Abstract
Aspergillus fumigatus is a filamentous fungus which can cause invasive disease in immunocompromised individuals. A. fumigatus can grow in medium containing up to 80% human serum, despite very low concentrations of free iron. The purpose of this study was to determine the mechanism by which A. fumigatus obtains iron from the serum iron-binding protein transferrin. In iron-depleted minimal essential medium (MEM), A. fumigatus growth was supported by the addition of holotransferrin (holoTf) or FeCl3 but not by the addition of apotransferrin (apoTf). Proteolytic degradation of transferrin by A. fumigatus occurred in MEM-serum; however, transferrin degradation did not occur until late logarithmic phase. Moreover, transferrin was not degraded by A. fumigatus incubated in MEM-holoTf. Urea polyacrylamide gel electrophoresis showed that in MEM-holoTf, holoTf was completely converted to apoTf by A. fumigatus. In human serum, all of the monoferric transferrin was converted to apoTf within 8 h. Siderophores were secreted by A. fumigatus after 8 h of growth in MEM-serum and 12 h in MEM-holoTf. The involvement of small molecules in iron acquisition was confirmed by the fact that transferrin was deferrated by A. fumigatus even when physically separated by a 12-kDa-cutoff membrane. Five siderophores were purified from A. fumigatus culture medium, and the two major siderophores were identified as triacetylfusarinine C and ferricrocin. Both triacetylfusarinine C and ferricrocin removed iron from holoTf with an affinity comparable to that of ferrichrome. These data indicate that A. fumigatus survival in human serum in vitro involves siderophore-mediated removal of iron from transferrin. Proteolytic degradation of transferrin may play a secondary role in iron acquisition.
Aspergillus fumigatus is an opportunistic fungal pathogen which can cause life-threatening invasive aspergillosis in immunocompromised individuals. Susceptible groups include bone marrow and solid organ transplant recipients (48), cancer patients receiving cytotoxic chemotherapy (5), AIDS patients (31), and patients with chronic granulomatous disease (44). The antifungal drugs amphotericin B and itraconazole are used in the treatment of invasive aspergillosis, but even with prophylaxis and treatment with amphotericin B, mortality rates average 65% for pulmonary aspergillosis and approach 100% if the disease spreads to the central nervous system (10). The survival of A. fumigatus within the bloodstream indicates that this fungus possesses mechanisms for obtaining essential nutrients for its growth and reproduction. Serum is inhibitory to the growth of many microorganisms, including most fungi, because free iron concentrations in serum are too low to support growth. Nevertheless, previous work by Gifford et al. showed that A. fumigatus can grow in media containing up to 80% human serum (15), indicating that it possesses an effective mechanism for acquiring iron from serum constituents.
With the exception of some Lactobacillus and Borrelia species (39, 41), all organisms require iron as an important cofactor. Free iron is limiting in the human body because it is complexed with iron-binding molecules, such as ferritin and heme compounds, intracellularly or with transferrin or lactoferrin in extracellular fluids (8, 42). These iron-binding molecules are responsible for lowering the free iron concentration in serum to 10−18 M (7). During infections, nonspecific host defenses decrease the level of free iron even further by increasing ferritin synthesis and releasing lactoferrin from neutrophils. Therefore, successful human pathogens must possess mechanisms to compete with the host for its tightly bound iron.
There is some indirect evidence that iron plays a role in the virulence of fungi. The risk of invasive aspergillosis was linked indirectly to serum iron levels in a study by Iglesias-Osma et al. (21). These researchers showed that the iron saturation of transferrin increased 1.6-fold during neoplastic episodes and that this level corresponded to an increased risk of aspergillosis (21). It was demonstrated that deferoxamine, a hydroxamate siderophore produced by actinomycetes, can stimulate the growth of Rhizopus spp. in iron-loaded patients (6). Deferoxamine chelates transferrin-bound iron, and the ferrated siderophore then can support the growth of Rhizopus spp.
Several strategies are used by pathogenic microorganisms to access transferrin-bound iron. A number of bacterial species, such as Neisseria spp. (42), Staphylococcus spp. (32), and Haemophilus spp. (42), express transferrin receptors and acquire iron by binding transferrin directly. Alternatively, many bacteria and fungi acquire iron by reducing ferric iron at the cell surface. Ferric reductases have been characterized in several yeast pathogens, including Candida albicans (33), Histoplasma capsulatum (46), and Cryptococcus neoformans (22, 36). Another strategy for iron uptake is the secretion of siderophores (42). Siderophores strongly bind Fe3+ and deliver the iron to the microbe via high-affinity siderophore uptake systems. Many microorganisms, including many filamentous fungi, produce siderophores in response to a low concentration of free iron (9). Iron binding affinities can be expressed as pM values, defined as −log[Fe3+] at pH 7.4 for solutions containing 10−6 M(total) iron and 10−5 M (total) ligand (16). Siderophores bind iron with pM values in the range of 22 to 50, sufficiently strong to remove iron attached to molecules such as transferrin, for which the pM value for complexation with iron is 23.6 (16).
Some bacterial siderophores have been shown to play a role in virulence. Pseudomonas aeruginosa produces two siderophores, pyoverdin and pyochelin. A pyoverdin-deficient mutant of P. aeruginosa exhibited severely restricted growth in human serum, while mutants deficient in the production of both siderophores showed attenuated virulence in a mouse model (45). Pyoverdin production also was shown to correlate with virulence in a burned-mouse infection model (30). Disruption of siderophore biosynthesis also decreased the virulence of Yersinia pestis (4) and Vibrio vulnificus (18).
The siderophores of pathogenic fungi have received far less study, partly because siderophores have not been detected in the budding and fission yeasts (19). Hydroxamate siderophores are produced by H. capsulatum (20) and Aspergillus species. A. fumigatus is known to secrete several hydroxamate siderophores, including triacetylfusarinine C and ferricrocin (12, 35, 49); however, there is not yet any evidence that siderophores produced by A. fumigatus are involved in iron uptake in vivo.
A. fumigatus can flourish in media containing high concentrations of human serum (15). Therefore, the purpose of this study was to determine whether the growth of A. fumigatus in human serum was the result of its ability to remove iron from transferrin. The specific objectives were to determine the extent to which A. fumigatus was able to grow in the presence of transferrin as the sole iron source and to examine whether transferrin was deferrated during incubation with A. fumigatus. Because A. fumigatus is an abundant producer of proteinases, we also investigated the possibility that the proteolytic degradation of transferrin released free iron into solution. Finally, we quantified siderophore secretion, purified siderophores from A. fumigatus cultures, and measured their ability to remove transferrin-bound iron.
