Summary
Background
We evaluated the relation of systemic blood pressure with intraday variations in ocular perfusion pressure and intraocular pressure in patients with primary open-angle glaucoma in a telemedical home monitoring scenario.
Material/Methods
In the project Teletonometry Mecklenburg-Vorpommern (TTMV) patients were equipped with a home monitoring system for 24-hour self-measurements of intraocular pressure and blood pressure for a period of six months. All measurements were transmitted via telephone modem to an electronic patient record. Ocular perfusion pressure (OPP) was automatically calculated from self-measurements of intraocular pressure (IOP), systolic (SBP) and diastolic blood pressure (DBP) using the equation: OPP=[2/3*(2/3*DBP+1/3*SBP)]–IOP. We present the temporal characteristics of 70 patients with primary open-angle glaucoma based on 3282 self-measurements.
Results
The diurnal ocular perfusion pressure trend showed four characteristic phases (7am – 12am, 12am – 6pm, 6pm – 10pm, and 10pm – 7am). Between 7am and 12am ocular perfusion pressure and simultaneously systolic and diastolic blood pressure were significantly depressed compared to all other phases (p<0.05) whereas intraocular pressure showed no significant shifting. Instead intraocular pressure was significantly depressed between 6pm and 10pm (p<0.05) where ocular perfusion pressure reached the highest intraday values.
Conclusions
We found that ocular perfusion pressure in patients with primary open-angle glaucoma showed remarkable circadian fluctuations. A significant decrease in the morning was associated with significantly depressed systolic and diastolic blood pressure levels. In addition we observed normal intraocular pressure values in the morning but a significant decrease in the evening which did not affect ocular perfusion pressure. These conclusions strengthen the evidence that systemic blood pressure fundamentally influences ocular circulation and consequently glaucoma progression.
Keywords: glaucoma, ocular perfusion, homemonitoring, 24 h ambulatory blood pressure monitoring, intraocular pressure, selftonometry
Background
In 2002 glaucoma jumped from third to second place on the list of the leading causes of blindness globally, which is published by the World Health Organization [1,2]. Glaucoma is becoming increasingly important in the context of an ageing world population. It poses a considerable challenge to public health, because blindness and visual impairment caused by glaucoma are irreversible. As a chronic eye disease glaucoma leads to a loss of retinal ganglion cells over the years, which results in characteristic optic nerve damage and typical visual field defects. In the early stages of glaucoma the patients affected may not realize that they are in danger of losing their sight, usually they only notice the few symptoms of the gradual loss of vision when retinal nerve fibres are already irreversibly lost. To avoid this feasible prevention, effective diagnostics and appropriate treatment are required, but this can only be achieved, if we improve our general understanding of glaucoma: “The medical understanding of the nature of glaucoma has changed profoundly in the past few years and a precise comprehensive definition and diagnostic criteria are yet to be finalised [3].”
Main risk factors for glaucoma development and progression are age and genetic predisposition, both parameters without interventional potential [4]. The only parameter subject to treatment is intraocular pressure, which is also correlated to glaucoma. However, this is insufficient because we observe both: individuals with elevated intraocular pressure without glaucomatous retinal changes (ocular hypertension) and individuals with normal intraocular pressure, who develop glaucoma (normal tension glaucoma). Furthermore, there are patients who develop glaucoma progression despite optimal treatment with successful intraocular depression [5–7]. The first evidence for the role of vascular components in the pathogenesis of primary open-angle glaucoma was reported in 1953 [8] and several studies have followed in the last few years [9–15]. In 1993 Kaiser et. al. demanded that blood pressure be considered in glaucoma treatment [16]. The impact of blood pressure and ocular perfusion pressure on glaucoma has received greater attention as evidence mounts [17].
Material and Methods
Patients
In the home monitoring project Teletonometry Mecklenburg-Vorpommern (TTMV) 153 probands were selected to evaluate the benefits of innovative telemedical glaucoma management. Eligible probands were individuals aged 18 years or older who had any type of glaucoma, ocular hypertension or suspected glaucoma. The main exclusion criteria were: blepharospasm, extreme visual impairment or blindness, diseases of the anterior segment, allergic inflammation, intraocular inflammation in the past six months or acute glaucoma. During the 12-month monitoring period 24 probands had to quit the study due to job-related or health-impaired factors (e.g. relocation, shift working or hospital admission due to accidents). The data presented in this study is exclusively based on the measured values of 70 patients with primary open-angle glaucoma. 33 patients were female, 37 were male. The average age of all 70 patients was 60.3±9.6 (mean ± SD). All participants were volunteers and gave their written consent to take part in this study. Local ethics committee approval was obtained to carry out this study.
