Summary
Background
Thermal ablation procedures, including radiofrequency ablation (RFA) or laser-induced interstitial thermotherapy (LITT), are now well established in the treatment of malignant unresectable hepatic tumors. But the impact of partial ablation (PA) on long-term survival following computed tomography (CT)-guided radiofrequency ablation and laser- induced interstitial thermotherapy of unresectable malignant liver lesions and the associated risk factors of PA remain partially unknown.
Material/Methods
This study included 254 liver tumors in 91 consecutive patients (66 men and 25 women; age 60.9±10.4 years; mean tumor size 25±14 mm [range 5–70 mm]) who underwent thermal ablation (RFA or LITT) between January 2000 and December 2007. Mean follow-up period was 21.1 month (range 1–69 months). Survival rate and local progression-free survival (PFS) were calculated for patients with complete ablation (CA) vs. patients with partial ablation (PA) to assess the impact on long-term survival.
Results
Median survival after CA was 47 months compared to 25 months after PA (P=0.04). The corresponding 5-year survival rates were 44% vs. 20%. Median PFS for CA was 11 months compared to 7 months for PA (P=0.118). The sole statistically significant risk factor for PA was tumor size (>30 mm; P=0.0003). Sustained complete ablation was achieved in 71% of lesions ≤30 mm vs. 47% of lesions >30 mm.
Conclusions
We conclude that achievement of complete ablation is a highly important predictor of long-term survival and that tumor size is by far the most important predictor of the likelihood of achieving complete ablation.
Keywords: radiofrequency ablation, laser induced interstitial thermotherapy, liver tumors, liver metastases
Background
Thermal ablation procedures, including radiofrequency ablation (RFA) or laser-induced interstitial thermotherapy (LITT), are now well established in the treatment of malignant unresectable hepatic tumors. This includes primary malignant liver tumors such as hepatocellular carcinoma (HCC) as well as metastases, especially from colorectal cancer (CRC) [1–6]. The main objective of thermal ablation is to induce in situ coagulation necrosis for complete destruction of targeted tumors with only limited damage to the surrounding normal tissues. Compared to other in situ ablation techniques such as microwave, cryoablation, high-intensity focused ultrasound (HIFU), and percutaneous ethanol injection (PEI), RFA and LITT seem to be the best options as both of them produce a comparatively large coagulation necrosis and are associated with a low rate of complications [7]. In RFA, a shielded needle electrode is inserted directly to the tumor under image guidance. The RF energy heats tissue by applying alternating current, resulting in ionic agitation. This local temperature rise induces a necrotic lesion around the needle tip. Similarly, in LITT laser light is directly transmitted percutaneously through flexible bare fibers into the tumor tissue. The biologic effect of the laser light is based on the absorption of laser photon energy by the tissue, leading to a local temperature rise. The aim is to induce coagulation necrosis of the treatment volume. Tumors in a variety of organs, including the lungs and kidneys [2,8–12] have been treated with thermal ablation, but thus far the liver has been the organ of primary interest. This is because thermal ablation of malignant liver lesions is a potentially curative treatment, it is less invasive, and it results in fewer complications compared with surgery [13–15]. Thermal ablation has shown promising results in local tumor control [14,16,17], but only when a complete thermal coagulation of the tumor, with no viable tumor cells in the treated area, is achieved. However, partial ablation of liver tumors is achieved in up to 52% of cases [18–21]. The purpose of this study was to assess the impact of partial ablation on the long-term survival of patients with malignant liver lesions.
Material and Methods
Patients and indications for thermal ablation
This retrospective study reviewed 91 consecutive patients with a total of 254 primary or secondary hepatic malignancies treated by thermal ablation (RFA: n=79, LITT: n= 12) between January 2000 and December 2007 (Table 1). All patients were referred from a multidisciplinary team. The indications for thermal ablation were extensively discussed at the visceral tumor board and only patients not eligible for liver resection underwent ablation. The exclusion criteria for RFA were as follows: eligibility for liver resection, presence of more than 5 intrahepatic nodules, a maximum tumor size of more than 7 cm, and extrahepatic disease. The procedures for thermal ablation of malignant liver lesions have been previously described in detail [22,23]. All ablations were performed percutaneously under analgo-sedation, and by CT guidance.