MATERIALS AND METHODS
Strains and growth conditions.
A. fumigatus (ATCC 13073) was obtained from the American Type Culture Collection and maintained on YM slants (0.3% malt extract, 0.3% yeast extract, 0.5% peptone, 0.5% glucose) at 4°C. A. fumigatus was cultured on YM plates at 28°C for 5 to 10 days until fully conidiated. Conidia were harvested by flooding the culture plates with phosphate-buffered saline containing 0.05% Tween 20 and swabbing with a sterile cotton swab. The conidia were vortexed, centrifuged, resuspended in phosphate-buffered saline, and filtered through a plug of sterile glass wool to remove hyphae. Concentrations of conidia were determined by counting in a hemacytometer.
A. fumigatus was cultured in minimal essential medium (MEM) (pH 7.4; Life Technologies, Burlington, Ontario, Canada) at 5-ml volumes in 25-ml culture flasks. A. fumigatus conidia were added to the medium at a final concentration of 106/ml, and the flasks were incubated at 37°C and 150 rpm for various times. Traces of iron were removed from glassware by overnight treatment with 1 mM EDTA, followed by 18 h in 5% HCl and thorough rinsing with deionized water. The iron-chelating agent 2,2′-dipyridyl (ICN Biomedicals Inc., Aurora, Ohio) was filter sterilized and added to MEM at a concentration of 250 μM, a concentration empirically determined to inhibit the growth of A. fumigatus. Human holotransferrin and apotransferrin were obtained from Sigma (Oakville, Ontario, Canada). Human serum (male) was obtained from Sigma, stored frozen until use, and added to MEM at a concentration of 10%. Dry weights of A. fumigatus cultures were measured by filtering the entire contents of each culture flask through Miracloth (Calbiochem, La Jolla, Calif.) and rinsing thoroughly with deionized water to remove all traces of culture medium. Mycelia then were transferred to preweighed microcentrifuge tubes, lyophilized overnight, and weighed.
Electrophoretic analysis of transferrin.
Proteolytic cleavage of transferrin during the growth of A. fumigatus was monitored by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (PAGE). Media were withdrawn from A. fumigatus cultures and electrophoresed according to the procedure of Laemmli (26). Gels were silver stained or transferred to polyvinylidene difluoride membranes (Bio-Rad), blocked with 5% bovine serum albumin, probed with a rabbit immunoglobulin G fraction of anti-human transferrin (1:1,000 dilution; Rockland Inc., Gilbertsville, Pa.), and treated with goat anti-rabbit horseradish peroxidase. Bands were visualized by adding the substrate diaminobenzidine.
Urea-PAGE was carried out as described by Wolz et al. (52) with a Protean II xi cell (Bio-Rad). Gels were stained with SYPRO orange (Molecular Probes, Eugene, Oreg.) and scanned with a Typhoon 9410 imager (Amersham).
Quantification of siderophores.
Total siderophore concentrations were measured by using chrome Azurol S (CAS) assay shuttle solution (43). Culture supernatants of A. fumigatus were diluted and mixed with an equal volume of CAS shuttle solution. The absorbance at 630 nm was measured. Dilutions of desferriferrichrome (Sigma) were used to generate a standard curve.
Removal of iron from transferrin sequestered within a dialysis membrane.
Holotransferrin (25 μM) was dissolved in MEM and sealed within a dialysis bag (nominal molecular weight cutoff, 12,000 to 14,000; Fisher). The dialysis bag then was immersed in 25 ml of MEM in a 125-ml flask. The medium in the flask was inoculated with 2.5 × 107 conidia and incubated at 37°C with slow shaking for 48 h. As a control, an uninoculated flask was maintained under the same conditions.
Siderophore purification.
Hydroxamate siderophores were purified from culture supernatants of A. fumigatus by using a modification of the method described by Payne (40). A. fumigatus was cultured in acid-washed flasks containing 4 liters of modified Grimm-Allen medium ([containing, per liter, 1 g of KHSO4, 3 g of K2HPO4, 3 g of (NH4)SO4, 20 g of sucrose, 1 g of citric acid, 2 mg of thiamine, 20 μg of CuSO4, 1 mg of MnSO4, 5.5 mg of ZnSO4, and 810 mg of MgSO4 (pH 6.9)]. This medium was inoculated with 4 × 109 conidia, and the flasks were incubated at 150 rpm and 37°C for 72 h. The cultures were filtered through Miracloth to remove mycelia, and the filtrate was concentrated under vacuum to 350 ml. Ammonium sulfate (50% saturation) and 5 g of FeCl3/liter were added, and the solution was stirred at 4°C for 16 h. The concentrated filtrate was filtered through Whatman paper and extracted five times with 50 ml of benzyl alcohol. Anhydrous ethyl ether (750 ml) was added to the combined benzyl alcohol fractions, and the siderophores were extracted eight times into 15 ml of double-distilled H2O. The aqueous layer was washed with diethyl ether and lyophilized to dryness. Siderophores were separated by flash column chromatography with dichloromethane and methanol. The separation of siderophores was confirmed by thin-layer chromatography.
Desferritriacetylfusarinine C was extracted from the medium by the same procedure but without the addition of FeCl3. Purification was achieved by preparative thin-layer chromatography with precleaned Silica Gel 60 F254 plates and 1-butanol- ethanol- water (5:3:2). Bands were visualized under UV light, and iron-reactive layers were scraped, extracted with water, and lyophilized.
Iron was removed from ferricrocin by treatment with 8-hydroxyquinoline. Ferricrocin was dissolved in slightly alkaline water (10 mg/ml), and a 10-fold (wt/wt) excess of 8-hydroxyquinoline was added. This mixture was heated for 30 min at 60°C and allowed to stand overnight at room temperature. Most of the 8-hydroxyquinoline was removed by centrifugation, and the remainder was removed from the supernatant by five extractions with chloroform.
Siderophore identification.
Matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectra were obtained for samples dispersed in a α-cyano-4-hydroxycinnamic acid matrix (triacetylfusarinine C and desferritriacetylfusarinine C) or a 2,5-dihydroxybenzoic acid matrix (ferricrocin and desferriferricrocin) by using a PerSeptive Biosystems Voyager-DE instrument.
