Summary
Background
Stabilization and bone healing of fractures in weight-bearing long bones are challenging. This study was conducted to evaluate the effect of a scaffold composed of chitosan fiber and calcium phosphate ceramics (CF/CPC scaffold) on stability and fracture repair in weight-bearing long bones.
Material/Methods
Comminuted fractures of paired radiuses were created in 36 healthy, mature dogs. The left radius of each dog was classified in the experimental group and treated with CF/CPC scaffold, and the right one was not filled, and was used as a blank control. Of the 12 animals in each group that were killed at week 4, 8, and 12 after the operation, 6 were used for histological analysis, and the other 6 used were for biomechanical studies. Both radiuses from each animal were dissected free and stored for these analyses. All the animals underwent X-ray radiograph pre- and post-operatively. Computer-aided rapid-prototyping technologies were adopted for the fabrication of three-dimensional scaffolds with precise geometric control.
Results
X-ray showed that the bone fracture area in the experimental group was filled with callus at week 12 after surgery. Histological examination detected slow resorption of the cement and new bone formation since week 4. At week 12, the scaffold material partially degraded and was still present in all specimens. Mechanical testing revealed that the failure strength of the radiuses treated with CF/CPC scaffolds was about 3 times that of the radiuses without implanted scaffolds.
Conclusions
The effect of using CF/CPC scaffold in treating comminuted weight-bearing long bone fractures is satisfactory.
Keywords: calcium phosphate cement, chitosan fiber, comminuted long-bone fracture, rapid prototyping, bone morphogenetic protein
Background
Fractures in weight-bearing long bones usually occur after substantial trauma such as a vehicular accident. The therapeutic methods used to treat these fractures vary, but reduction and stabilization have been without exception the major principle. However, comminuted long-bone fractures, caused by high energy injury, are very unstable, and the reduction and stabilization, as well as bone healing, are challenging due to the high risk of malunion and nonunion. This kind of fracture is generally not amenable to conservative repair. Traditionally, the fragments of the fractures are reduced and individually stabilized using pins, wires and screws [1–3]. But the effects of these methods are far from satisfactory, and thus synthetic materials have been developed. One of the most commonly used synthetic alternatives is calcium phosphate ceramics (CPC). However, this material has some disadvantages, for example, poor mechanical resistance and secondary migration of the ceramic particles. Interestingly, chitosan fiber (CF), which has excellent properties such as biocompatibility, biodegradability and nontoxicity [4–6], can significantly improve the mechanical properties of CPC [7,8]. A synthetic material composed of both calcium phosphate ceramics and chitosan fiber (CF/CPC) might overcome these problems as fillers.
With the development of material processing techniques, bone substitutes that best match human anatomy can be fabricated with mixed biomaterials using advanced engineering techniques, such as rapid prototyping (RP). RP technique can fabricate any part with complex interior or exterior shapes through a layer-by-layer building process. RP and computer aided design (CAD) are commonly combined to realize customized designs based on patients’ CT images [9,10].
Previously, we developed a novel CF/CPC scaffold and presented its structure and manufacture [11,12]. The study of its mechanical properties and osteoconductivity [13] has revealed that it has many advantages, such as strong proliferation ability and easy preparation. Our previous findings demonstrated that the CF/CPC scaffold is suitable for bone tissue engineering. In the present study, the performance of the CF/CPC scaffold in comminuted long-bone fractures was evaluated.
Material and Methods
Animals
Thirty-six healthy, mature, male mongrel dogs, weighing 20 to 25 kilograms, were used. All animals were in good physical condition and their growth plates were closed. Principles of laboratory animal care were followed. The animals were caged, and water and food were supplied ad libitum. All procedures were conducted according to guidelines established by the National Institutes of Health. The study protocol was approved by the Ethics Committee of the Second Affiliated Hospital, Xi’an Jiaotong University School of Medicine.
