Abstract
In cystic fibrosis, a recessive genetic disease caused by defects in the cystic fibrosis conductance regulator (CFTR), the main cause of death is lung infection and inflammation. Nutritional deficits have been proposed to contribute to the excessive host inflammatory response in both humans and Cftr-knockout mice. Cftr-knockout mice and gut-corrected Cftr-knockout mice expressing human CFTR primarily in the gut were challenged with Pseudomonas aeruginosa-laden agarose beads; they responded similarly with respect to bronchoalveolar lavage cell counts and levels of the acute-phase cytokines tumor necrosis factor alpha, interleukin-1β (IL-1β), and IL-6. Wild-type mice fed the liquid diet used to prevent intestinal obstruction in Cftr-knockout mice had inflammatory responses to P. aeruginosa-laden agarose beads similar to those of wild-type mice fed an enriched solid diet, so dietary effects are unlikely to account for differences between wild-type mice and mice with cystic fibrosis. Finally, since cystic fibrosis patients and Cftr-knockout mice have an imbalance in fatty acids (significantly lower-than-normal levels of docosahexaenoic acid), the effects of specific supplementation with docosahexaenoic acid of wild-type and Cftr-knockout mice on their inflammatory responses to P. aeruginosa-laden agarose beads were tested. There were no significant differences (P = 0.35) in cumulative survival rates between Cftr-knockout mice and wild-type mice provided with either the liquid diet Peptamen or Peptamen containing docosahexaenoic acid. In conclusion, diet and docosahexaenoic acid imbalances alone are unlikely to explain the differences in the host response to lung infections with mucoid P. aeruginosa between mice with cystic fibrosis and their wild-type counterparts.
Cystic fibrosis is caused by a defect in the cystic fibrosis transmembrane conductance regulator gene (CFTR) in humans. Although cystic fibrosis affects many organ systems, such as the intestinal tract, liver, pancreas, and lung, the vast majority of the morbidity and mortality derives from chronic bronchopulmonary infections with mucoid Pseudomonas aeruginosa. Genetically altered mice bearing a knockout mutation in the murine homologue to CFTR (Cftr) experience greater mortality and an increased inflammatory response compared to their normal littermates in response to challenge with mucoid P. aeruginosa incorporated into agarose beads (13, 14). The exact reason(s) why Cftr-knockout mice respond differently than wild-type mice has not been fully elucidated.
One possible contributing factor to this difference is the nutritional deficit in cystic fibrosis patients and mice. Patients with pancreatic sufficiency have significantly better pulmonary function for age (5, 24, 28), though whether this is due to their better nutritional state or to other genetic factors is not clear. However, Konstan and coworkers (16) have shown that a nutritional deficit at age 3 years predicts poorer pulmonary function at age 6 years. Yu and colleagues (30) showed that calorie-restricted mice were less able to withstand challenge with P. aeruginosa than those that were nutritionally replete. In addition, they suggested that defective Cftr in the intestinal tract leads to nutritional deficiency, which in turn contributes to compromised host responses to aerosol exposures of nonmucoid P. aeruginosa laboratory strain PAO1 with regard to innate lung defenses, bacterial colonization, and excessive inflammation in the cystic fibrosis respiratory tract (30). However, studies directly investigating the role of nutrition in the response of Cftr-knockout mice to bronchopulmonary infection with mucoid P. aeruginosa have not been reported.
Others have implicated more-specific nutritional deficits in the excess inflammatory response. Docosahexaenoic acid (DHA) is an essential fatty acid known to down regulate arachidonic acid incorporation into membrane phospholipids (11, 22, 23). Arachidonic acid is a precursor to potent inflammatory agents (18). DHA and arachidonic acid compete for the same elongation and desaturation enzymes and for the site of esterification of phospholipids. Cftr-knockout mice exhibit a marked imbalance in phospholipid-bound arachidonic acid and DHA in several organs, including the lung. Correction of the fatty acid imbalance in Cftr-knockout mice has been achieved by supplementing the diet with DHA. Cftr-knockout mice treated in this way normalized their inflammatory response to pulmonary instillation of P. aeruginosa lipopolysaccharide (LPS) (12), though the response to whole bacteria following DHA supplementation was not tested.
The purpose of this study was to determine if differences in nutrition could alter the response of mice to challenge with mucoid P. aeruginosa. In one study, the inflammatory responses of Cftr-knockout and gut-corrected Cftr-knockout mice (Cftr-knockout mice also bearing the human CFTR transgene driven by the fatty acid-binding promoter, which leads to CFTR expression in the gut) to challenge with mucoid P. aeruginosa-laden agarose beads were compared. In a second study, the diet provided to Cftr-knockout mice (Peptamen) and a fortified rodent chow (Harlan-Teklad solid rodent chow) were fed to wild-type mice, which were then infected with P. aeruginosa-laden agarose beads; the host inflammatory response was then assessed. Measures of inflammation included changes in body weight, cell content of bronchoalveolar lavage fluid (BALF), and levels in epithelial lining fluid (ELF) of inflammatory mediators such as the acute-phase mediators tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β), the immunomodulatory cytokine IL-6, and the murine neutrophil chemokines macrophage inflammatory protein 2 (mip-2) and keratinocyte chemoattractant (KC). In a third study, dietary supplementation with DHA of Cftr-knockout mice and their wild-type littermates was tested to determine if correction of the fatty acid defect by DHA supplementation would improve survival of the Cftr-knockout mice compared to that of the sham-treated controls when the mice were challenged with P. aeruginosa-laden agarose beads.