1H and 13C nuclear magnetic resonance (NMR) spectra were recorded at 293 K by using a Bruker AMX-400 NMR spectrometer or a Varian Inova 500-MHz NMR spectrometer. All chemical shifts are reported relative to tetramethyl-silane. Correlation spectroscopy (COSY), total correlation spectroscopy (TOCSY), nuclear Overhauser effect spectroscopy (NOESY), and rotating-frame Overhauser, effect spectroscopy (ROESY) studies of desferriferricrocin allowed assignment of all signals to specific amino acid residues.
Incubation of A. fumigatus desferrisiderophores with holotransferrin.
Desferritriacetylfusarinine C, desferriferricrocin, and desferriferrichrome were diluted to concentrations ranging from 5 mM to 5 μM and incubated with holotransferrin (25 μM) in 50 mM Tris- 150 mM NaCl- 20 mM NaHCO3 (pH 7.4) buffer for 16 h at 37°C. The extent of iron saturation of transferrin at the end of the incubation period was determined by urea-PAGE.
RESULTS
Holotransferrin but not apotransferrin supports the growth of A. fumigatus in iron-depleted medium.
MEM was made iron limiting by the addition of the iron-chelator 2,2′-dipyridyl. A concentration of 250 μM 2,2′-dipyridyl was empirically determined to be the MIC for A. fumigatus ATCC 13073 (data not shown). The addition of apotransferrin to MEM containing 2,2′-dipyridyl did not support the growth of A. fumigatus, whereas the addition of either 25 μM holotransferrin or 50 μM FeCl3 to iron-limited MEM promoted statistically significant growth of A. fumigatus (Table 1). These data indicate that transferrin-bound iron is available to A. fumigatus.
TABLE 1.
Growth of A. fumigatus in MEM (5 ml) containing a 250 μM concentration of the iron chelator 2,2′-dipyridyl and supplemented with holotransferrin, apotransferrin, or FeCl3
| Iron source | Mean ± SD mycelial dry wt after 96 h (mg) |
|---|---|
| None (MEM and 2,2′-dipyridyl alone) | 0.13 ± 0.07 |
| Holotransferrin (25 μM) | 1.30 ± 0.90a |
| Apotransferrin (25 μM) | 0.02 ± 0.05 |
| FeCl3 (50 μM) | 0.80 ± 0.40a |
Growth was significantly greater than that in the absence of any iron source (none) (P < 0.05).
Serum transferrin is not degraded during the growth of A. fumigatus.
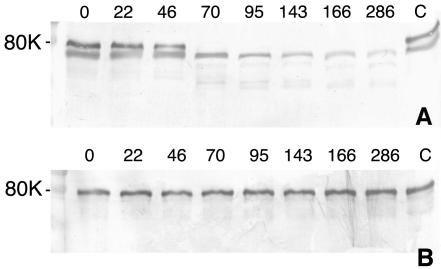
Like many fungi, A. fumigatus is a prolific producer of proteinases. Previous results obtained by Gifford et al. showed that growth in human serum, an iron-deficient medium, was accompanied by increased secretion of proteinases (15). In theory, these proteinases could degrade human transferrin and release iron, which then would be available for uptake by fungal cells. Degradation of human transferrin was monitored by culturing A. fumigatus in MEM containing either 2.5 μM purified human transferrin or 10% human serum. As expected, the addition of serum to MEM stimulated proteinase secretion, as evidenced by the degradation of serum transferrin beginning at 46 h of culturing. However, transferrin was stable for at least the first 22 h of incubation. There was a small decrease in the amount of transferrin at 46 h, and considerable degradation was observed at all later time points (Fig. 1A). Stationary phase was reached at between 22 and 46 h of growth (data not shown); therefore, serum transferrin degradation by A. fumigatus did not occur until after the beginning of stationary phase. In contrast, transferrin was stable in serum for at least 286 h in control flasks, which contained no A. fumigatus (Fig. 1A, lane C). When holotransferrin alone was added to MEM, it was not degraded by A. fumigatus after 286 h of incubation (Fig. 1B). Because transferrin degradation was not observed in either medium during the early growth of A. fumigatus, when the organism has the greatest requirement for iron, it is unlikely that transferrin proteolysis is the primary mechanism of iron acquisition by A. fumigatus under conditions of low free iron concentrations.
FIG. 1.
Degradation of transferrin by A. fumigatus in liquid cultures. A. fumigatus was incubated in MEM containing 10% human serum (A) or 2.5 μM holotransferrin (B). Supernatants were withdrawn from the cultures after the number of hours indicated above the lanes, and the presence of transferrin was determined by Western blotting following sodium dodecyl sulfate-PAGE. Controls (lanes C) were uninoculated samples incubated for 286 h. The band underneath transferrin in panel A is another protein that cross-reacted with the polyclonal antitransferrin antibody.
A. fumigatus can remove iron from holotransferrin.
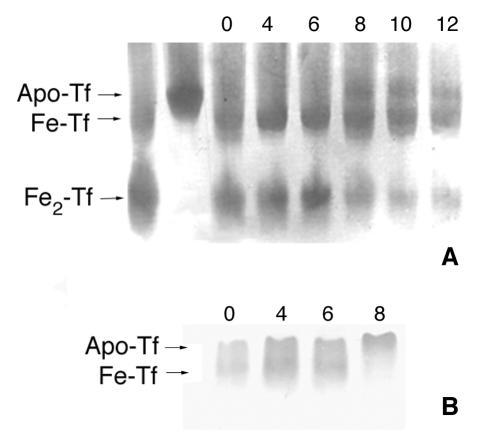
The ability of A. fumigatus cultures to remove iron from holotransferrin was investigated with urea-PAGE. Urea-PAGE can be used to distinguish among holotransferrin, monoferric transferrin, and apotransferrin. A. fumigatus was cultured in MEM containing either 10% human serum or 2.5 μM human holotransferrin. In medium containing 2.5 μM holotransferrin, the relative amount of holotransferrin decreased and apotransferrin was detected within 8 h (Fig. 2A). The human serum contained a mixture of apotransferrin and monoferric transferrin, but within 8 h of incubation with A. fumigatus, monoferric transferrin was no longer detected in the human serum; only apotransferrin was present (Fig. 2B). Since transferrin was not degraded until more than 22 h of culturing (Fig. 1), the iron was removed from intact holotransferrin.