Materials
Calcium phosphate cement (CPC) was purchased from Shanghai Rebone Biomaterials Co. Ltd. (China). Chitosan fiber (CF) was provided by Weifang Youngdeok Chitosan Co., Ltd (China). Bone morphogenetic protein-2 (BMP-2) was purchased from SinoBio Biotech (China). Epoxy resin was developed by the Institute of Advanced Manufacturing Technology (IAMT) in Xi’an Jiaotong University.
Model building
The operation was performed under general anesthesia. Animals were anesthetized by intraperitoneal injection of sodium pentobarbital 33 mg/kg body weight. The left foreleg was depilated and disinfected routinely for surgery. A 3-cm longitudinal lateral incision was made in the middle segment of the foreleg to expose the radial shaft. The radius, together with periosteum, was completely sawed off at 4 cm distal to the head of radius with a wire saw under constant irrigation, and then the radius was sawed off at 3 cm distal to the first osteotomy line. Sawed bone was minced by rongeur in situ to create a comminuted, mid-diaphyseal radial fracture 3 cm in length. Comminuted bone fragments were not removed from the fracture site. Muscle and skin were closed in a layered fashion.
Scaffold manufacture
All dogs underwent helical computerized tomography scanning (CT). Images of bilateral radius were obtained, with a slice thickness of 1.3 mm, a reconstruction interval of 0.6 mm, and a resolution of 512±512. The original CT images were processed to get new binary images, which were imported into Mimics Software (Materialise NV, Belgium). The medullary canal of radius was reconstructed and visualized in a three-dimensional (3D) display using the regional growing technique. Meanwhile, considering the reliable fixation between the medullary canal scaffold and the host bone, a cylindrical connector was also designed on both ends to fix the scaffold into the proximal and distal radius. The resin prototype was fabricated quickly and automatically using Stereolithography (SLA) with data-processing software (RP-Data, IAMT).
CPC was composed of powder and fluid. BMP-2 was dissolved in a setting fluid (10 μg: 1.0 ml). CF was broken into bits and mixed with CPC powder (1:20), and then the mixture was added into the solution. The solution was then stirred into paste, poured into custom-made molds and compressed for shaping. After the paste entirely dried, the resin molds were removed and the CF/CPC scaffolds were obtained (Figure 1).
Figure 1.
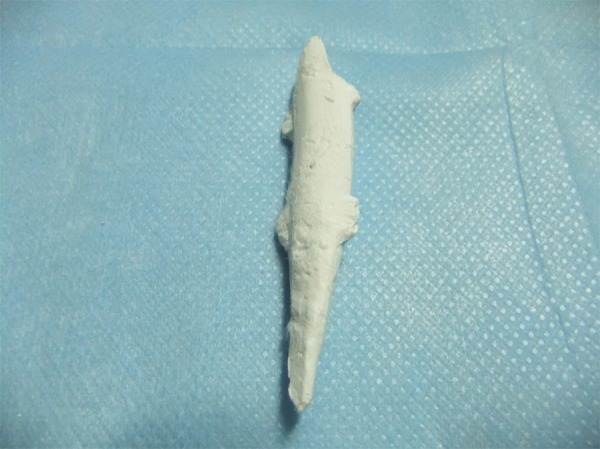
Chitosan fiber/calcium phosphate ceramics composite scaffold.
Surgical technique of fixation
Paired radiuses were harvested from the forelegs of the dogs. The left radius of each dog was classified in the experimental group and treated with scaffold, and the right one was not filled, and was used as a blank control. All radiuses were fixed with pure titanium panels.
The scaffold was placed through the fracture site and into the medullary canal of the left radius of each dog, and the comminuted bone fragments were inflicted at the scaffold. The right radius, as blank control, was filled with nothing and the bone fragments were left alone. Titanium panels with brackets and screws were used to locate the radiuses, then the incisions were closed. After surgery, each studied limb was manually tested and none of the specimens showed any sign of instability. All animals were permitted full weight bearing upon recovery and were given conventional feeding.