MATERIALS AND METHODS
Mice.
Breeding pairs of the congenic B6.129P2-Cftrtm1Unc mice (Cftr knockout; stock no. 2196) and STOCK Cftrtm1Unc-TgN(FABPCFTR)#Jaw mice (gut-corrected Cftr knockout; stock no. 2364) were originally obtained from The Jackson Laboratory and maintained by breeding heterozygotes or homozygotes, respectively, by inbreeding (brother-sister mating). C57BL/6J mice were purchased from The Jackson Laboratory and acclimated for at least 3 days prior to initiation of an experiment. Only male mice were used in these experiments.
Mice were maintained in specific pathogen-free conditions and housed in sterilized microisolator units with corncob bedding as described elsewhere (26). Autoclaved tap water was provided ad libitum. Cftr-knockout mice were fed the liquid diet Peptamen (Nestle Clinical Nutrition, Deerfield, Ill.) after weaning to reduce the incidence of intestinal obstruction experienced by this strain of mice with cystic fibrosis (10). The breeders were fed solid rodent chow (irradiated Harlan Teklad 7960; Harlan Teklad, Madison, Wis.), and wild-type mice were fed either irradiated Harlan Teklad 7960 or irradiated ProLab RMH 3000 (Purina Mills, Inc., St. Louis, Mo.), both solid rodent chows, unless otherwise indicated.
Growth curve study.
Pups from litters of heterozygote breeding pairs of B6.129P2-Cftrtm1Unc mice and pups from litters of breeding pairs of STOCK Cftrtm1Unc-TgN(FABPCFTR)#Jaw mice were weighed once weekly, starting at 7 days of life, by using an electronic balance (Scout series; Ohaus Corp., Florham Park, N.J.). These pups were used for other experiments at the completion of the weighing period, as needed. Mice not needed for other experiments were euthanized by carbon dioxide.
P. aeruginosa-laden agarose beads.
Mucoid P. aeruginosa clinical strain PA M57-15, generously provided by Michael Tosi, was embedded into agarose beads as described elsewhere (26). Briefly, bacteria were grown to late log phase in a shaking incubator at 37°C. Two percent agarose (50 ml of low electroendosmotic agarose; Fisher Scientific, Hanover Park, Ill.) in phosphate-buffered saline (PBS), pH 7.4, was mixed with a 5-ml aliquot of the bacterial broth. The agarose-broth mixture was added to heavy mineral oil that was equilibrated at 50 to 55°C, rapidly stirred for 6 min at room temperature, and then cooled over 10 min. The agarose beads were washed once with 0.5% deoxycholic acid-sodium salt (SDC) in PBS, once with 0.25% SDC in PBS, and 3 to 4 times with PBS. The bead slurry was allowed to settle, and a final volume to 75% of the bead slurry was prepared. Quantitative bacteriology was performed on an aliquot of homogenized bead slurry. Bead diameter was measured by using an inverted light microscope in several fields with the software package Image ProPlus (Media Cybernetics, Baltimore, Md.). P. aeruginosa-laden agarose beads were prepared the day before inoculation and stored overnight at 4°C, and a different bead preparation was used for each experiment.
Inoculating mice with P. aeruginosa-laden agarose beads.
Mice were inoculated preferentially into the right mainstem bronchus, as described elsewhere (26). Briefly, mice were anesthetized with 2.5% Avertin (0.015 ml/g of body wt intraperitoneally), the ventral cervical region was surgically prepared, and a 1-cm skin incision was made just cranial to the thoracic inlet. A 27-gauge 1-in. over-the-needle intravenous catheter (angiocatheter) was used to cannulate the trachea, which was visualized by blunt dissection. The original bead slurries were diluted 10- or 20-fold in sterile PBS for the mice that were to be sacrificed 3 or 10 days after inoculation, respectively. A 0.05-ml aliquot of diluted bead slurry was injected as a bolus. Following inoculation, mice were allowed to recover from the anesthesia. Heat was supplied by careful use of a heat lamp or by use of a circulating-water heating pad (Gaymar T/Pad TP22G and T/Pump set to 38°C; Gaymar Industries Inc., Orchard Park, N.Y.) until the mice were mobile, after which the mice were placed in a fresh, autoclaved microisolator cage.