FIG. 2.
Iron removal from transferrin by A. fumigatus. A. fumigatus was cultured in MEM containing 2.5 μM purified human holotransferrin (A) or 10% human serum (B). Culture media were withdrawn, and the iron saturation of transferrin was analyzed by urea-PAGE. Transferrin was visualized by Western blotting. The numbers above the lanes represent the hours of incubation with A. fumigatus. Fe2-Tf, holotransferrin; Apo-Tf, apotransferrin.
A. fumigatus secretes siderophores in the early phase of growth in transferrin-containing medium.
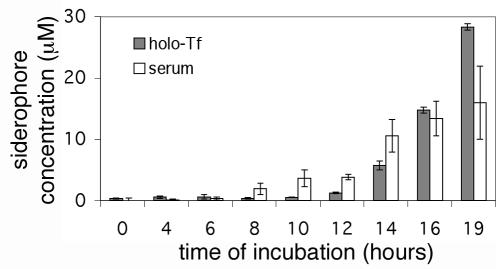
Total siderophore secretion by A. fumigatus was quantified by using CAS shuttle solution and desferriferrichrome to generate a standard curve. Cultures were monitored during the early phase of growth to determine whether siderophore secretion could be responsible for the removal of iron from transferrin observed in the first 8 h of culturing. In MEM containing 10% human serum, significant levels of siderophores were first detected by the CAS assay at 8 h. In MEM supplemented with 2.5 μM holotransferrin, significant levels of siderophore secretion were observed at 12 h (Fig. 3). Thus, siderophore secretion occurs early in the growth of A. fumigatus, coinciding with the first observed removal of transferrin-bound iron after 8 to 12 h of incubation (Fig. 2).
FIG. 3.
Siderophore secretion by A. fumigatus in MEM containing holotransferrin or human serum. A. fumigatus spores (106/ml) were added to MEM containing 2.5 μM holotransferrin (holo-Tf) or 10% human serum at time zero. The cultures were incubated at 37°C and 150 rpm. The siderophore concentrations in the culture supernatants were determined by the CAS assay. Error bars indicate standard deviations.
A. fumigatus can remove iron from transferrin across a dialysis membrane.
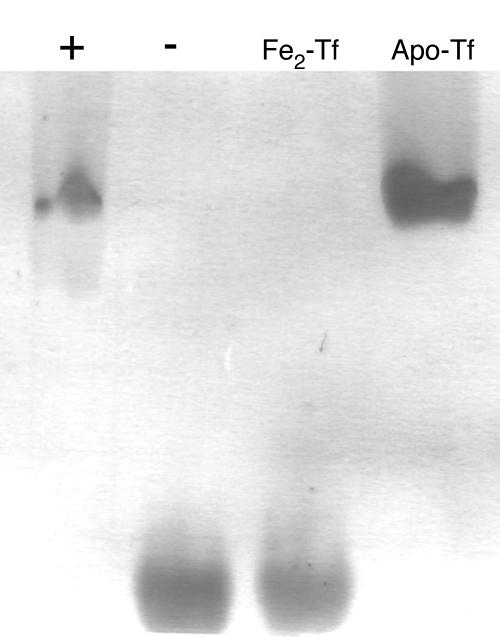
Holotransferrin was placed within a dialysis bag to determine whether small molecules produced by A. fumigatus, such as siderophores, were responsible for the removal of iron from transferrin. Holotransferrin (25 μM) was sealed within a dialysis bag with a molecular mass cutoff of 12 to 14 kDa. Most known fungal siderophores are smaller than 1 kDa and so should readily pass through the dialysis membrane. The dialysis bag was suspended in MEM inoculated with A. fumigatus and incubated at 37°C for 48 h. The iron-binding state of the transferrin was monitored by urea-PAGE. Despite physical separation from A. fumigatus, holotransferrin contained within the dialysis bag was almost completely deferrated during incubation with A. fumigatus (Fig. 4). In the uninoculated control, no deferration of transferrin was observed. Acid production did not cause the release of iron from transferrin because the pH of MEM did not drop below 7.0 during the incubation period (data not shown).
FIG. 4.
A. fumigatus can transport iron from transferrin across a dialysis membrane. A. fumigatus was inoculated into MEM in which a dialysis bag containing holotransferrin (25 μM) was suspended. A. fumigatus was incubated for 48 h, and then transferrin was withdrawn from the dialysis bag and analyzed by urea-PAGE (lane +). An uninoculated control flask containing MEM plus transferrin in a dialysis bag also was examined (lane −). Pure holotransferrin (Fe2-Tf) and apotransferrin (Apo-Tf) standards also were run.
Purification of siderophores from A. fumigatus culture medium.
Siderophores were purified from A. fumigatus culture medium as described in Materials and Methods. Extraction of the culture medium with benzyl alcohol revealed five different iron-binding compounds, which could be distinguished by their Rf values after thin-layer chromatography (Table 2). These compounds were purified by flash column chromatography. The red, orange, and yellow compounds lost their color when treated with the iron chelator 8-hydroxyquinoline. Fraction 3 contained by far the most abundant siderophore produced under these culture conditions, with fractions 1, 2, and 5 producing much smaller amounts (Table 2).
TABLE 2.
Siderophores produced by A. fumigatus ATCC 13073
The solvent was chloroform-benzyl alcohol-methanol (2:1:1 [vol/vol/vol]), except for fraction 5, for which the solvent was present at 2:1:2.
Dry weight of pure compound isolated per liter of culture medium. A. fumigatus was inoculated into Grimm-Allen medium and grown for 72 h at 37°C and 150 rpm.
Fractions 1 and 2 together gave a yield of 15 mg.
Identification of siderophores.
MALDI-TOF mass spectra, 1H NMR, and 13C NMR were used to identify the two most abundant A. fumigatus siderophores as triacetylfusarinine C and ferricrocin.
MALDI-TOF mass spectra were obtained for both the ferrated and the desferri forms of both siderophores. The mass ions observed (Table 3) are in agreement with the identification of the siderophores as triacetylfusarinine C (C39O15N6H57Fe, 905.78) and ferricrocin (C28O13N9H44Fe, 770.58).
TABLE 3.