Observing parameters
General data: diet, activity, and wound response of animals were observed after operation.
X-ray radiograph
Radiographs of the experimental limbs were obtained before the operation, and also at weeks 4, 8 and 12 after reduction and fixation to evaluate the condition of bridging callus formation and the degradation of scaffold.
Histological examination
Six specimens were randomly selected at weeks 4, 8, and 12 postoperatively. Experimental and control radiuses were dissected free of soft tissue, and were immediately fixed with 4% paraformaldehyde for 72 h at room temperature. A 0.5 cm fragment was cut off from the bone defect area of each radius to prepare histological sections. These sections were made in a transverse direction perpendicular to the axis of the implant.
Mechanical testing
Six dogs were randomly selected at the 4th, 8th, and 12th weeks postoperatively and sacrificed. Paired radiuses were harvested and excess tissue was removed. The bone was placed upright at a crosshead speed of 1 mm/min on a computer-controlled universal testing machine (Instron 1195, Instron, Canton, MA) after removal of periosteum and soft tissue to run the static test. Failure of the bone or bone-implant system was taken as a stopping criterion. Six samples were tested in each group in order to get statistically reliable values.
Statistical analysis
All data were collected and statistically processed with SPSS13.0. One-way analysis of variance was applied when inter- and intra-group comparisons at different time points were conducted. A significance level of p = 0.05 was set for all statistical tests.
Results
General data
All the animals recovered to normal diet and activity after surgery. No significant swelling and effusion were seen in the affected limbs. No death was observed during follow-up.
X-ray radiograph
The postoperative radiographs demonstrated that the scaffolds were placed in the medullary canals of the left radiuses and were in close contact to the bone. Material degradation was observed beginning at week 4 after surgery, and the bone contact at the juncture was getting closer at week 8. When the experiment ended at week 12, the bone fracture area was filled with callus and the medullary cavity was partially recanalized (Figure 2).
Figure 2.
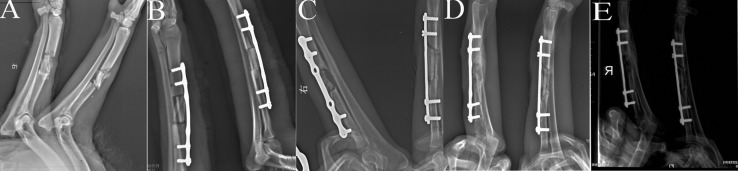
Radiographic view of the both radiuses in adult dogs. The right radiuses were the experimental group treated with scaffold, the left were black control. (A) Before implantation with CF/CPC. (B) 0 week after implantation with CF/CPC, (C) 4 weeks after implantation with CF/CPC. (D) 8 weeks after implantation with CF/CPC. (E) 12 weeks after implantation with CF/CPC.
In the right radiuses, certain periosteal reaction was observed at week 4 following surgery. At week 12, no bone callus was seen in the bone fracture area, and capitulum was ossified with medullary cavity blocked. These indicated that the bone fracture was not repaired (Figure 2).
Histological examination
Histological examination demonstrated slow resorption of the cement and new bone formation originating from the edge of the fracture in the experimental group 4 weeks after the operation. At week 4, the material partially degraded and a tiny gap formed between the material and the bone tissues. There was contact between the material and the surrounding bone tissues. The material kept degrading and being absorbed, and the bone tissues partially integrated into the material at week 8. The gap in between became less obvious. At week 12, the material was still present in the medullary area in all the sections. With the increase of time in vivo, the cement was penetrated by small blood vessels and was surrounded by circumferential lamellae of bone (Figure 3).
Figure 3.
