111:1229–33. doi: 10.1001/archopht.1993.01090090081024. [DOI] [PubMed] [Google Scholar]
- 13.Wakakura M, Ishikawa S. Central serous chorioretinopathy complicating systemic corticosteroid treatment. Br J Ophthalmol. 1984;68:329–31. doi: 10.1136/bjo.68.5.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karadimas P, Bouzas EA. Glucocorticoid use represents a risk factor for central serous chorioretinopathy: a prospective, case-control study. Graefes Arch Clin Exp Ophthalmol. 2004;242(9):800–2. doi: 10.1007/s00417-004-0885-z. [DOI] [PubMed] [Google Scholar]
- 15.Polak BCP, Baarsma GS, Snyers B. Diffuse retinal pigment epitheliopathy complicating systemic corticosteroid treatment. Br J Ophthalmol. 1995;79:922–25. doi: 10.1136/bjo.79.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho-Recchia CA, Yannuzzi LA, Negrão S, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109(10):1834–37. doi: 10.1016/s0161-6420(02)01117-x. [DOI] [PubMed] [Google Scholar]
- 17.Gass JDM, Slamovits TL, Fuller DG, et al. Posterior chorioretinopathy and retinal detachment after organ transplantation. Arch Ophthalmol. 1992;110:1717–22. doi: 10.1001/archopht.1992.01080240057030. [DOI] [PubMed] [Google Scholar]
- 18.Gass JDM. Central serous chorioretinopathy and white subretinal exudation during pregnancy. Arch Ophthalmol. 1991;109:677–81. doi: 10.1001/archopht.1991.01080050091036. [DOI] [PubMed] [Google Scholar]
- 19.Sunness JS, Haller JA, Fine SL. Central serous chorioretinopathy and pregnancy. Arch Ophthalmol. 1993;111:360–64. doi: 10.1001/archopht.1993.01090030078043. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984;68:815–20. doi: 10.1136/bjo.68.11.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein ML, van Buskirk EM, Friedman E, et al. Experience with nontreatment of central serous chorioidopathy. Arch Ophthalmol. 1974;91:247–50. doi: 10.1001/archopht.1974.03900060257001. [DOI] [PubMed] [Google Scholar]
- 22.Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86:126–45. doi: 10.1111/j.1600-0420.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 23.Souied E, Mimoun G, Leys A. Formes frontiers. Degenerescence maculaire liee a l’age. In: Soubrane G, Coscas G, Souied E, editors. Les DMLAs. Paris: Elsevier Masson; 2007. pp. 379–87. [Google Scholar]
- 24.Spaide RF. Central serous chorioretinopathy. In: Holz FG, Spaide RF, editors. Essentials in Ophthalmology Medical Retina. Berlin Heidelberg: Springer-Verlag; 2005. pp. 77–94. [Google Scholar]
- 25.Lafaut BA, Salati C, Priem H, De Laey JJ. Indocyanine green angiography is of value for the diagnosis of chronic central serous chorioretinopathy in elderly patients. Graefe’s Arch Clin Exp Ophthalmol. 1998;256:513–21. doi: 10.1007/s004170050114. [DOI] [PubMed] [Google Scholar]
- 26.Yang CS, Chen K, Lee SM, Lee FL. Photodynamic therapy in the treatment of choroidal neovascularization complicating central serous chorioretinopathy. J Chin Med Assoc. 2009;72:501–5. doi: 10.1016/S1726-4901(09)70417-4. [DOI] [PubMed] [Google Scholar]
- 27.Dickhoff KV, Hoffren M, Laatikainen L. Les modifications de l’epithelium pigmentaire retinien en rapport avec la retinopathie sereuse centrale. J Fr Ophtalmol. 1989;12:877–81. [PubMed] [Google Scholar]
- 28.Bandello F, Virgili G, Lanzetta P, et al. ICG angiography and retinal pigment epithelial decompensation (CRSC and epitheliopathy) J Fr Ophtalmol. 2001;24:448–51. [PubMed] [Google Scholar]
- 29.Berger RA, Olk RJ, Burgess D. Central serous chorioretinopathy in patients over fifty years of age. Ophthalmic Surg. 1991;22:583–90. [PubMed] [Google Scholar]
- 30.Ergun E, Tittl M, Stur M. Photodynamic Therapy With Verteporfin in Subfoveal Choroidal Neovascularization Secondary to Central Serous Chorioretinopathy. Arch Ophthalmol. 2004;122:37–41. doi: 10.1001/archopht.122.1.37. [DOI] [PubMed] [Google Scholar]
- 31.Yoneya S, Saito T, Komatsu Y, et al. Binding properties of indocyanine green in human blood. Invest Ophthalmol Vis Sci. 1998;39:1286–90. [PubMed] [Google Scholar]
- 32.Guyer DR, Yannuzzi LA, Slakter JS, et al. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112:1057–62. doi: 10.1001/archopht.1994.01090200063023. [DOI] [PubMed] [Google Scholar]