Calculated and measured mass ions for ferrated and desferri forms of fractions 3 and 4
| Form | Mass ion
|
|||
|---|---|---|---|---|
| Calculated for triacetylfusarinine C | Observed for fraction 3 | Calculated for ferricrocin | Observed for fraction 4 | |
| Ferrated MH+ | 906.79 | 906.77 | 771.59 | 771.37 |
| Ferrated MNa+ | 928.79 | 928.76 | 793.57 | 793.38 |
| Desferri MH+ | 853.97 | 853.74 | 718.76 | 718.33 |
| Desferri MNa+ | 875.97 | 875.67 | 740.75 | 740.37 |
1H NMR and 13C NMR spectra of the deferrated siderophores (Tables 4, 5, and 6) were in agreement with published spectra of desferritriacetylfusarinine C (24) and desferriferricrocin (17, 27, 28). The 1H NMR spectrum of desferriferricrocin has not been fully reported; therefore, detailed proton assignments were determined by using a combination of COSY, TOCSY, NOESY, and ROESY NMR spectra with dimethyl sulfoxide (DMSO-d6) solvent at 293 K (Table 5).
TABLE 4.
NMR chemical shifts observed for purified desferri form of fraction 3 at 293 K confirm its identity as triacetylfusarinine C
| Structural group | Signal for:
|
|||
|---|---|---|---|---|
|
1H NMR in DMSO-d6
|
13C NMR in CDCl3
|
|||
| Publisheda | Fraction 3 | Publisheda | Fraction 3 | |
| αCH | 4.18 (m) | 4.06-4.23 (m) | 52.5 | 52.6 |
| βCH2 | 1.62 (m) | 1.44-1.73 (m) | 29.3 | 28.9 |
| γCH2 | 1.62 (m) | 1.44-1.73 (m) | 23.3 | 23.1 |
| δCH2 | 3.48 (m) | 3.50 (m) | 48.1 | 47.1 |
| —COO— | 170.9 | 171.2 | ||
| Nδ-Acyl-CH= | 6.22 (s) | 6.31 (s) | 118.4 | 119.0 |
| Nδ-Acyl-CH2— | 2.64 (t) | 2.66 (m) | 32.4 | 31.7 |
| Nδ-Acyl-CH2O— | 4.18 (m) | 4.06-4.23 (m) | 62.9 | 62.7 |
| Nδ-Acyl-CH3 | 1.87 (s) | 1.86 (s) | 24.4 | 24.4 |
| Nδ-Acyl=C< | 149.1 | 149.0 | ||
| Nα-Acetyl-CH3 | 1.84 (s) | 1.83 (s) | 22.9 | 22.8 |
| Nα-Acetyl>C=O | 172.0 | 171.2b | ||
| Hydroxamic>C=O | 172.0 | 172.1b | ||
| Nδ-OH | 9.74 | |||
| NαH | 8.21 | |||
values are from reference 24.
Assignments may be reversed.
TABLE 5.
1H NMR chemical shifts for desferri form of fraction 4 at 293 K in DMSO-d6 confirm its identity as desferriferricrocin
| Structural group | Signal(s) at the following residuea:
|
|||||
|---|---|---|---|---|---|---|
| Glycine (1) | Serine (2) | Glycine (3) |
N5-Acetyl-N5-hydroxyl-l-ornithine
|
|||
| 4 | 5 | 6 | ||||
| NH | 8.54 | 8.08 | 8.35 | 8.08 | 7.91 | 7.84 |
| αCH | 3.90, 3.41 | 4.19 | 3.77, 3.69 | 3.97 | 4.17 | 4.00 |
| βCH | 3.66, 3.55 | 1.69, 1.56 | 1.67, 1.57 | 1.62, 1.49 | ||
| δCH | 1.50, 1.50 | 1.52, 1.52 | 1.53, 1.43 | |||
| γCH | 3.45, 3.45 | 3.41, 3.49 | 3.44, 3.44 | |||
| CH3 | 1.96 | 1.96 | 1.93 | |||
| OH | 3.50 | 9.72b | ||||
Numbers refer to the order in which residues occur in the ferricrocin molecule.
Broad NMR signal centered at 9.72 ppm and compatible with ornithine OH.
TABLE 6.
13C NMR assignments for desferri form of fraction 4 (desferriferricrocin) at 293 K in D2O
| Structural group | Signal(s) at the following residue:
|
||
|---|---|---|---|
| Glycine | Serine | N5-Acetyl-N5-hydroxyl-l-ornithine | |
| αCH | 43.6, 43.8 | 56.2 | 54.8, 54.8, 55.0 |
| βCH | 61.3 | 28.4, 29.2, 29.5 | |
| δCH | 23.2, 23.3, 23.4 | ||
| γCH | 48.2, 48.3, 48.3 | ||
| Acetyl-CH3 | 20.2 | ||
| C=O | 172.3, 172.6 | 172.9 | 174.5, 174.7, 174.9 |
| Hydroxamic C=O | 174.1 | ||
Incubation of A. fumigatus siderophores with holotransferrin.
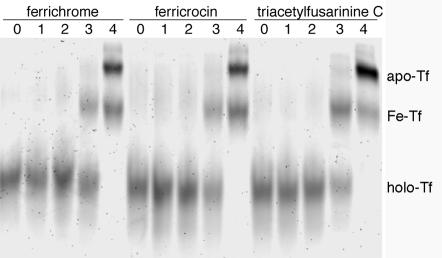
To determine whether individual A. fumigatus siderophores were able to compete for transferrin-bound iron, holotransferrin was incubated with dilutions of purified desferritriacetylfusarinine C and desferriferricrocin. Holotransferrin also was incubated with commercially available desferriferrichrome for comparison. The iron saturation of transferrin was monitored by urea-PAGE. The results were similar for all three desferrisiderophores. Desferritriacetylfusarinine C, desferriferricrocin, and desferriferrichrome all were able to remove some iron from holotransferrin (25 μM) when present at 500 μM, although the complete absence of holotransferrin was not observed until the desferrisiderophore concentration reached 5 mM (Fig. 5). These results confirm that at least two of the A. fumigatus siderophores can remove iron from human transferrin. The siderophore concentrations necessary to remove iron from transferrin in vivo will depend on the relative local concentrations of the siderophore and transferrin and also may be affected by the presence of cells which actively take up the ferrated siderophore and alter the iron uptake equilibrium.
FIG. 5.