Evaluation of mice postoperatively.
The mice were observed daily for clinical signs such as coat quality, posture, ambulation, hydration status, and body weight. Mice that were moribund (could not right themselves after being placed in lateral recumbency) were sacrificed before termination of the experiment. This was the only clinical sign that could definitely predict death. The mice were sacrificed 3 or 10 days after inoculation by using carbon dioxide narcosis followed by exsanguination. Gross lung pathology was noted. All procedures were approved by Case Western Reserve University's Animal Care and Use Committee, an institution accredited by the Association for Assessment and Accreditation of Laboratory Animal Care-International.
BAL.
Following sacrifice, bronchoalveolar lavage (BAL) was performed in situ by using a 22-gauge bead-tipped feeding needle ligated to the trachea to prevent backflow. Lavage was conducted with three 1-ml aliquots of sterile PBS, which were then pooled. Qualitative bacteriology was performed on a 10-μl aliquot of unprocessed BALF on tryptic soy agar plates. BALF was treated with 100 mM phenylmethylsulfonyl fluoride and 5 mM EDTA and then centrifuged for 10 min at 100 × g at 4°C. The supernatant was sterile-filtered (22-μm syringe filters, Millex-GV; Millipore Corp., Bedford, Mass.) and stored at −70°C until cytokine analysis could be performed. Pellets were resuspended in 1 ml of PBS. A cell count was performed by using a hemacytometer. Cytocentrifuge preparations (Cytospin 3; Shandon, Pittsburg, Pa.) were stained with hematoxylin and eosin by using standard techniques, and a differential cell count was performed. The cytokines measured were the murine proinflammatory mediators TNF-α and IL-1β, the murine immunomodulatory cytokine IL-6, and the murine neutrophil chemokines mip-2 and KC KC/N51. They were measured by enzyme-linked immunosorbent assay according to the manufacturer's recommendations (R&D Systems, Minneapolis, Minn.). Values that fell below the limits of detection for the assay were assigned a value equal to the lowest limit of detection for each assay. Cytokine concentrations were normalized for urea dilution (20) and expressed as nanograms/milliliter of ELF.
Comparing Cftr-knockout mice with gut-corrected Cftr-knockout mice.
Gut-corrected Cftr-knockout and Cftr-knockout mice were fed the solid rodent chow Prolab 3000 or the liquid diet Peptamen, respectively, following weaning. Mice ranging in age between 6.9 to 8.9 weeks were inoculated with P. aeruginosa-laden agarose beads (5.4 × 104 CFU/mouse; average bead size, 165 μm in diameter) and sacrificed 3 days later. BAL was performed for enumeration of cells and cytokine analysis.
Comparison of diets in wild-type mice.
C57BL/6J males were fed either the liquid diet Peptamen or the solid Harlan Teklad 7960 rodent chow starting at 3 weeks of age until the termination of the study. Mice were inoculated with mucoid P. aeruginosa-laden agarose beads (1.9 × 104 CFU/mouse; average bead diameter, 123 μm) at 6.3 weeks of age. The Harlan Teklad diet was chosen instead of the Prolab diet because the former contains 9% crude fat and the latter contains 5% crude fat; Peptamen contains 33% fat. Three days following infection, the mice were sacrificed, after which BAL was performed for cell counts and cytokine analysis.
Supplemental DHA.
Cftr-knockout mice and their homozygous wild-type littermates were housed individually so that diet intake could be measured to ensure appropriate dosage of the fatty acid supplement DHA per animal. DHA was sonicated into the Peptamen to deliver 40 mg/day per os starting 7 days before inoculation with P. aeruginosa-laden agarose beads (0.5 × 105 to 1.3 × 105 CFU/mouse). Mice were 7.1 ± 1.1 weeks of age when infected with P. aeruginosa. Since mice infected with P. aeruginosa do not eat as much as normal mice the first few days after infection (27), mice were fed by gavage daily starting 4 days prior to infection with a 50-μl bolus of Peptamen or Peptamen containing 40 mg of DHA to acclimate them to the procedure, which was continued until the completion of the study. The mice tolerated this procedure well. Mice were monitored daily for body weight and clinical appearance. Ten days after infection, the surviving mice were sacrificed by carbon dioxide in accordance with the 2000 Report of the AVMA Panel on Euthanasia followed by exsanguination by direct cardiac puncture. Cells in the BALF were enumerated.
Statistics.
Changes in body weight are represented as the percent change in body weight from day 0, when mice were inoculated with P. aeruginosa-laden agarose beads. Neutrophil counts are represented as the absolute number of neutrophils per milliliter of BALF and as the relative number or percentage of cells per ml of BALF. Since the absolute cell counts were not expected to follow a normal distribution, the natural log was taken for use in statistical analysis. Values of zero were assigned a value of one before log transformation of the data. As indicated, the Student t test, one-way analysis of variance (ANOVA), or the Kruskal-Wallis test was performed to assess differences between the different cohorts, followed by pairwise tests using Bonferroni's or Tukey's method to correct for multiple comparisons. Data are represented as means ± standard deviations unless otherwise indicated. Cumulative survival rates were compared by using an exact χ2 test. The criterion for statistical significance was P ≤ 0.05.