Study design
This study was designed as an observational study in two groups, conducted at the University Eye Hospital in Greifswald, Germany. Probands were recruited at the hospital itself, at ophthalmologic practices and through the media. They were randomly assigned in a 1:1 ratio to one of two groups. The first group underwent 6 months of telemedical home monitoring followed by 6 months conventional glaucoma management, whilst the second group started with 6 months conventional glaucoma management followed by 6 months of telemedical home monitoring. All probands were examined at the beginning, after 6 months and at the end of the study using all glaucoma relevant diagnostics.
Study assessments
All patients were equipped with devices for telemedical home monitoring. For a period of 6 months they performed self-measurements of intraocular pressure and blood pressure at home and subsequently transmitted the data via a telemedical interface to a server in the hospital. The data was stored in electronic patient records, which were accessed by the ophthalmologists using a web frontend [18,19].
After extensive personal instruction in device handling the probands installed the devices at home and measured intraocular pressure with the Ocuton S (EPSa GmbH, Saalfeld, Germany), a self-tonometer using the Goldmann applanation principle, and blood pressure with the boso medicus PC (Bosch + Sohn GmbH u. Co. KG, Jungningen, Germany). We handed out a measuring schedule, that instructed the probands to check blood pressure and intraocular pressure in the morning and evening once a week and in addition to perform 24-hour profiles every 4 weeks. The majority of the probands decided to perform even more measurements than requested. The electronic patient record automatically calculated ocular perfusion pressure using the equation:
where:
OPP – ocular perfusion pressure,
DBP – diastolic blood pressure,
SBP – systolic blood pressure,
IOP – intraocular pressure.
Statistical analysis