Iron saturation of transferrin following incubation with A. fumigatus siderophores. Purified desferrisiderophores were incubated with holotransferrin (25 μM) at 37°C for 16 h. Desferriferrichrome, desferriferricrocin, and desferritriacetylfusarinine C were serially diluted to final concentrations of 5 μM (lanes 1), 50 μM (lanes 2), 500 μM (lanes 3), and 5 mM (lanes 4). Controls containing holotransferrin alone also were run (lanes 0). holo-Tf, holotransferrin; Fe-Tf, monoferric transferrin; apo-Tf, apotransferrin.
DISCUSSION
Siderophore production by microorganisms allows their growth in environments where iron concentrations are limiting. In aerobic environments, ferric ions are abundant but are largely bound as insoluble ferric hydroxides (34). At a neutral pH, the equilibrium concentration of Fe3+ in water cannot exceed 10−17 M (2), yet iron is required in micromolar amounts for fungal growth (51). Therefore, soil microorganisms, such as A. fumigatus, have adapted to iron-limited environments by producing a variety of siderophores. Organisms that secrete siderophores to access iron from environmental ferric hydroxides also may effectively scavenge transferrin-bound iron in vivo.
Previous work by Gifford et al. demonstrated that A. fumigatus can grow in human serum (15), and the results from the present study indicate that A. fumigatus overcomes the iron limitation of serum by secreting hydroxamate siderophores which remove iron from serum transferrin. Proteolytic cleavage of transferrin by A. fumigatus is a secondary mechanism by which it can obtain iron.
In this study, holotransferrin, but not apotransferrin, promoted the growth of A. fumigatus in iron-deficient medium, indicating that A. fumigatus can use holotransferrin as an iron source. Urea-PAGE can distinguish among the four different forms of transferrin—Fe2-transferrin (holotranferrin), FeC-transferrin, transferrin-FeN, and apotransferrin—based upon their different degrees of resistance to denaturation in 6 M urea. Holotransferrin incubated with A. fumigatus conidia was converted to apotransferrin within 8 h, approximately the same time at which siderophores were detected by the CAS assay and very soon after the germination of conidia. Unlike proteinase secretion (15), siderophore production occurs early in the growth of A. fumigatus. These data suggest that siderophore-mediated removal of iron from human transferrin is important in the growth of A. fumigatus. The removal of iron from transferrin across a dialysis membrane further supports the hypothesis that A. fumigatus uses siderophores to obtain iron, as opposed to expressing transferrin receptors or ferric reductase proteins.
Five siderophores were purified from A. fumigatus cultures, and the two major siderophores were identified as triacetylfusarinine C and ferricrocin. To date, all A. fumigatus siderophores that have been characterized are hydroxamate siderophores; these include triacetylfusarinine C and siderophores of the ferrichrome class, such as ferricrocin. Nilius and Farmer reported the production of six siderophores by A. fumigatus, with triacetylfusarinine C being the most prominent, followed by ferricrocin (35). Other studies have detected ferricrocin and ferrirubin in A. fumigatus cultures (49), although the type and ratio of siderophores produced appeared to vary from strain to strain (12). Siderophores observed in other Aspergillus species include ferrichrome, fusigen, ferrichrysin, ferrirhodin, and ferrirubin (35) and the asperchromes (23). Aspergillus nidulans produces triacetylfusarinine C and ferricrocin as the two major siderophores. Eisendle et al. (13) showed that a mutant of A. nidulans deficient in hydroxamate siderophore production was unable to grow unless the medium was supplemented with siderophores or ferrous iron.
Triacetylfusarinine C is a siderophore common in Aspergillus species (12, 13), while ferricrocin is thought to be an important intracellular iron storage compound in fungi such as A. nidulans (13) and Neurospora crassa (29). Ferricrocin is produced by a wide variety of fungi, including Cenococcum geophilum (17), Phialocephala fortinii (3), and Colletotrichum gloeosporioides (37). Triacetylfusarinine C has been reported to bind iron with a pM of 31.8 in phosphate buffer, at pH 6.8, and at 30°C (1), and ferricrocin and ferrichrome have reported pM values of 26.5 (53) and 25.2 (11), respectively. The high iron affinities of these compounds theoretically enable these siderophores to remove iron from ferrated transferrin. This theory was confirmed in our study, as all three siderophores removed transferrin-bound iron. Hydroxamate siderophores have been reported to remove iron from transferrin in other studies. These include rhodotorulic acid (47), a dihydroxamate siderophore with a pM of 21.9 (16), and aerobactin (25), a bacterial siderophore with a prototypical hydroxamate-citrate structure and a pM of 23.3 (16). Escherichia coli strains bearing the plasmid for aerobactin production can grow in the presence of transferrin, and virulence is associated with the synthesis of aerobactin (50).
Microorganisms probably use several mechanisms to ensure a continuous supply of iron for growth. We therefore postulated that A. fumigatus may possess more than one mechanism for obtaining iron from serum. A. fumigatus produces proteinases when cultured in serum-containing media (15), and these proteinases could allow iron release from transferrin, making it available to the fungus. In the present study, a small amount of degradation of transferrin was apparent after 46 h, and transferrin was completely degraded within 70 h. When grown in MEM containing human serum, A. fumigatus reaches stationary phase after approximately 25 h, whereas the peak of proteinase production occurs after 40 to 48 h (15). The fact that transferrin is not hydrolyzed until late logarithmic phase also may be related to the relative resistance of holotransferrin to proteolytic cleavage compared to that of apotransferrin (14). Because the demand for iron is highest during logarithmic phase, when active growth is occurring, proteolytic degradation of transferrin is unlikely to be the primary mechanism by which A. fumigatus obtains iron. This conclusion was further supported by results showing that A. fumigatus was able to grow in MEM containing transferrin alone without any degradation of transferrin.
The relative importance of hydrolysis of iron-binding proteins and siderophore secretion has been evaluated with two bacterial pathogens. Using chemical mutagenesis, Okujo et al. (38) created a mutant of V. vulnificus that was deficient in the secretion of an extracellular protease (VVP) but was still able to secrete the siderophore vulnibactin. They compared the growth in holotransferrin of this mutant and a VVP-secreting strain that produced only small amounts of the siderophore. Their results indicated that siderophore production rather than VVP secretion was necessary for growth when ferrated transferrin was the sole iron source. In another study, Wolz et al. (52) created a strain of Pseudomonas aeruginosa that was unable to produce LasB, the only secreted protease capable of transferrin hydrolysis. LasB mutants were still able to remove iron from transferrin by use of the P. aeruginosa siderophore pyoverdin, suggesting that siderophore production alone was sufficient to obtain iron. However, when pyoverdin and transferrin were present in equimolar concentrations, iron exchange was enhanced by the proteolytic degradation of transferrin by LasB (52). A similar scenario could be envisioned for A. fumigatus growing in serum during late logarithmic phase, when protease secretion is maximal.