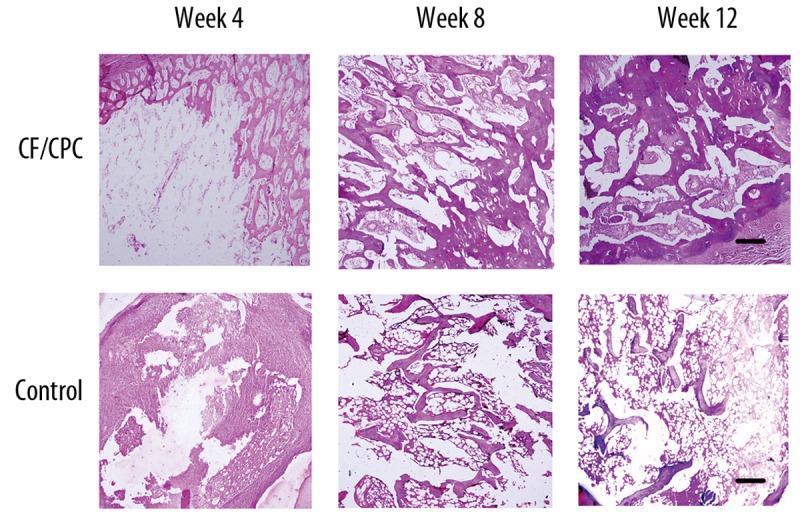
Histological sections from various time points. The bar indicates 100 μm.
In the control group, no bone connection was identified in the bone fracture area at week 12, when the experiment ended. The result was similar to that identified by X-ray radiographs. There were no signs of an inflammatory response (no inflammatory cells were seen) (Figure 3).
Statistical analysis
The inter-group comparative study identified statistically significant biomechanical difference at weeks 4, 8 and 12 (P<0.05). The compressive strength of the experimental group at week 12 was 1766±221 N, much higher than 595±96 N in the control group,. The intra-group analysis revealed that the compressive strength in week 4 was significantly lower than that in week 8, and the strength in week 12 was the largest (P<0.05) (Table 1).
Table 1.
Biomechanical test results of the experimental and control groups (mean ±SD, n=6).
| Time | Experimental group (N) | Control group(N) |
|---|---|---|
| Week 4 | 524.9971±137.6440 | 169.1710±49.7467 |
| Week 8 | 1240.4250±206.9098 | 394.9199±60.0185 |
| Week 12 | 1766.3640±221.3034 | 595.0188±95.9176 |
Discussion
In this study, CF was added into CPC to form a synthesized material, CF/CPC, so as to overcome the disadvantage of CPC as fillers. The scaffolds used in the experiments were manufactured with CF/CPC by RP, and the performance of the CF/CPC scaffold in treating comminuted long-bone fractures was tested. Our results demonstrate that CF/CPC scaffold perfectly matches the surrounding tissues and is helpful in promoting the actual bone remodeling process. This is, to our knowledge, the first time that CF/CPC scaffold was found to have good potential for treating comminuted long-bone fractures, and suggests a promising future for the application of CF/CPC scaffolds.
Comminuted long-bone fractures are profound clinical problems and have remained challenging to orthopedic surgeons. They frequently result in disabilities, and, in the worst cases, amputation [14]. Since amputation significantly compromises quality of life, a severely comminuted long bone is usually salvaged from amputation by internal or external fixation. Anatomic reduction involves reconstruction of the bony column to restore stability. However, this type of fracture is difficult to adequately anatomically reduce and stabilize. As the long bone is curved and the medullary cavity is not of uniform diameter throughout its length, the frequently used intramedullary pins cannot completely fill the entire length of the cavity, which may reduce the fixation rigidity and lead to fracture displacement. These implants do not possess the property of individual matching and cannot completely fuse with the host bone at the interface. Therefore, custom scaffold is a key part of the restoration of biological and mechanical function.