The Kolmogorov-Smirnov and Shapiro-Wilk tests showed no normal distribution of the data, so we analyzed significance using the non-parametric Mann-Whitney and Waller-Duncan tests. All reported P values are two-sided. Statistical analysis was performed with the software SPSS.
Results
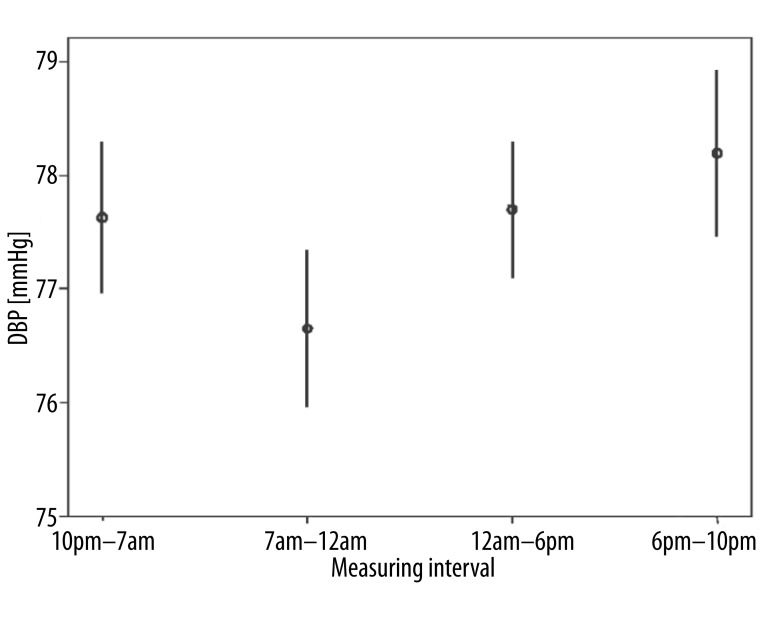
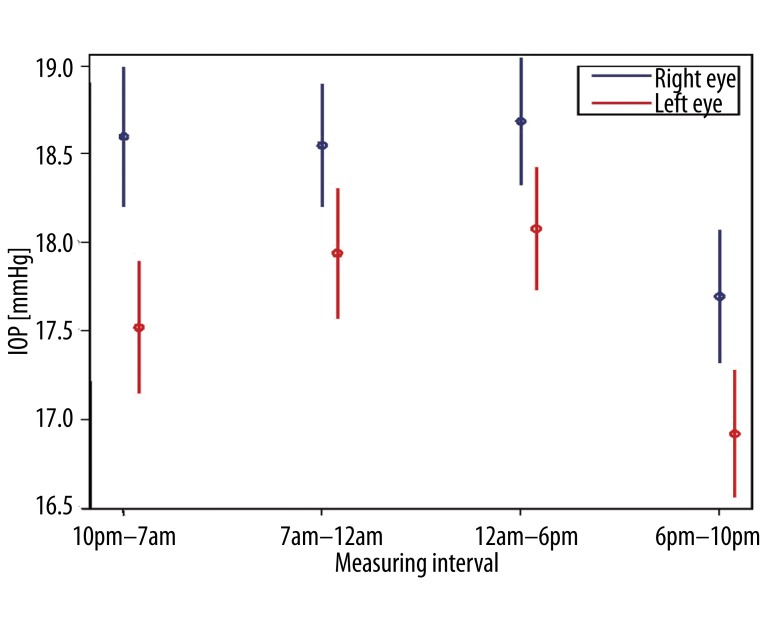
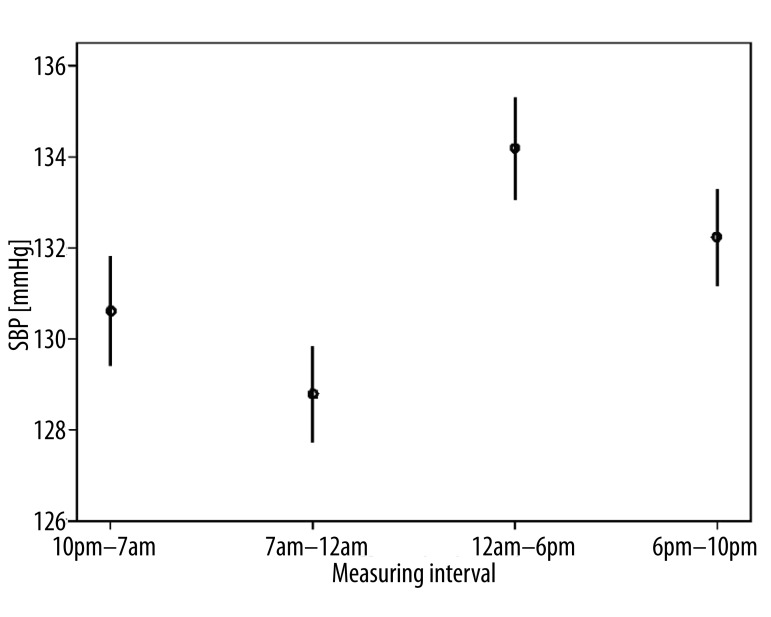
All 70 patients with primary open-angle glaucoma performed a total number of 3282 self-measurements at home and transmitted the data to the electronic patient record, which then calculated ocular perfusion pressure automatically from the submitted values. We analyzed intraday variations of ocular perfusion pressure and determined four characteristic phases: 7am – 12am, 12am – 6pm, 6pm – 10pm, 10pm – 7am [20]. Between 7am and 12am ocular perfusion pressure (Figure 1) and simultaneously systolic and diastolic blood pressure were significantly depressed (Figure 2) compared to all other phases (p<0.05) whereas intraocular pressure showed no significant shifting (Figure 3). Instead intraocular pressure was significantly depressed between 6pm and 10pm (p<0.05) where ocular perfusion pressure reached the highest intraday values (Figures 1, 3). Systolic blood pressure was significantly lowered from 7am – 12am (p<0.05) and significantly increased from 12am – 6pm (Figure 4).
Figure 1.
Characteristic phases in diurnal variation of ocular perfusion pressure (OPP) in 70 patients with primary open-angle glaucoma (n=3282 self-measurements). Between 7am and 12am ocular perfusion pressure was significantly depressed compared to all other phases (p<0.05). Indicated is the mean OPP with 95% confidence intervals.
Figure 2.
Characteristic phases in diurnal variation of diastolic blood pressure (DBP) in 70 patients with primary open-angle glaucoma (n=3282 self-measurements). Between 7am and 12am diastolic blood pressure was significantly depressed compared to all other phases (p<0.05). Indicated is the mean diastolic blood pressure with 95% confidence intervals.
Figure 3.