We have provided evidence that the growth of A. fumigatus in human serum in vitro is supported by the production of siderophores. However, we cannot exclude the possibility that low-molecular-weight reductants also participate in iron acquisition, as has been shown for H. capsulatum (47) and C. neoformans (36). Direct evidence for a role of A. fumigatus siderophores in virulence awaits the study of siderophore-negative mutant strains.
Acknowledgments
We thank B. Johnston, Department of Chemistry, Simon Fraser University, for assistance with the MALDI-TOF analysis and A. Tracey and M. Tracey, Department of Chemistry, Simon Fraser University, for obtaining and interpreting NMR spectra.
Financial support from the Natural Sciences and Engineering Research Council of Canada is gratefully acknowledged.
Editor: T. R. Kozel
REFERENCES
- 1.Adjimani, J. P., and T. Emery. 1987. Iron uptake in Mycelia sterilia EP-76. J. Bacteriol. 169:3664-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aisen, P. 1998. Transferrin, the transferrin receptor, and the uptake of iron by cells. Met. Ions Biol. Syst. 35:585-631. [PubMed] [Google Scholar]
- 3.Bartholdy, B. A., M. Berreck, and K. Haselwandter. 2001. Hydroxamate siderophore synthesis by Phialocephala fortinii, a typical dark septate fungal root endophyte. Biometals 14:33-42. [DOI] [PubMed] [Google Scholar]
- 4.Bearden, S. W., and R. D. Perry. 1999. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol. Microbiol. 32:403-414. [DOI] [PubMed] [Google Scholar]
- 5.Bodey, G., B. Bueltmann, W. Duguid, D. Gibbs, H. Hanak, M. Hotchi, G. Mall, P. Martino, F. Meunier, S. Milliken, et al. 1992. Fungal infections in cancer patients: an international autopsy survey. Eur. J. Clin. Microbiol. Infect. Dis. 11:99-109. [DOI] [PubMed] [Google Scholar]
- 6.Boelaert, J. R., M. de Locht, J. Van Cutsem, V. Kerrels, B. Cantinieaux, A. Verdonck, H. W. Van Landuyt, and Y. J. Schneider. 1993. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J. Clin. Investig. 91:1979-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullen, J. J. 1981. The significance of iron in infection. Rev. Infect. Dis. 3:1127-1138. [DOI] [PubMed] [Google Scholar]
- 8.Byers, B. R., and J. E. Arceneaux. 1998. Microbial iron transport: iron acquisition by pathogenic microorganisms. Met. Ions Biol. Syst. 35:37-66. [PubMed] [Google Scholar]
- 9.Dave, B. P., and H. C. Dube. 2000. Regulation of siderophore production by iron Fe(III) in certain fungi and fluorescent pseudomonads. Indian J. Exp. Biol. 38:297-299. [PubMed] [Google Scholar]
- 10.De Bock, R. 1994. Epidemiology of invasive fungal infections in bone marrow transplantation. Bone Marrow Transplant. 14:S1-S2. [PubMed] [Google Scholar]
- 11.Dhungana, S., S. Heggemann, P. Gebhardt, U. Mollmann, and A. L. Crumbliss. 2003. Fe(III) coordination properties of a new saccharide-based exocyclic trihydroxamate analogue of ferrichrome. Inorg. Chem. 42:42-50. [DOI] [PubMed] [Google Scholar]
- 12.Diekmann, H., and E. Krezdorn. 1975. Metabolic products of microorganisms. 150. Ferricrocin, triacetylfusigen and other sideramines from fungi of the genus Aspergillus, group Fumigatus. Arch. Microbiol. 106:191-194. (In German.) [DOI] [PubMed] [Google Scholar]
- 13.Eisendle, M., H. Oberegger, I. Zadra, and H. Haas. 2003. The siderophore system is essential for viability of Aspergillus nidulans: functional analysis of two genes encoding 1-ornithine N 5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC). Mol. Microbiol. 49:359-375. [DOI] [PubMed] [Google Scholar]
- 14.Esparza, I., and J. H. Brock. 1980. The effect of trypsin digestion on the structure and iron-donating properties of transferrins from several species. Biochim. Biophys. Acta 622:297-307. [DOI] [PubMed] [Google Scholar]
- 15.Gifford, A. H., J. R. Klippenstein, and M. M. Moore. 2002. Serum stimulates growth of and proteinase secretion by Aspergillus fumigatus. Infect. Immun. 70:19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris, W. R., C. J. Carrano, S. R. Cooper, S. R. Sofen, A. Avdeef, J. V. McArdle, and K. N. Raymond. 1979. Coordination chemistry of microbial iron transport compounds. 19. Stability constants and electrochemical behavior of ferric enterobactin and model complexes. J. Am. Chem. Soc. 101:6097-6104. [Google Scholar]
- 17.Haselwandter, K., and G. Winkelmann. 2002. Ferricrocin—an ectomycorrhizal siderophore of Cenococcum geophilum. Biometals 15:73-77. [DOI] [PubMed] [Google Scholar]
- 18.Henderson, D. P., and S. M. Payne. 1994. Vibrio cholerae iron transport systems: roles of heme and siderophore iron transport in virulence and identification of a gene associated with multiple iron transport systems. Infect. Immun. 62:5120-5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard, D. H. 1999. Acquisition, transport, and storage of iron by pathogenic fungi. Clin. Microbiol. Rev. 12:394-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard, D. H., R. Rafie, A. Tiwari, and K. F. Faull. 2000. Hydroxamate siderophores of Histoplasma capsulatum. Infect. Immun. 68:2338-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iglesias-Osma, C., L. Gonzalez-Villaron, J. F. San Miguel, M. D. Caballero, L. Vazquez, and S. de Castro. 1995. Iron metabolism and fungal infections in patients with haematological malignancies. J. Clin. Pathol. 48:223-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson, E. S., A. P. Goodner, and K. J. Nyhus. 1998. Ferrous iron uptake in Cryptococcus neoformans. Infect. Immun. 66:4169-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jalal, M. A., R. Mocharla, C. L. Barnes, M. B. Hossain, D. R. Powell, D. L. Eng-Wilmot, S. L. Grayson, B. A. Benson, and D. van der Helm. 1984. Extracellular siderophores from Aspergillus ochraceus. J. Bacteriol. 158:683-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jalal, M. A. F., and D. van der Helm. 1991. Isolation and spectroscopic identification of fungal siderophores, p. 235-269. In G. Winkelmann (ed.), Handbook of microbial iron chelates. CRC Press, Inc., Boca Raton, Fla.