Currently, high quality, reasonably strong, reproducible scaffolds can be produced due to rapidly developing computer-based techniques such as RP. An advantage of RP is that it can fabricate parts with complex interior or exterior shapes through a layer-by-layer building process. This is particularly useful in tissue engineering, since it allows very good reproducibility and the production of almost any kind of structure within the limitations of each technique used [15]. A CAD model can be obtained from CT, allowing the external shape of the scaffold to match that of the damaged tissue site. With CAD-based RP, the space between fracture fragments can be eliminated or minimized, and primary or direct healing is promoted through osteon remodeling. In our previous research, we have already produced scaffolds with CF/CPC material by RP, and the mechanical properties and osteoconductivity of the scaffolds have also been studied [11–13]. Since the cortical tube is hollow, a bone void filling agent is required to achieve accurate reduction. The scaffolds used in this experiment were tailored to match the shape and size of the medullary cavity of bones in comminuted long-bone fractures.
Scaffold material is a key influential factor for the clinical application of tissue engineered bone. CPC, as a type of widely studied and commonly used biodegradable polymer, possesses great potential as a carrier in bone tissue engineering due to its biocompatibility, degradability and osteoconductivity. However, its degradation products are acidic. It has been definitively established that synthetic materials have excellent chemical and mechanical properties that natural polymers usually do not possess. To develop a synthesized material, CF, a deacetylated derivative of chitin and linear polysaccharide is added. CF can induce osteoconductivity, endochondral ossification and direct membranous osteoinduction to promote bone formation [16,17]. Additionally, it is inexpensive and readily available. The synthesized material CF/CPC combines the advantages of both CPC and CF, and the composite CF/CPC scaffold has appropriate chemical and mechanical properties.
Tissue engineered bone reconstruction involves 2 mutually acting processes, scaffold degradation and new bone formation [18]. Non-biodegradable scaffold requires a second surgery to remove it, so biodegradable scaffold is preferred in treating comminuted long-bone fractures, and the degradation of scaffolds can affect cell growth, tissue regeneration and host response [19]. Biodegradable scaffold, which functions as a structural support, is a delivery vehicle for osteoinductive molecules to increase bone formation [20]. It has been demonstrated that a variety of growth factors and cytokines are normally released in the posttraumatic phase [21,22]. Scaffolds with growth factors may induce endogenous osteoblasts and their progenitors in situ for subsequent bone regeneration. In this experiment, the scaffold was used not only as a temporary load-bearing device, but also as a carrier for BMP-2. BMPs are soluble, bioactive signaling proteins that include many factors implicated in osteoinduction [23]. They stimulate pluripotent mesenchymal stem cells to proliferate and differentiate into osteoblastic, chondroblastic or fibroblastic lineages, depending upon the local environment [24]. And they are also known to induce the formation of cartilage and bone.
Scaffolds serve primarily as osteoconductive moieties on which newly formed bone deposits through creeping substitution from adjacent living bone [25]. They provide structural support for fractures and should be able to withstand a certain level of loading. It is necessary to optimize their mechanical response so that they can provide appropriate support in the process of bone regeneration and are simultaneously slowly resorbed [26]. Rigid fixation during the initial healing phase is crucial for successful bone healing. Highly comminuted fractures in weight-bearing areas must be adequately stabilized with metallic implants. In our experiment, a plate was utilized for fracture fixation to guarantee the normal length of bone.
The X-ray observation in our study showed that the bone remodeling function was progressively increased as time was prolonged in the experimental groups, confirming the function of scaffold in remodeling. The histological observation identified a greater volume of bone ingrowth in the experimental group as compared with the control group. No significant inflammatory reactions were observed in the 12 weeks after implantation, which suggests good biocompatibility of the implanted material. Hematoxylin-eosin staining detected an increase of new bone volume between weeks 4 and 12 after surgery. These findings indicate that the scaffolds promoted a greater volume of bone ingrowth since they were implanted. The compressive strength of radius in situ was observed and recorded so as to identify the scaffold-facilitated bone healing. The compressive test at week 12 after surgery revealed the instability of the fractures without scaffolds. The compressive strength sufficient to destroy the radiuses in the control group was 595.0188±95.9176 N, whereas the radiuses in the scaffold group broke at about 1750 N, which was about 3 times that of the former. In a word, the fractured radiuses treated with CF/CPC scaffolds remodeled better, and the scaffolds made of the synthesized material CF/CPC have improved bone healing.