Table 1.
Characteristics of 254 liver tumors in patient characteristics.
| Characteristics | No. of tumors (no. of patients) or mean ± SD | |
|---|---|---|
| Gender | Male | (66) |
| Female | (25) | |
| Age (years) | 60.92±10.39 | |
| Tumor size (cm) | 2.54±1.40 | |
| No. of lesions | 2.79±1.45 | |
| Tumor type | ||
| HCC | 18 (8) | |
| Metastatic liver malignancies | 236 (83) | |
| Colorectal cancer | (54) | |
| Other | (29) | |
Postoperative evaluation and follow-up
All patients underwent a CT scan at 4–6 weeks after ablation to assess and evaluate the results of the procedure, at 4, 8, and 12 months following the thermal ablation treatment, and subsequently every 6 months. Complete ablation (CA) was defined as complete necrosis of all malignant liver lesions, without any contrast enhancement at any phase of the exploration and no recurrent disease within a 10 mm margin of the ablated regions during the follow-up period. Partial ablation (PA) was defined as the persistence of vascular enhancement peripheral to or at the treatment site at the first follow-up scan, or recurrent disease within a 10 mm margin during follow-up.
Statistical analysis
All data were statistically analyzed by using Microsoft Excel (Microsoft, Inc., Redmond, WA) and SPSS (SPSS for Windows, version 17, SPSS Inc., Chicago, Illinois). We applied a cut-off for continuous-type variables (e.g., size or age) to convert them to an ordinal scale corresponding to categories used in the literature. Univariate analysis was used to describe differences among groups defined according to the characteristics mentioned above. Chi-square or Fisher’s exact tests were used to compare the proportions for qualitative variables. Overall survival from the time of the first thermal ablation treatment to the death of the patient or end of observation was calculated for all patients using the Kaplan-Meier method. Difference among groups was determined by the log-rank test. A value of P<0.05 was considered statistically significant. All data are expressed as mean ±SD or median + range.
Results
A total of 91 patients with liver malignancies underwent thermal ablation during the study period (Table 1). There were 66 men (73%) and 25 women (27%) with a mean age of 60.9 years (±10.4 years). Eighty-three (91.2%) patients had metastatic liver malignancies, of which 54 were colorectal liver metastases (65%), and 8 (8.8%) patients had HCC. The median number of tumors per patient was 3 (range 1–5). A total of 254 tumors were treated in these 91 patients. Among these tumors, 236 (92.9%) were liver metastases and 18 (7.1%) were hepatic primary (HCC). Thermal ablation was used to treat a single tumor in 24.2% of cases. Two tumors in 24.2% of cases. Three nodules were treated during the same procedure in 18.7% of cases, 4 nodules in 14.3%, and 5 nodules were treated in 18.7% of cases. Mean size of the ablated tumor was 25 mm in diameter (range 5–70 mm). Treatment with thermal ablation, always performed under CT guidance, was technically feasible in all cases. Thirty-day mortality was 0%, with no deaths related to postprocedural complications or morbidity. Median follow-up time from ablation until death or end of observation was 19 months (mean, 21.1 months), with a range of 1 to 69 months.