RESULTS
Growth curve of Cftr-knockout mice, gut-corrected Cftr-knockout mice, and wild-type mice.
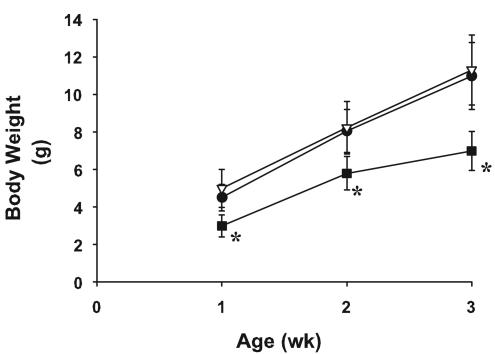
Figure 1 illustrates the growth of Cftr-knockout mice, their wild-type littermates, and the gut-corrected Cftr-knockout mice. Cftr-knockout mice weighed significantly less (P < 0.001) than their control littermates or gut-corrected Cftr-knockout mice at 1, 2, and 3 weeks of age. The gut-corrected Cftr-knockout mice and wild-type mice were similar in weight at weeks 1, 2, and 3.
FIG. 1.
Growth of Cftr-knockout mice and wild-type mice. Cftr-knockout mice (closed squares) (n = 14 to 15/time point), their homozygous wild-type littermates (closed circles) (n = 34 to 36/time point), and gut-corrected Cftr-knockout mice (open triangles) (n = 33 to 37/time point) were weighed weekly starting at 1 week of age. Data are expressed as means ± standard deviations. Cftr-knockout mice weighed significantly less than the wild-type or gut-corrected Cftr-knockout mice at 1 (3.0 ± 0.6, 4.5 ± 0.7, and 5.0 ± 1.0 g, respectively), 2 (5.8 ± 0.9, 8.1 ± 1.1, and 8.2 ± 1.4 g, respectively) and 3 weeks (7.0 ± 1.1, 11.0 ± 1.8, and 11.3 ± 1.9 g, respectively) of age. An asterisk (*) indicates a result that is significantly different from that for wild-type and gut-corrected Cftr-knockout mice (one-way ANOVA and pairwise comparisons made by using a Tukey test; P < 0.001).
Response of Cftr-knockout mice and gut-corrected Cftr-knockout mice to lung infections with mucoid P. aeruginosa.
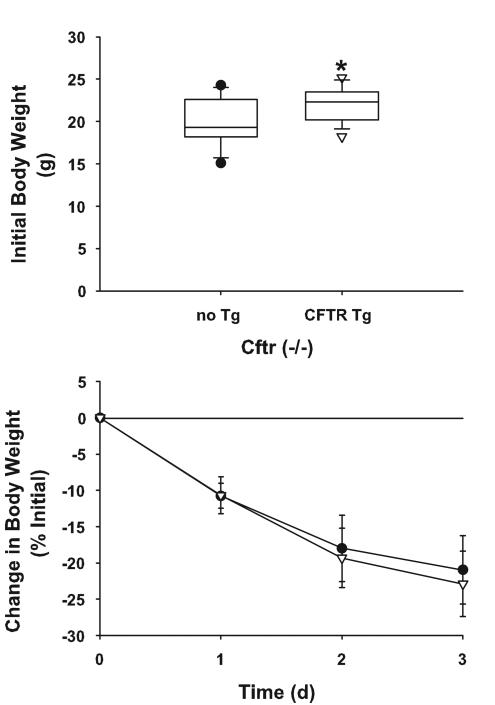
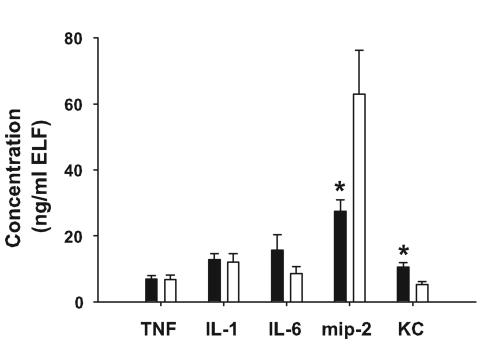
Cftr-knockout mice and gut-corrected Cftr-knockout mice were inoculated with mucoid P. aeruginosa-laden agarose beads (5.4 × 104 CFU/mouse; average bead size, 165 μm in diameter). Three days later, the mice were sacrificed and BALF was collected. Although the gut-corrected Cftr-knockout mice weighed significantly more than the Cftr-knockout mice at the onset of the study (P = 0.019), weight loss was comparable in the two groups following P. aeruginosa lung infection at all time points studied (P > 0.05) (Fig. 2). In addition, there were no significant differences between the two cystic fibrosis strains in the numbers of cells in BALF 3 days after infection (Table 1). Although there were no significant differences (P > 0.05) in the levels of TNF-α, IL-1β, and IL-6 between the two strains of mice with cystic fibrosis, there were significant differences (P ≤ 0.015) in the levels of murine neutrophil chemokines mip-2 and KC (Fig. 3). For KC, the gut-corrected Cftr-knockout mice had higher levels, whereas for mip-2, the Cftr-knockout mice had higher levels.