- 33.Tsujikawa A, Ojima Y, Yamashira K, et al. Punctate hyperfluorescent spots associated with central serous chorioretinopathy as seen on indocyanine green angiography. Retina. 2010;30:801–9. doi: 10.1097/IAE.0b013e3181c72068. [DOI] [PubMed] [Google Scholar]
- 34.Kitaya N, Nagaoka T, Hikichi T, et al. Features of abnormal choroidal circulation in central serous chorioretinopathy. Br J Ophthalmol. 2003;87:709–12. doi: 10.1136/bjo.87.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheider A, Nasemann JE, Lund OE. Fluorescein and indocyanine green angiograpies of central serous chorioretinopathy by scanning laser ophthalmolscopy. Am J Ophthlmol. 1993;115:50–56. doi: 10.1016/s0002-9394(14)73524-x. [DOI] [PubMed] [Google Scholar]
- 36.Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina. 1994;14:231–42. doi: 10.1097/00006982-199414030-00008. [DOI] [PubMed] [Google Scholar]
- 37.Menchini U, Virgili G, Lanzetta P, Ferrari E. Indocyanine green angiography in central serous chorioretinopathy. International Ophthalmol. 1997;21:57–67. doi: 10.1023/a:1005880129005. [DOI] [PubMed] [Google Scholar]
- 38.Caccavale A, Imparato M, Romanazzi F, et al. A new strategy of treatment with low-dosage acetyl salicylic acid in patients affected by central serous chorioretinopathy. Med Hypotheses. 2009;73:435–37. doi: 10.1016/j.mehy.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 39.van Velthoven ME, Verbraak FD, Garcia PM, et al. Evaluation of central serous retinopathy with en face optical coherence tomography. Br J Ophthalmol. 2005;89:1483–88. doi: 10.1136/bjo.2005.073056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawa M, Gomi F, Harino S. Three-dimensional optical coherence tomographic findings of idiopatic multiple serous retinal pigment epithelial detachment. Arch Ophthalmol. 2005;123:122–23. doi: 10.1001/archopht.123.1.122. [DOI] [PubMed] [Google Scholar]
- 41.de Venecia G, Jampol LM. The eye in accelerated hypertension. II. Localized serous detachments of the retina in patients. Arch Ophthalmol. 1984;102:68–73. doi: 10.1001/archopht.1984.01040030052033. [DOI] [PubMed] [Google Scholar]
- 42.Sivaraj RR, Durrani OM, Denniston AK, et al. Ocular manifestations of systemic lupus erythematosus. Rheumatology. 2007;46(12):1757–62. doi: 10.1093/rheumatology/kem173. [DOI] [PubMed] [Google Scholar]
- 43.Sakaue M, Hoffman BB. Glucocorticoids induce transcription and expression of the a13B adrenergic receptor gene in DTT1 MF-2 smooth muscle cells. J Clin Invest. 1991;88:385–89. doi: 10.1172/JCI115315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karpik AG, Schwartz MM, Dickey LE, et al. Ocular immune reactants in patients dying with systemic lupus erythematosus. Clin Immunol Immunopathol. 1985;35:295–312. doi: 10.1016/0090-1229(85)90091-1. [DOI] [PubMed] [Google Scholar]
- 45.Modrzejewska M, Ostanek L, Bobrowska-Snarska D, et al. Ocular circulation in systemic lupus erythematosus. Med Sci Monit. 2009;15(11):CR573–78. [PubMed] [Google Scholar]
- 46.Konstantinidis L, Mantel I, Zografos L, Ambresin A. Intravitreal ranibizumab in the treatment of choroidal neovascularization associated with idiopathic central serous chorioretinopathy. Eur J Ophthalmol. 2010;20:955–58. doi: 10.1177/112067211002000524. [DOI] [PubMed] [Google Scholar]
- 47.Ha TW, Ham DI, Kang SW. Management of choroidal neovascularization following laser photocoagulation for central serous chorioretinopathy. Korean J Ophthalmol. 2001;16:88–92. doi: 10.3341/kjo.2002.16.2.88. [DOI] [PubMed] [Google Scholar]
- 48.Miller H, Miller B, Ryan SJ. The role of retinal pigment epithelium in the evolution of subretinal neovascularization. Invest Ophthalmol. 1986;27:1644–52. [PubMed] [Google Scholar]
- 49.Blumenkranz MS, Moshfeghi DM. Pharmacotherapy of age-related macular degeneration. In: Ryan SJ, editor. Retina. 4th ed. USA: Elsevier Mosby; 2006. pp. 1212–39. [Google Scholar]
- 50.Wu J, Zhou X, Su L, et al. A newly-designed positioning system for simultaneous indocyanine green and fluorescein anterior segment angiography of the albino rabbit. Med Sci Monit. 2010;16(8):BR246–49. [PubMed] [Google Scholar]
- 51.Killory BD, Nakaji P, Gonzales F, et al. Prospective evaluation of surgical microscope – integrated intraoperative near-infrared indocyanine green angiography during cerebral arteriovenous malformation surgery. Neurosurgery. 2009;65:456–62. doi: 10.1227/01.NEU.0000346649.48114.3A. [DOI] [PubMed] [Google Scholar]