Survival
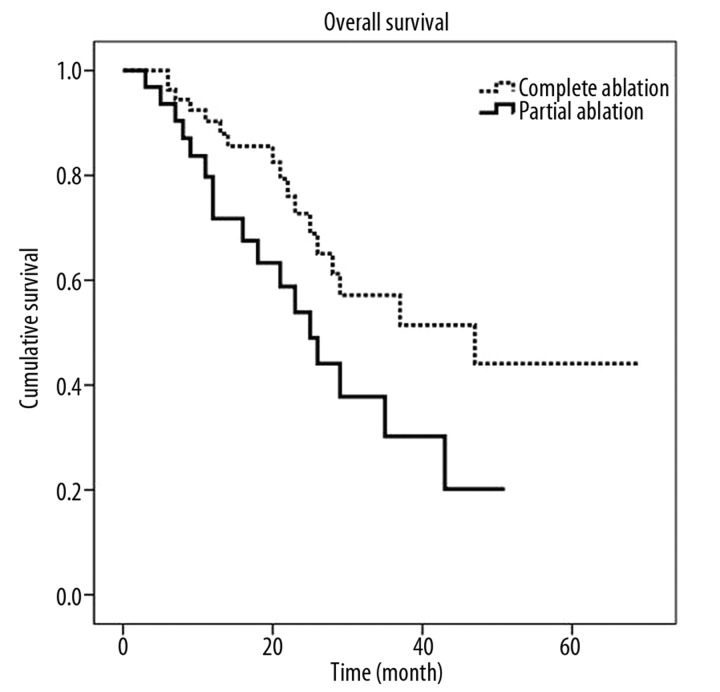
The estimated median survival for all patients who had thermal ablation of their malignant liver lesions was 29 months (95% CI 18–40 month). The estimated median survival for patients who had a complete ablation or a partial ablation was 47 months (95% CI 17.9–76.0 month) and 25 months (18.1–31.9 months), respectively. The 5-year overall survival after a complete ablation was 44%, while the 5-year survival after a partial ablation was 20%, as shown in Figure 1. This difference was statistically significant (P=0.041).
Figure 1.
Cumulative survival curves for complete and partial ablation calculated by the Kaplan-Meier method.
Progression-free survival (PFS)
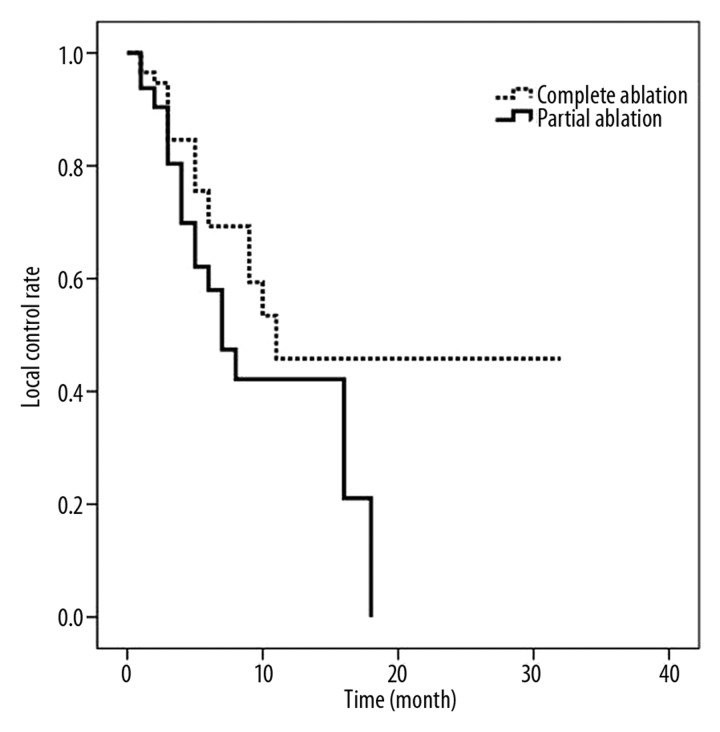
The median time to progression of disease for patients who had a PA was 7 months (95% CI: 4.46–9.54 months), compared to 11 months (95% CI: 4.23–17.77 month) for patients with a CA (P=0.118). The corresponding mean time to progression was 9.81±1.34 months for partial ablations and 16.05±2.76 months for a complete ablation (Figure 2). Complete tumor ablation was achieved in 96% of cases at the first follow-up CT scan (at 4–6 weeks). The long-term complete ablation figure was 62% after a mean follow-up of 21.1 months (range 1–69).