FIG. 2.
Cftr-knockout mice (closed circles) (n = 15) and gut-corrected Cftr-knockout mice (open triangles) (n = 11) were weighed on day 0, after which the mice were inoculated preferentially in the right mainstem bronchus with mucoid P. aeruginosa-laden agarose beads. The mice were weighed daily thereafter. The gut-corrected Cftr-knockout mice weighed significantly more on day 0 than the Cftr-knockout mice (21.9 ± 2.0 and 19.7 ± 2.6 g, respectively; the unpaired Student t test, P = 0.019). However, there were no significant differences (the unpaired Student t test, P > 0.05) in weight loss between the two cohorts following infection with mucoid P. aeruginosa. Data are expressed as means ± standard deviations. Tg, transgene; d, day.
TABLE 1.
Number of inflammatory response cells in BALF 3 days after challenge with mucoid P. aeruginosa in two strains of mice with cystic fibrosisa
| Mouse strain | No. of samples | Treatment(s)b | Relative no. (%) of indicated type of cell in BALF
|
Absolute no. of indicated type of cell in BALF (million cells/ml)
|
||||
|---|---|---|---|---|---|---|---|---|
| AMc | PMNd | Lymphe | Leukocyte | AMc | PMNd | |||
| Cftr-knockout | 10 | P. aeruginosa beads in liquid diet | 9.7 ± 14.5 | 90.1 ± 14.7 | 0.1 ± 0.3 | 0.75 ± 0.50 | 0.06 ± 0.07 | 0.69 ± 0.48 |
| Gut-corrected Cftr-knockout | 15 | P. aeruginosa beads in solid chow | 6.1 ± 7.9 | 93.7 ± 7.9 | 0.2 ± 0.3 | 1.19 ± 0.43 | 0.05 ± 0.04 | 1.13 ± 0.43 |
Data are presented as means ± standard deviations.
P. aeruginosa beads, mucoid P. aeruginosa-laden agarose beads.
AM, alveolar macrophage.
PMN, neutrophil.
Lymph, lymphocyte.
FIG. 3.
Cftr-knockout mice (open bars) (n = 7 to 8) and gut-corrected Cftr-knockout mice (solid bars) (n = 15) were inoculated with mucoid P. aeruginosa-laden agarose beads. Three days later, the mice were sacrificed and BAL was performed. Inflammatory mediators were measured in the ELF by enzyme-linked immunosorbent assay. Gut-corrected Cftr-knockout mice had significantly lower levels of mip-2 (27.46 ± 13.33 and 62.93 ± 37.50 ng/ml of ELF, respectively) and significantly greater amounts of KC (10.60 ± 4.93 and 5.32 ± 2.53 ng/ml of ELF, respectively) than Cftr-knockout mice. Data are expressed as means ± standard errors of the means. An asterisk (*) indicates results that were significantly different from those for gut-corrected Cftr-knockout mice (P ≤ 0.015).
Comparing diets in wild-type mice.
C57BL/6J weanlings (20-days old) were fed either a diet of standard solid rodent chow, Prolab 3000, or the human liquid elemental diet Peptamen for 3 weeks prior to inoculation with mucoid P. aeruginosa-laden agarose beads (1.9 × 104 CFU/mouse) on day 0. Three days later, the mice were sacrificed and BAL was performed for cell counts and cytokine analysis. The initial body weights of mice fed the different diets were similar, and following infection there were no differences in weight loss at any time point studied (Table 2). In addition, there were no significant differences in concentrations of proinflammatory mediators in ELF (Table 3) or in cell numbers (Table 4) between the two groups of mice.
TABLE 2.
Body weights of wild-type C57BL/6U mice fed different diets prior to and after lung infection with P. aeruginosaa
| Dietb | No. of samples | Initial body wt (g) | Change in body wt (% initial)
|
||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |||
| Solid chow + P. aeruginosa beads | 9 | 23.0 ± 2.3 | −9.3 ± 2.7 | −14.7 ± 5.3 | −14.3 ± 8.2 |
| Liquid diet + P. aeruginosa beads | 9 | 22.6 ± 0.7 | −8.7 ± 1.5 | −17.6 ± 2.3 | −19.4 ± 3.1 |
Data are presented as means ± standard deviations.