Characteristic phases in diurnal variation of intraocular pressure (IOP) in 70 patients with primary open-angle glaucoma (n=3282 self-measurements). Intraocular pressure showed no significant shifting between 7am and 12am while ocular perfusion pressure was significantly depressed (p<0.05) in the same time interval. Instead intraocular pressure decreased significantly between 6pm and 10pm (p<0.05) without affecting ocular perfusion pressure. Indicated is the mean IOP with 95% confidence intervals.
Figure 4.
Characteristic phases in diurnal variation of systolic blood pressure (SBP) in 70 patients with primary open-angle glaucoma (n=3282 self-measurements). Systolic blood pressure was significantly lowered from 7am – 12am (p<0.05) and significantly increased from 12am – 6pm (p<0.05). Indicated is the mean systolic blood pressure with 95% confidence intervals.
Discussion
The vascular role in glaucoma pathophysiology has been studied intensely, but the etiology of primary open-angle glaucoma still remains unclear and relations between risk-factors appear to be controversial. Newer studies conclude that ocular perfusion pressure is strongly associated with glaucoma especially in persons with hypertension and hypertensive therapy [21]. Thus we have to reconsider the general impact of this diagnostic parameter as well as its relation to intraocular pressure. A recently published review provides a detailed and extensive overview of several milestone glaucoma studies [5]. The authors not only confirmed the role of ocular perfusion pressure as an important risk factor for the development and progression of glaucoma but furthermore pointed out that this parameter perfectly combines the vascular and mechanical components of glaucoma.
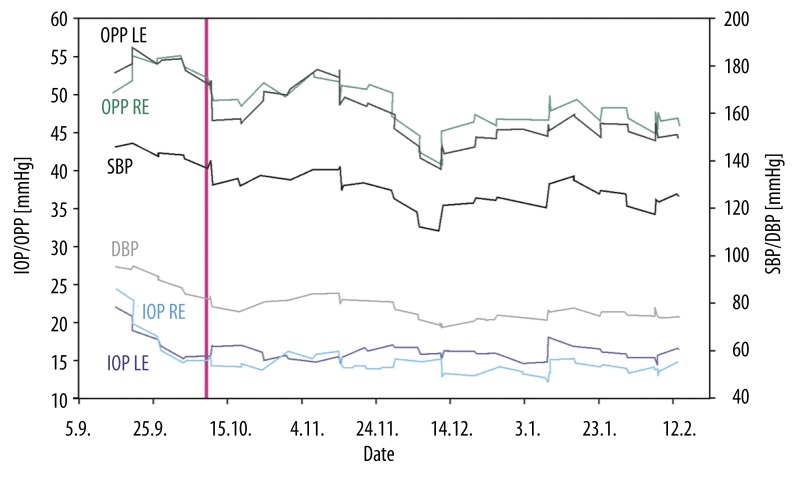
Since there is no feasible method to measure ocular perfusion pressure directly we investigated the approximated value calculated from home monitoring self-measurements of blood pressure and intraocular pressure. This framework demonstrated how easily ocular perfusion pressure can be obtained; nevertheless this important parameter has not yet been included continuously in large studies or clinical routine. Although self-tonometry is not yet common, we were able to observe several benefits for glaucoma patients: 24-hour profiles without hospital admission [22], completed diurnal documentation without office hours related gaps [22], optimized healthcare management for elderly patients in remote areas [23], positive health economics evaluation [24], and finally it opened new ways to analyze the relation of intraocular pressure, blood pressure and ocular perfusion pressure. The results of this study are not only important for ophthalmologists who still focus on intraocular pressure [4] and morphological or functional changes as treatment relevant parameters without regarding systemic blood pressure. We now have strong evidence that the glaucomatous effects of intraocular pressure depend on the characteristics of systemic blood pressure. In Figure 5 we present a clinical case report of a female patient, whose glaucoma medication was modified by her ophthalmologist while at the same time the general practitioner changed her hypertensive therapy, neither informed the other of the new treatment regime. They both regarded their intervention as successful because the patient’s intraocular pressure and blood pressure were lowered to appropriate levels. However both were unaware that as a result patient’s ocular perfusion pressure dropped under 45 mmHg, a level that increases the risk of glaucomatous damage progression [11,17]. This report illustrates that glaucoma is a systemic disease which requires interdisciplinary cross-sectional treatment. Consequently hypertensive therapy has to be adapted to ocular circulation in glaucoma patients to reduce the risk of morphologic damage. Our study has some limitations. Ocular perfusion pressure (OPP) was not measured directly instead it was calculated by taking mean arterial blood pressure and subtracting intraocular pressure (IOP). Since perfusion pressure is defined as the difference between arterial and venous pressure, and in the eye venous pressure is slightly higher than or even equal to IOP, for practical purposes the calculated OPP is a good approximation to quantify vascular changes [25,26]. Furthermore it was reported that the self-tonometer Ocuton S tended to over- or underestimate IOP compared to the gold standard Goldmann applanation tonometry (GAT) even though it uses the GAT principle to measure IOP [27,28]. However measurement accuracy of the self-tonometer was already improved after the implementation of a new firmware [29].