Figure 2.
Time to local progress for patients with complete or partial ablation. Median time to progression for PA was 7 months, compared to 11 months for patients with a CA.
Determinants of partial ablation
Based on these significant differences in survival times for complete and partial ablation, we analyzed potential variables that could have an impact on partial ablation of tumors (Table 2). The following variables were analyzed: sex, tumor size (>30 mm vs. ≤30 mm), tumor histopathology (HCC vs. liver metastases) and patient age (>70 years vs. ≤70 years). Of 254 lesions that were evaluated during follow-up, complete and sustained ablation was achieved in 96% of cases by the first follow-up CT scan (at 4–6 weeks). The sex, age, tumor type and number of lesions of patients with residual tumor or recurrence of disease were not statistically different from those of patients with a complete ablation of the treated lesions. Only lesion size had a statistically significant impact on the risk of residual tumor or recurrence (p=0.0003). The complete ablation rate for tumors ≤30 mm was 71% compared to 47% for lesions larger than 30 mm.
Table 2.
Factors associated with partial ablation.
| Lesion/patient based* factors associated with incomplete ablation: univariate analysis | |||
|---|---|---|---|
| Variable | Complete ablation | Partial ablation | P |
| N=157 | N=97 | ||
| Gender | NS | ||
| Male | 120 | 70 | |
| Female | 37 | 27 | |
| Age | NS | ||
| Above 70 | 34 | 20 | |
| Below 70 | 123 | 77 | |
| Tumor type | NS | ||
| HCCs | 9 | 9 | |
| Mets | 148 | 88 | |
| Tumor size | .0003 | ||
| ≤30 mm | 113 | 48 | |
| >30 mm | 44 | 49 | |
| No. lesions* | NS | ||
| 1 | 14 | 8 | |
| 2 | 16 | 6 | |
| 3 | 12 | 5 | |
| 4 | 10 | 3 | |
| 5 | 7 | 10 | |
Discussion
Surgical resection is currently the only therapeutic option with a potential curative effect for patients with liver metastases. However, in more than 70% of cases the surgical approach is contraindicated due to poor liver reserve, insufficient health status, or an overly advanced tumor stage [18,24–28]. For this reason, there is a need for alternative treatment options for patients with unresectable liver malignancies [29–31]. Thermal ablation techniques, such as RFA and LITT, have gained a high degree of acceptance and are the therapeutic option of choice in the treatment of unresectable liver malignancies [32–35]. The main objective of thermal ablation of malignant liver tumors is to achieve a complete thermal coagulation of the tumor, thus leaving no viable tumor cells in the treated area. Nevertheless, partial ablation or recurrent disease is a frequent problem in thermal ablation procedures, where rates of up to 52% have been cited [18,21]. In our retrospective study the rate of local recurrence was 38%, which is in line with the reported figures. Even though there are many available case series in the literature on thermal ablation, there is only limited data on recurrence risk factors [36], predictive factors for survival, and on long-term results [37–39]. The interim results of the only prospective randomized phase II study (CLOCC trial [chemotherapy plus local ablation vs. chemotherapy]) initiated by the European Organization for Research and Treatment of Cancer could not demonstrate a statically significant impact of RFA plus chemotherapy compared to chemotherapy alone for patients with an advanced stage of unresectable metastatic colorectal cancer liver disease [40]. The 30-month overall survival rates were 64.9% (95% CI, 51.13–77.09) for RFA plus chemotherapy and 56.9% (95% CI 43.23–69.84) for chemotherapy alone. There was, however, a significant trend in the prolongation of progression-free survival for patients who were treated with RFA (p=0.025). No prospective trial comparing RFA to surgical resection in patients with similar extent of liver metastases has been