P. aeruginosa beads, mucoid P. aeruginosa-laden agarose beads.
TABLE 3.
Inflammatory mediator production in ELF of wild-type C57BL/bu mice fed different diets 3 days after inoculation with mucoid P. aeruginosa-laden agarose beadsa
| Dietb | No. of samples | Concentration of indicated inflammatory mediator in ELF (ng/ml)
|
||||
|---|---|---|---|---|---|---|
| TNF-α | IL-1β | IL-6 | mip-2 | KC | ||
| Solid chow + P. aeruginosa beads | 9 | 7.04 ± 6.95 | 4.84 ± 7.12 | 6.46 ± 4.74 | 23.47 ± 33.74 | 4.29 ± 2.81 |
| Liquid diet + P. aeruginosa beads | 9 | 12.17 ± 6.04 | 5.95 ± 5.16 | 9.57 ± 4.93 | 34.85 ± 25.19 | 8.62 ± 5.62 |
Data are presented as means ± standard deviations.
P. aeruginosa beads, mucoid P. aeruginosa-laden agarose beads.
TABLE 4.
Number of inflammatory response cells in BALF of C57BL/bu mice 3 days after challenge with mucoid P. aeruginosa
| Treatmentb | No. of samples | Relative no. (%) of indicated type of cell in BALF
|
Absolute no. of indicated type of cell in BALF (million cells/ml)
|
||||
|---|---|---|---|---|---|---|---|
| AMc | PMNd | Lymphe | Leukocyte | AMc | PMNd | ||
| Solid chow + P. aeruginosa beads | 9 | 8.6 ± 5.1 | 90.9 ± 4.9 | 0.4 ± 0.5 | 0.25 ± 0.19 | 0.02 ± 0.01 | 0.23 ± 0.18 |
| Liquid diet + P. aeruginosa beads | 9 | 4.9 ± 2.4 | 94.3 ± 2.5 | 0.8 ± 0.6 | 0.25 ± 0.10 | 0.01 ± 0.01 | 0.24 ± 0.10 |
Data are represented as means ± standard deviation, and sample sizes are indicated in parentheses.
P. aeruginosa beads, mucoid P. aeruginosa-laden agarose beads.
AM, alveolar macrophage.
PMN, neutrophil.
Lymph, lymphocyte.
Dietary supplementation of Cftr-knockout mice with DHA.
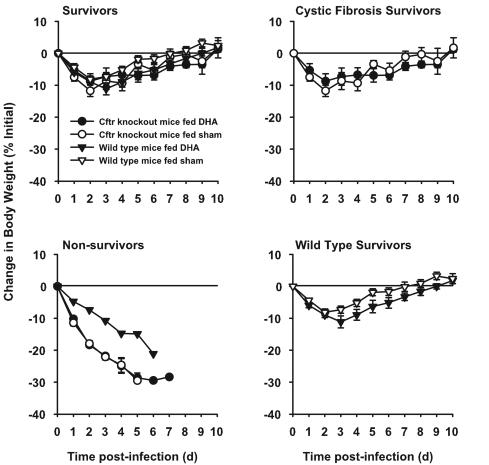
Starting 7 days before infection with P. aeruginosa-laden agarose beads and then throughout the remainder of the 10-day study, wild-type mice and Cftr-knockout mice were treated daily with DHA. All mice lost weight following infection (Fig. 4). ANOVA (with Bonferroni's multiple comparisons) showed that the baseline body weights of wild-type mice supplemented with Peptamen with or without DHA (25.6 ± 2.2 and 27.3 ± 3.2, respectively) did not differ significantly (the unpaired Student t test, P > 0.21) and that there were no significant differences (the unpaired Student t test, P = 0.28) between the starting weights of similarly treated Cftr-knockout mice (20.9 ± 1.3 and 21.9 ± 2.4 g, respectively). However, Cftr-knockout mice weighed significantly less (the unpaired Student t test, P < 0.001) than their wild-type counterparts (21.4 ± 1.9 and 26.5 ± 2.8 g, respectively). Subsequent analysis indicated that percent weight loss was not significantly related to initial body weight; therefore, there was no adjustment made for initial body weight in further analysis. The weight loss of mice that died followed a much different (more downward) trajectory than that of mice that survived the course of the 10-day experiment (Fig. 4). Because the pattern of weight loss in mice that died was much different from that of those that survived, comparisons of weight loss among the four groups were made 4 days after infection (when no deaths had occurred due to the lung infection) and among survivors 10 days after infection. Four days after infection, there were no significant differences in weight loss among the four groups (P = 0.09, one-way ANOVA), and post hoc Bonferroni's pairwise comparisons did not reveal any pairwise differences to be significant. There was a trend for the weight loss to be greater in the groups that later had deaths (wild-type mice supplemented with DHA and Cftr-knockout mice supplemented with Peptamen alone or containing DHA) than in the groups where no deaths occurred (wild-type mice fed Peptamen alone). The mean weight change 10 days after infection did not differ among survivors in the four groups (P = 0.97).