Conclusions
The scaffolds made with CF/CPC material can be used in combination with bone plating to successfully treat comminuted long-bone fractures in canine radiuses. The composite scaffolds excellently match with the surrounding tissues. The gross observation, imaging analysis, histological examination and mechanical testing showed that our scaffolds exhibit superior biocompatibility and can facilitate cell growth. However, the stability of the fracture and the desirable rate and concentration of the released BMP were not studied in the present research. The minimal scaffold resorption at week 12 postoperation indicates the need of either a modification of the scaffold design or a longer time of observation after implantation. Our results also need to be confirmed by a larger number of animal models. This study may provide valuable insight into the application of bone tissue engineering in the treatment of comminuted long-bone fractures, and pave the way for future work aimed at optimizing the mechanical performance of such scaffolds.
Footnotes
Conflict of interest
The authors declare no conflicts of interests.
Source of support: Sci-technical Development Foundation of Shaanxi Province (2010K13-02-10)
References
- 1.Raju P, Kini SG. Loss of correction in unstable comminuted distal radius fractures with external fixation and bone grafting--a long term followup study. J Orthop Surg Res. 2011;21(6):23. doi: 10.1186/1749-799X-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartl C, Stengel D, Bruckner T, et al. Open reduction and internal fixation versus casting for highly comminuted and intra-articular fractures of the distal radius (ORCHID): protocol for a randomized clinical multi-center trial. Trials. 2011;22(12):84. doi: 10.1186/1745-6215-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fjalestad T, Hole MØ, Jørgensen JJ, et al. Health and cost consequences of surgical versus conservative treatment for a comminuted proximal humeral fracture in elderly patients. Injury. 2010;41(6):599–605. doi: 10.1016/j.injury.2009.10.056. [DOI] [PubMed] [Google Scholar]
- 4.Kumar MNVR. A review of chitin and chitosan applications. React Funct Polym. 2000;46(1):1–27. [Google Scholar]
- 5.Ohashi E, Karube I. Development of a thin membrane glucose sensor using β-type crystalline chitin for implantable biosensor. J Biotechnol. 1995;40(1):13–19. doi: 10.1016/0168-1656(95)00028-o. [DOI] [PubMed] [Google Scholar]
- 6.Leroux L, Hatim Z, Frèche M, Lacout JL. Effects of various adjuvants (lactic acid, glycerol, and chitosan) on the injectability of a calcium phosphate cement. Bone. 1999;25(2 suppl):31S–34S. doi: 10.1016/s8756-3282(99)00130-1. [DOI] [PubMed] [Google Scholar]
- 7.Xu HH, Quinn JB. Calcium phosphate cement containing resorbable fibers for short-term reinforcement and macroporosity. Biomaterials. 2002;23(1):193–202. doi: 10.1016/s0142-9612(01)00095-3. [DOI] [PubMed] [Google Scholar]
- 8.Xu HH, Simon CG., Jr Fast setting calcium phosphate–chitosan scaffold: mechanical properties and biocompatibility. Biomaterials. 2005;26(12):1337–48. doi: 10.1016/j.biomaterials.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 9.Shuxian Z, Wanhua Z, Bingheng L. 3D reconstruction of the structure of a residual limb for customising the design of a prosthetic socket. Med Eng Phys. 2005;27(1):67–74. doi: 10.1016/j.medengphy.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Lin YP, Wang CT, Dai KR. Reverse engineering in CAD model reconstruction of customized artificial joint. Med Eng Phys. 2005;27(2):189–93. doi: 10.1016/j.medengphy.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Lian Q, Li D, Zhang Y. Novel architecture of bone scaffold and its bionic functions. Chinese journal of mechanical engineering. 2006;42(1):121–25. [Google Scholar]