conducted to date. However, the better survival of patients with CA when compared to patients with PA might indicate the effectiveness of local thermo- therapies. This study aimed to identify predictive factors for effective percutaneous thermal ablation and evaluate the impact of complete ablation on overall survival. As has been reported before by Ayav et al. [41], our study reiterates that tumor size is a significant factor associated with partial ablation or local recurrence. For tumors larger than 30 mm the local recurrence was 53% (49 of 93 tumors >30 mm). However, for tumors smaller or equal to 30 mm the respective number was 29% (48 of 161 tumors ≤30 mm). Lam et al. [42] reported that the median age of patients with local recurrence after RFA was 66 vs. 60 for patients without local recurrence (p=0.05). We did not observe this trend in our study. As previously reported, we confirmed that tumor pathology had no influence on partial ablation or local recurrence [43,44]. Age and sex where also not significant predictors of partial ablation. In order to evaluate the therapeutic impact of complete ablation we analyzed its impact on long-term survival. For patients with a partial ablation, median survival was 25 months and the 5-year survival was 20%. However, for patients with a complete ablation, median survival was 47 months and the 5-year survival was 44%, comparable to similar survival rates following surgical resection which have been widely reported in the literature [45,46]. This result, showing better local success in smaller lesions, might indicate that the concept of a safety margin is not only useful in the surgical approach but also for thermal ablation. According to these findings, we now caution against the use of thermal ablation for tumors >30 mm. Our retrospective study has certain limitations, such as the nonuniform histopathologic types of liver tumors (i.e. HCC and metastatic liver tumor from various organs) as well as the small number of patients evaluated.
Conclusions
Thermal ablation has proved to be an effective treatment option for local control of small, unresectable hepatic malignancies. We demonstrated with statistical significance that achievement of complete ablation is a highly important predictor of long-term survival and that tumor size is by far the most important predictor of achieving complete ablation. We conclude that lesions of less than or equal to 30 mm have a higher probability of achieving a complete ablation and are thus recommendable candidates for thermal ablation techniques.
Footnotes
Source of support: Departmental sourcers
References
- 1.Curley SA, Izzo F. Radiofrequency ablation of primary and metastatic hepatic malignancies. Int J Clin Oncol. 2002;7(2):72–81. doi: 10.1007/s101470200010. [DOI] [PubMed] [Google Scholar]
- 2.Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities – part II. J Vasc Interv Radiol. 2001;12(10):1135–48. doi: 10.1016/s1051-0443(07)61670-4. [DOI] [PubMed] [Google Scholar]
- 3.Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217(3):633–46. doi: 10.1148/radiology.217.3.r00dc26633. [DOI] [PubMed] [Google Scholar]
- 4.Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103(6):1201–9. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 5.Vogl TJ, Straub R, Eichler K, et al. Colorectal carcinoma metastases in liver: laser-induced interstitial thermotherapy – local tumor control rate and survival data. Radiology. 2004;230(2):450–58. doi: 10.1148/radiol.2302020646. [DOI] [PubMed] [Google Scholar]
- 6.Witczak-Malinowska K, Zadrozny D, Studniarek M, et al. Preliminary assessment of utility of radiofrequency ablation technique in treatment of primary hepatocellular carcinoma (HCC) in patients with hepatic cirrhosis. Med Sci Monit. 2003;9(Suppl 3):68–72. [PubMed] [Google Scholar]