FIG. 4.
Change in body weight after infection with P. aeruginosa, with or without dietary supplementation with DHA. Mice with cystic fibrosis (circles) and wild-type littermates (triangles) were given supplements of DHA (closed symbols) prior to infection with P. aeruginosa-laden agarose beads on day 0. The controls were provided with Peptamen alone (open symbols). Body weights were determined starting just before infection and daily thereafter. Sample sizes at the beginning were 8 to 9 per group. Data is presented as the percent change from the initial body weight on day 0. Data are shown as means ± standard errors of the means. Note that none of the wild-type mice on which gavage was performed with a sham treatment died. d, day.
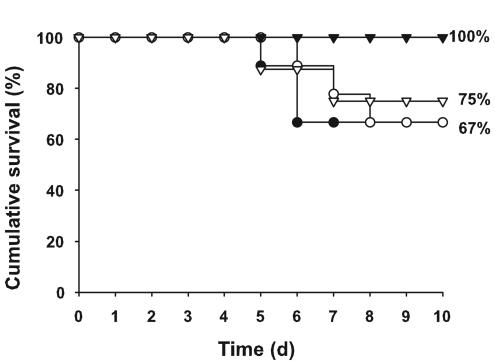
Mortality of infected mice began 5 days after infection. The cumulative survival rates of the different cohorts of mice were not significantly different (P = 0.35; exact χ2 test) (Fig. 5). The mortality rates of Cftr-knockout mice were the same regardless of treatment (33.3%). Wild-type mice who received supplements of DHA experienced a 25% mortality rate, but none of their sham-treated counterparts died.
FIG. 5.
Cumulative survival rate after infection with P. aeruginosa, with or without dietary supplementation with DHA. Mice with cystic fibrosis (circles) and normal, wild-type littermates (triangles) were supplemented with DHA (open symbols) prior to infection with P. aeruginosa-laden agarose beads on day 0. The controls were provided with Peptamen alone (closed symbols). Sample sizes at the beginning were 8 to 9 per group. There were no significant differences between the groups (exact χ2 test; P = 0.35). d, day.
Cell counts were determined from the BALFs of surviving mice (Table 5). There were no significant differences in relative or absolute macrophage or neutrophil numbers among survivors in the four groups. Relative numbers of lymphocytes were significantly different among the cohorts (P = 0.023; Kruskal-Wallis test); however, the only significant difference among the groups as determined by pairwise comparisons was between the Cftr-knockout mice receiving DHA and wild-type mice receiving a sham treatment. There were no significant differences in raw absolute cell counts (one-way ANOVA); however, statistical analysis using the natural log transformation of the data indicated that there were significant differences among the cohorts with respect to leukocyte counts (P = 0.035). As determined by using post hoc Bonferroni's pairwise comparisons, only the Cftr-knockout mice that received supplements of DHA and wild-type mice that received supplements of Peptamen alone differed (P < 0.05).
TABLE 5.
Leukocyte counts in BALF after supplementation with DHA
| Mouse strain | No. of samples | Supplement | Relative no. (%) of indicated type of cell in BALF
|
Absolute no. of indicated type of cell in BALF (thousand cells/ml)
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| AMd | PMNe | Lymphf | Leukocytes [ln(Leuk)]a | AMd [ln(AM)] | PMNe [ln(PMN)] | Lymphf [ln(Lymph)] | |||
| Wild typeg | 8 | Sham | 74.8 ± 14.8 | 14.3 ± 6.0 | 10.9 ± 10.7 | 40.0 ± 34.9 [3.3 ± 1.0] | 32.4 ± 31.3 [3.0 ± 1.1] | 5.4 ± 4.8 [1.3 ± 1.1] | 2.2 ± 1.4 [0.5 ± 0.9] |
| 6 | DHA | 69.2 ± 20.0 | 28.6 ± 20.9 | 2.2 ± 2.6 | 55.8 ± 20.0 [4.0 ± 0.4] | 37.7 ± 16.9 [3.6 ± 0.4] | 16.7 ± 13.9 [2.5 ± 1.0] | 1.5 ± 1.9 [−0.3 ± 1.3] | |
| Cftr knockout | 6 | Sham | 66.9 ± 15.7 | 30.1 ± 16.3 | 3.1 ± 2.5 | 61.3 ± 37.4 [4.0 ± 0.6] | 39.5 ± 19.0 [3.5 ± 0.6] | 20.2 ± 20.4 [2.6 ± 1.0] | 1.7 ± 1.3 [−0.7 ± 3.1] |
| 6 | DHA | 66.6 ± 29.9 | 32.5 ± 30.1 | 0.9 ± 1.0b | 102.8 ± 57.5 [4.5 ± 0.6]b | 59.1 ± 35.1 [4.0 ± 0.5] | 42.7 ± 60.0 [2.9 ± 1.6] | 1.1 ± 1.2 [−2.3 ± 3.8] | |
Significantly different (one-way ANOVA or Kruskal-Wallis test; P ≤ 0.05).