- 12.Lian Q, Li D, Li A, et al. Mechanical performance of chitosan fiber/calcium phosphate cement composite for artificial bone. Chinese Journal of Mechanical Engineering. 2008;44(6):49–53. [Google Scholar]
- 13.Lian Q, Li D, Lu B. Fabrication of fiber-reinforced CPC composite artificial bone by RP/RT. Materials Science Forum. 2006;532–33:745–48. [Google Scholar]
- 14.Giannoudis PV, Pountos I. Tissue regeneration: the past, the present and the future. Injury. 2005;36(Suppl 4):S2–5. doi: 10.1016/j.injury.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Lacroix D, Planell JA, Prendergast PJ. Computer-aided design and finite-element modelling of biomaterial scaffolds for bone tissue engineering. Philos Transact A Math Phys Eng Sci. 2009;367(1895):1993–2009. doi: 10.1098/rsta.2009.0024. [DOI] [PubMed] [Google Scholar]
- 16.Muzzarelli RAA. Biochemical significance of exogenous chitin and chitosans in animals and patients. Carbohydrat Polym. 1993;20(1):7–16. [Google Scholar]
- 17.Muzzarelli RA, Mattioli-Belmonte M, Tietz C, et al. Stimulatory effect on bone formation exerted by a modified chitosan. Biomaterials. 1994;15(13):1075–81. doi: 10.1016/0142-9612(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 18.Adachi T, Osako Y, Tanaka M, et al. Framework for optimal design of porous scaffold microstructure by computational simulation of bone regeneration. Biomaterials. 2006;27(21):3964–72. doi: 10.1016/j.biomaterials.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 19.Babensee JE, Anderson JM, McIntire LV, Mikos AG. Host response to tissue engineered devices. Adv Drug Deliv Rev. 1998;33(1–2):111–39. doi: 10.1016/s0169-409x(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 20.Sitharaman B, Shi X, Walboomers XF, et al. In vivo biocompatibility of ultra-short single-walled carbon nanotube/biodegradable polymer nanocomposites for bone tissue engineering. Bone. 2008;43(2):362–70. doi: 10.1016/j.bone.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Bolander ME. Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med. 1992;200(2):165–70. doi: 10.3181/00379727-200-43410a. [DOI] [PubMed] [Google Scholar]
- 22.Andrew JG, Hoyland J, Andrew SM, et al. Demonstration of TGF-beta 1 mRNA by in situ hybridization in normal human fracture healing. Calcif Tissue Int. 1993;52(2):74–78. doi: 10.1007/BF00308311. [DOI] [PubMed] [Google Scholar]
- 23.Wozney JM. Overview of bone morphogenetic proteins. Spine (Phila Pa 1976) 2002;27(16 Suppl 1):S2–8. doi: 10.1097/00007632-200208151-00002. [DOI] [PubMed] [Google Scholar]
- 24.Kirker-Head CA, Boudrieau RJ, Kraus KH. Use of bone morphogenetic proteins for augmentation of bone regeneration. J Am Vet Med Assoc. 2007;231(7):1039–55. doi: 10.2460/javma.231.7.1039. [DOI] [PubMed] [Google Scholar]
- 25.Groeneveld EH, van den Bergh JP, Holzmann P, et al. Mineralization processes in demineralized bone matrix grafts in human maxillary sinus floor elevations. J Biomed Mater Res. 1999;48(4):393–402. doi: 10.1002/(sici)1097-4636(1999)48:4<393::aid-jbm1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 26.Adachi T, Osako Y, Tanaka M, et al. Framework for optimal design of porous scaffold microstructure by computational simulation of bone regeneration. Biomaterials. 2006;27(21):3964–72. doi: 10.1016/j.biomaterials.2006.02.039. [DOI] [PubMed] [Google Scholar]