Figure 5.
Clinical case report of a female 56 year old patient with primary open-angle glaucoma and systemic hypertension. Diagrammatic overview of the dynamic of intraocular pressure (IOP), ocular perfusion pressure (OPP), systolic (SBP) and diastolic (DBP) blood pressure based on 77 self-measurements during a period of six months home monitoring (all lines show moving average smoothed series). The red line indicates the date when both the ophthalmologist and the general practitioner modified glaucoma medication (topical clonidine was replaced by latanoprost) and respectively hypertensive therapy (angiotensin II receptor antagonist was added to initial combination of ACE-inhibitor, diuretic and calcium ion antagonist). This reduced intraocular pressure and blood pressure successfully, whereas the resulting decrease of ocular perfusion pressure remained unnoticed.
Conclusions
Our findings strengthen the evidence that blood pressure may contribute to optic disc damage in glaucoma patients. Still future research is needed to demonstrate how glaucoma patients may benefit from telemedical home monitoring in terms of slowing or even stopping morphological changes and glaucoma progression. Since glaucoma progresses slowly over several years this would require a long-lasting study setup. This would probably answer the question how strong perfusion pressure is as prognostic factor. Further efforts will have to be spent on improving the usability and reliability of the self-tonometer to advance measuring accuracy.
Acknowledgements
We would like to thank Maria Moynihan from the Division of Research Development at the University of Greifswald for proofreading our manuscript as native English speaker. The project Teletonometry Mecklenburg-Vorpommern was supported by the German Federal Ministry of Education and Research under grant 03/2707A. Publication fee was gratefully sponsored by Pharm-Allergan GmbH, Germany.
Footnotes
Conflicts of interest
None.
Source of support: This study was granted by the German Federal Ministry of Research
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- 2.Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004;82:887–88. [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Priority Eye Diseases: Glaucoma [WHO website] Jan 3, 2011. [accessed November 17, 2011]. http://www.who.int/blindness/causes/priority/en/index7.html.
- 4.Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360:1113–24. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study monograph. An ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults. 1973–1975. Surv Ophthalmol. 1980;24(Suppl):335–610. [PubMed] [Google Scholar]
- 6.Quaranta L, Gandolfo F, Turano R, et al. Effects of topical hypotensive drugs on circadian IOP, blood pressure, and calculated diastolic ocular perfusion pressure in patients with glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2917–23. doi: 10.1167/iovs.05-1253. [DOI] [PubMed] [Google Scholar]
- 7.Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090–95. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- 8.Duke-Elder S. Primary glaucoma as a vascular disease; the James A. Craig prize lecture. Ulster Med J. 1953;22:3–16. [PMC free article] [PubMed] [Google Scholar]
- 9.Hulsman CA, Vingerling JR, Hofman A, et al. Blood pressure, arterial stiffness, and open-angle glaucoma: the Rotterdam study. Arch Ophthalmol. 2007;125:805–12. doi: 10.1001/archopht.125.6.805. [DOI] [PubMed] [Google Scholar]