- 7.McGhana JP, Dodd GD., III Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001;176(1):3–16. doi: 10.2214/ajr.176.1.1760003. [DOI] [PubMed] [Google Scholar]
- 8.Veltri A, Garetto I, Pagano E, et al. Percutaneous RF thermal ablation of renal tumors: is US guidance really less favorable than other imaging guidance techniques? Cardiovasc Intervent Radiol. 2009;32(1):76–85. doi: 10.1007/s00270-008-9414-5. [DOI] [PubMed] [Google Scholar]
- 9.de Baere T. Lung tumor radiofrequency ablation: where do we stand? Cardiovasc Intervent Radiol. 2011;34(2):241–51. doi: 10.1007/s00270-010-9860-8. [DOI] [PubMed] [Google Scholar]
- 10.Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study) Lancet Oncol. 2008;9(7):621–28. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- 11.Crocetti L, Lencioni R. Radiofrequency ablation of pulmonary tumors. Eur J Radiol. 2010;75(1):23–27. doi: 10.1016/j.ejrad.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Matuszewski M, Michajłwoski J, Bianek-Bodzak I, Krajka K. Radiofrequency ablation of small symptomatic angiomyolipomas of the kidney: Report of two cases. Pol J Radiol. 2010;75(3):68–71. [PMC free article] [PubMed] [Google Scholar]
- 13.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221(1):159–66. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 14.Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226(2):441–51. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 15.Szymański J, KuŸmcz M, Bakier J, Keniewski M. Percutaneous thermoablation of focal changes in the liver – our own experience. Pol J Radiol. 2004;69(Suppl 1):94. [Google Scholar]
- 16.Helmberger T, Dogan S, Straub G, et al. Liver resection or combined chemoembolization and radiofrequency ablation improve survival in patients with hepatocellular carcinoma. Digestion. 2007;75(2–3):104–12. doi: 10.1159/000104730. [DOI] [PubMed] [Google Scholar]
- 17.Wong SL, Mangu PB, Choti MA, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28(3):493–508. doi: 10.1200/JCO.2009.23.4450. [DOI] [PubMed] [Google Scholar]
- 18.Poon RT, Ng KK, Lam CM, et al. Effectiveness of radiofrequency ablation for hepatocellular carcinomas larger than 3 cm in diameter. Arch Surg. 2004;139(3):281–87. doi: 10.1001/archsurg.139.3.281. [DOI] [PubMed] [Google Scholar]
- 19.Ng KK, Poon RT, Lam CM, et al. Efficacy and safety of radiofrequency ablation for perivascular hepatocellular carcinoma without hepatic inflow occlusion. Br J Surg. 2006;93(4):440–47. doi: 10.1002/bjs.5267. [DOI] [PubMed] [Google Scholar]
- 20.Raut CP, Izzo F, Marra P, et al. Significant long-term survival after radiofrequency ablation of unresectable hepatocellular carcinoma in patients with cirrhosis. Ann Surg Oncol. 2005;12(8):616–28. doi: 10.1245/ASO.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Hori T, Nagata K, Hasuike S, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38(10):977–81. doi: 10.1007/s00535-003-1181-0. [DOI] [PubMed] [Google Scholar]
- 22.Guenette JP, Dupuy DE. Radiofrequency ablation of colorectal hepatic metastases. J Surg Oncol. 2010;102(8):978–87. doi: 10.1002/jso.21658. [DOI] [PubMed] [Google Scholar]
- 23.Stafford RJ, Fuentes D, Elliott AA, et al. Laser-induced thermal therapy for tumor ablation. Crit Rev Biomed Eng. 2010;38(1):79–100. doi: 10.1615/critrevbiomedeng.v38.i1.70. [DOI] [PubMed] [Google Scholar]
- 24.Poon RT, Fan ST, Lo CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234(1):63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–18. doi: 10.1097/00000658-199909000-00004. discussion 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helmberger T. [Interventional procedures for hepatic metastases]. Chirurg. 2010;81(6):542–50. doi: 10.1007/s00104-010-1888-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhou YM, Li B, Xu DH, Yang JM. Safety and efficacy of partial hepatectomy for huge (≥10 cm) hepatocellular carcinoma: a systematic review. Med Sci Monit. 2011;17(3):RA76–83. doi: 10.12659/MSM.881443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozłowska D, Szewczyk M, Banaszkiewicz Z, et al. Knowledge of symptoms and diagnostic possibilities of cancer diseases. Arch Med Sci. 2011;7(2):304–9. doi: 10.5114/aoms.2011.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker D, Hansler JM, Strobel D, Hahn EG. Percutaneous ethanol injection and radio-frequency ablation for the treatment of nonresectable colorectal liver metastases – techniques and results. Langenbecks Arch Surg. 1999;384(4):339–43. doi: 10.1007/pl00008077. [DOI] [PubMed] [Google Scholar]