- 25.Konopka, K., A. Bindereif, and J. B. Neilands. 1982. Aerobactin-mediated utilization of transferrin iron. Biochemistry 21:6503-6508. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 27.Llinas, M., W. J. Horsley, and M. P. Klein. 1976. Nitrogen-15 nuclear magnetic resonance spectrum of alumichrome. Detection by a double resonance Fourier transform technique. J. Am. Chem. Soc. 98:7554-7558. [DOI] [PubMed] [Google Scholar]
- 28.Llinas, M., M. P. Klein, and J. B. Neilands. 1972. Solution conformation of the ferrichromes. A comparative proton magnetic resonance study of glycine- and serine-containing ferrichromes. J. Mol. Biol. 68:265-284. [DOI] [PubMed] [Google Scholar]
- 29.Matzanke, B. F., E. Bill, A. X. Trautwein, and G. Winkelmann. 1987. Role of siderophores in iron storage in spores of Neurospora crassa and Aspergillus ochraceus. J. Bacteriol. 169:5873-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer, J. M., A. Neely, A. Stintzi, C. Georges, and I. A. Holder. 1996. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 64:518-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minamoto, G. Y., T. F. Barlam, and N. J. Vander Els. 1992. Invasive aspergillosis in patients with AIDS. Clin. Infect. Dis. 14:66-74. [DOI] [PubMed] [Google Scholar]
- 32.Modun, B., R. W. Evans, C. L. Joannou, and P. Williams. 1998. Receptor-mediated recognition and uptake of iron from human transferrin by Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 66:3591-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrissey, J. A., P. H. Williams, and A. M. Cashmore. 1996. Candida albicans has a cell-associated ferric-reductase activity which is regulated in response to levels of iron and copper. Microbiology 142:485-492. [DOI] [PubMed] [Google Scholar]
- 34.Neilands, J. B. 1995. Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem. 270:26723-26726. [DOI] [PubMed] [Google Scholar]
- 35.Nilius, A. M., and S. G. Farmer. 1990. Identification of extracellular siderophores of pathogenic strains of Aspergillus fumigatus. J. Med. Vet Mycol. 28:395-403. [DOI] [PubMed] [Google Scholar]
- 36.Nyhus, K. J., A. T. Wilborn, and E. S. Jacobson. 1997. Ferric iron reduction by Cryptococcus neoformans. Infect. Immun. 65:434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohra, J., K. Morita, Y. Tsujino, H. Tazaki, T. Fujimori, M. Goering, S. Evans, and P. Zorner. 1995. Production of the phytotoxic metabolite, ferricrocin, by the fungus Colletotrichum gloeosporioides. Biosci. Biotechnol. Biochem. 59:113-114. [DOI] [PubMed] [Google Scholar]
- 38.Okujo, N., T. Akiyama, S. Miyoshi, S. Shinoda, and S. Yamamoto. 1996. Involvement of vulnibactin and exocellular protease in utilization of transferrin- and lactoferrin-bound iron by Vibrio vulnificus. Microbiol. Immunol. 40:595-598. [DOI] [PubMed] [Google Scholar]
- 39.Pandey, A., F. Bringel, and J. M. Meyer. 1994. Iron requirement and search for siderophores in lactic acid bacteria. Appl. Microbiol. Biotechnol. 40:735-739. [Google Scholar]
- 40.Payne, S. M. 1994. Detection, isolation, and characterization of siderophores. Methods Enzymol. 235:329-344. [DOI] [PubMed] [Google Scholar]
- 41.Posey, J. E., and F. C. Gherardini. 2000. Lack of a role for iron in the Lyme disease pathogen. Science 288:1651-1653. [DOI] [PubMed] [Google Scholar]
- 42.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 43.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 44.Segal, B. H., E. S. DeCarlo, K. J. Kwon-Chung, H. L. Malech, J. I. Gallin, and S. M. Holland. 1998. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore) 77:345-354. [DOI] [PubMed] [Google Scholar]
- 45.Takase, H., H. Nitanai, K. Hoshino, and T. Otani. 2000. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect. Immun. 68:1834-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Timmerman, M. M., and J. P. Woods. 1999. Ferric reduction is a potential iron acquisition mechanism for Histoplasma capsulatum. Infect. Immun. 67:6403-6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Timmerman, M. M., and J. P. Woods. 2001. Potential role for extracellular glutathione-dependent ferric reductase in utilization of environmental and host ferric compounds by Histoplasma capsulatum. Infect. Immun. 69:7671-7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wald, A., W. Leisenring, J. A. van Burik, and R. A. Bowden. 1997. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect. Dis. 175:1459-1466. [DOI] [PubMed] [Google Scholar]
- 49.Wiebe, C., and G. Winkelmann. 1975. Kinetic studies on the specificity of chelate-iron uptake in Aspergillus. J. Bacteriol. 123:837-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams, P. H., and P. J. Warner. 1980. ColV plasmid-mediated, colicin V-independent iron uptake system of invasive strains of Escherichia coli. Infect. Immun. 29:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winkelmann, G. 1992. Structures and functions of fungal siderophores containing hydroxamate and complexone type iron binding ligands. Mycol. Res. 96:529-534. [Google Scholar]
- 52.Wolz, C., K. Hohloch, A. Ocaktan, K. Poole, R. W. Evans, N. Rochel, A. M. Albrecht-Gary, M. A. Abdallah, and G. Doring. 1994. Iron release from transferrin by pyoverdin and elastase from Pseudomonas aeruginosa. Infect. Immun. 62:4021-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong, G. B., M. J. Kappel, K. N. Raymond, B. Matzanke, and G. Winkelmann. 1983. Coordination chemistry of microbial iron transport compounds. 24. Characterization of coprogen and ferricrocin, two ferric hydroxamate siderophores. J. Am. Chem. Soc. 105:810-815. [Google Scholar]