- 10.Klein BE, Klein R, Knudtson MD. Intraocular pressure and systemic blood pressure: longitudinal perspective: the Beaver Dam Eye Study. Br J Ophthalmol. 2005;89:284–87. doi: 10.1136/bjo.2004.048710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leske MC, Wu SY, Hennis A, et al. BESs Study Group. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008;115:85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Orzalesi N, Rossetti L, Omboni S OPTIME Study Group (Osservatorio sulla Patologia glaucomatosa, Indagine Medico Epidemiologica); CONPROSO (Collegio Nazionale dei Professori Ordinari di Scienze Oftalmologiche) Vascular risk factors in glaucoma: the results of a national survey. Graefes Arch Clin Exp Ophthalmol. 2007;245:795–802. doi: 10.1007/s00417-006-0457-5. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, Iwase A, Araie M, et al. Tajimi Study Group. Risk factors for open-angle glaucoma in a Japanese population: the Tajimi Study. Ophthalmology. 2006;113:1613–17. doi: 10.1016/j.ophtha.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 14.Topouzis F, Coleman AL, Harris A, et al. Association of blood pressure status with the optic disk structure in non-glaucoma subjects: the Thessaloniki Eye Study. Am J Ophthalmol. 2006;142:60–67. doi: 10.1016/j.ajo.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Wang H, Wang Y, Jonas JB. Intraocular pressure correlated with arterial blood pressure: the Beijing eye study. Am J Ophthalmol. 2007;144:461–62. doi: 10.1016/j.ajo.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser HJ, Flammer J, Graf T, Stümpfig D. Systemic blood pressure in glaucoma patients. Graefes Arch Clin Exp Ophthalmol. 1993;231:677–80. doi: 10.1007/BF00919280. [DOI] [PubMed] [Google Scholar]
- 17.Costa VP, Arcieri ES, Harris A. Blood Pressure and Glaucoma. Br J Ophthalmol. 2009;93:1276–82. doi: 10.1136/bjo.2008.149047. [DOI] [PubMed] [Google Scholar]
- 18.Jürgens C, Grossjohann R, Meiering J, et al. Home-monitoring in ophthalmology-prerequisites for an improvement in quality of life: conclusions from the management research study Teletonometry Mecklenburg-Vorpommern. Klin Monatsbl Augenheilkd. 2009;226:459–65. doi: 10.1055/s-0028-1109407. [DOI] [PubMed] [Google Scholar]
- 19.Jürgens C, Antal S, Heydenreich F, et al. Digital patient record for remote monitoring of intraocular pressure, blood pressure and serum glucose. Klin Monatsbl Augenheilkd. 2006;223:757–64. doi: 10.1055/s-2006-926810. [DOI] [PubMed] [Google Scholar]
- 20.Antal S, Jürgens C, Grossjohann R, Tost FH. Diurnal variation of ocular pressure in open-angle glaucoma with telemonitoring. Klin Monatsbl Augenheilkd. 2009;226:168–75. doi: 10.1055/s-2008-1027918. [DOI] [PubMed] [Google Scholar]
- 21.Leske MC, Heijl A, Hyman L, et al. EMGT Group. Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114:1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Jürgens C, Antal S, Henrici K, et al. Fluctuation of intraocular pressure in 24-hour telemonitoring compared to tonometry during normal office hours. Klin Monatsbl Augenheilkd. 2009;226:54–59. doi: 10.1055/s-2008-1027730. [DOI] [PubMed] [Google Scholar]
- 23.Jürgens C, Tost F. Progress in geriatric care through telemedicine. Ophthalmologe. 2006;103:749–54. doi: 10.1007/s00347-006-1412-x. [DOI] [PubMed] [Google Scholar]
- 24.Swierk T, Jürgens C, Grossjohann R, et al. Health economical aspects of telemedical glaucoma monitoring. Ophthalmologe. 2011;108:342–50. doi: 10.1007/s00347-010-2253-1. [DOI] [PubMed] [Google Scholar]
- 25.Hayreh SS. The blood supply of the optic nerve head and the evaluation of it: myth and reality. Prog Retin Eye Res. 2001;20:563–93. doi: 10.1016/s1350-9462(01)00004-0. [DOI] [PubMed] [Google Scholar]
- 26.Hayreh SS. Blood flow in the optic nerve head and factors that may influence it. Prog Retin Eye Res. 2001;20:595–624. doi: 10.1016/s1350-9462(01)00005-2. [DOI] [PubMed] [Google Scholar]
- 27.Kóthy P, Nagymihály A, Holló G. The evaluation of tonometry and self-tonometry with Ocuton tonometers. Med Sci Monit. 2003;9(1):PI1–4. [PubMed] [Google Scholar]
- 28.Marchini G, Babighian S, Specchia L, Perfetti S. Evaluation of the new Ocuton S tonometer. Acta Ophthalmol Scand. 2002;80:167–71. doi: 10.1034/j.1600-0420.2002.800209.x. [DOI] [PubMed] [Google Scholar]
- 29.Lanfermann E, Jürgens C, Grossjohann R, et al. Intraocular pressure measurements with the newly reconfigured Ocuton S*TT-MV self-tonometer in comparison to Goldmann applanation tonometry in glaucoma patients. Med Sci Monit. 2009;15(11):CR556–62. [PubMed] [Google Scholar]