- 30.Boxberger F, Albrecht H, Konturek PC, et al. Neoadjuvant treatment with weekly high-dose 5-fluorouracil as a 24h-infusion, folinic acid and biweekly oxaliplatin in patients with primary resectable liver metastases of colorectal cancer: long-term results of a phase II trial. Med Sci Monit. 2010;16(2):CR49–55. [PubMed] [Google Scholar]
- 31.Wiggermann P, Sieron D, Brosche C, et al. Transarterial Chemoembolization of Child-A hepatocellular carcinoma: drug-eluting bead TACE (DEB TACE) vs. TACE with cisplatin/lipiodol (cTACE) Med Sci Monit. 2011;17(4):CR189–95. doi: 10.12659/MSM.881714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52(2):762–73. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 33.Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010;33(1):11–17. doi: 10.1007/s00270-009-9736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19(5):1206–13. doi: 10.1007/s00330-008-1258-5. [DOI] [PubMed] [Google Scholar]
- 35.Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47(1):82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 36.Paulet E, Aube C, Pessaux P, et al. Factors limiting complete tumor ablation by radiofrequency ablation. Cardiovasc Intervent Radiol. 2008;31(1):107–15. doi: 10.1007/s00270-007-9208-1. [DOI] [PubMed] [Google Scholar]
- 37.Mazzaglia PJ, Berber E, Milas M, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery. 2007;142(1):10–19. doi: 10.1016/j.surg.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 38.Siperstein AE, Berber E, Ballem N, Parikh RT. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg. 2007;246(4):559–65. doi: 10.1097/SLA.0b013e318155a7b6. discussion 65–67. [DOI] [PubMed] [Google Scholar]
- 39.Veltri A, Sacchetto P, Tosetti I, et al. Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol. 2008;31(5):948–56. doi: 10.1007/s00270-008-9362-0. [DOI] [PubMed] [Google Scholar]
- 40.Ruers T, Punt C, van Coevorden F, et al. Final results of the EORTC intergroup randomized study 40004 (CLOCC) evaluating the benefit of radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRCLM) J Clin Oncol. 2010;28(15 suppl (May 20 Supplement)):3526. [Google Scholar]
- 41.Ayav A, Germain A, Marchal F, et al. Radiofrequency ablation of unresectable liver tumors: factors associated with incomplete ablation or local recurrence. Am J Surg. 2010;200(4):435–39. doi: 10.1016/j.amjsurg.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Lam VW, Ng KK, Chok KS, et al. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg. 2008;207(1):20–29. doi: 10.1016/j.jamcollsurg.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 43.de Baere T, Elias D, Dromain C, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. AJR Am J Roentgenol. 2000;175(6):1619–25. doi: 10.2214/ajr.175.6.1751619. [DOI] [PubMed] [Google Scholar]
- 44.Chow DH, Sinn LH, Ng KK, et al. Radiofrequency ablation for hepatocellular carcinoma and metastatic liver tumors: a comparative study. J Surg Oncol. 2006;94(7):565–71. doi: 10.1002/jso.20674. [DOI] [PubMed] [Google Scholar]
- 45.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15(3):938–46. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 46.Scheele J, Altendorf-Hofmann A. Resection of colorectal liver metastases. Langenbecks Arch Surg. 1999;384(4):313–27. doi: 10.1007/s004230050209. [DOI] [PubMed] [Google Scholar]