Significantly different from wild-type, sham-treated mice (Bonferroni's multiple comparisons; P ≤ 0.05).
All mice were inoculated with P. aeruginosa-laden agarose beads on day 0, and the survivors were sacrificed on day 10. Data are represented as means ± standard deviations. Log transformation of data is indicated in brackets.
AM, alveolar macrophage.
PMN, neutrophil.
Lymph, lymphocyte.
Wild type, wild-type littermates of Cftr-knockout mice.
DISCUSSION
The lung disease in cystic arises from early bacterial infection, which is not readily cleared, and an exuberant inflammatory response by the host. The tendency toward bacterial infection and the excess inflammatory response, probably both contribute to the persistent, progressive airways disease characterized by chronic Pseudomonas aeruginosa bronchopulmonary infection, chronic influx of neutrophils into the airway lumen, and persistence of the neutrophil response throughout the course of the disease.
The best available model of the inflammatory response to bronchopulmonary infection with P. aeruginosa is the agarose bead model (6, 21, 26). In this model, P. aeruginosa is physically retained in the lungs by mechanical means, circumventing the normal host defenses, so this is not a model for the initial infection of the cystic fibrosis lung. However, mice develop a robust, though transient, neutrophil response to the P. aeruginosa-laden agarose beads (27), and the character of this inflammatory response resembles that observed in the cystic fibrosis lung both histologically and in the pattern of cytokines in ELF. In addition, the mucoid nature of the bacteria is retained in both Cftr-knockout mice and wild-type mice after inoculation with mucoid P. aeruginosa-laden agarose beads, as indicated by culturing BALF on tryptic soy agar (data not shown). Moreover, this model responds to several therapeutic interventions (e.g., ibuprofen, antibiotics, and vaccines) in a manner similar to that of cystic fibrosis patients (1, 2, 15, 17). This model was used to evaluate the effects of nutrition on the inflammatory response in cystic fibrosis.
Both Cftr-knockout mice and gut-corrected Cftr-knockout mice have lung inflammatory responses in excess of those of wild-type controls when they are challenged with P. aeruginosa-laden agarose beads (14, 25). The responses of Cftr-knockout mice and gut-corrected Cftr-knockout mice to P. aeruginosa-laden agarose bead challenge were very similar. The gut-corrected Cftr-knockout mice grow in a fashion indistinguishable from that of wild-type mice, and in adulthood their body weights are comparable to those of wild-type mice; therefore, they are probably nutritionally replete. On the other hand, the Cftr-knockout mice do not grow as well and may be considered nutritionally depleted. Since there are differences in the diets fed to these two strains of mice with cystic fibrosis (gut-corrected Cftr-knockout mice can survive on normal mouse chow, whereas the Cftr-knockout mice cannot and are fed a liquid diet), the effect of diet alone on mice challenged with P. aeruginosa-laden agarose beads was tested. No differences were found. Therefore, the hypothesis that the excess inflammatory response in the Cftr-knockout mice arises from nutritional depletion alone was not supported.
A more specific nutritional deficit, lack of DHA, was suggested to contribute to the pathophysiology of cystic fibrosis, since supplementation with DHA protected Cftr-knockout mice against the excess inflammatory response to intratracheal lipopolysaccharides (LPSs) (12). This finding suggested that DHA deficiency, or the accompanying increase in membrane arachidonic acid, contributes to the inflammatory response mounted by the cystic fibrosis lung. Therefore, the effect of DHA supplementation on the response of the cystic fibrosis lung to challenge with P. aeruginosa-laden agarose beads was tested, performed similarly to that for LPS challenge (12). DHA pretreatment did not improve mortality rates for either Cftr-knockout or wild-type mice challenged with P. aeruginosa-laden agarose beads. Mortality rates were not very high for either wild-type mice or Cftr-knockout mice in this study. It is possible that nutritional support provided by gavage, regardless of DHA content, improved the response to chronic lung infections with mucoid P. aeruginosa. In addition, the operator for making the agarose beads and the operator for inoculating the mice were different in this study than in the previous study (14), and different operators for either procedure can lead to differential results between experiments (26).
There may be several reasons why DHA protects Cftr-knockout mice against an increased inflammatory response to LPS challenge (12) but not against the challenge of P. aeruginosa-laden agarose beads. DHA is probably acting not simply to restore the normal DHA-to-arachidonic acid balance but rather in a pharmacologic manner, since DHA had similar effects on BAL lymphocytes and mortality in Cftr-knockout and wild-type mice. LPS is a classical macrophage stimulant (8) and has relatively little effect on the airway epithelial cells (19), whereas P. aeruginosa-laden agarose beads must challenge the epithelium as well as the professional immune cells. If DHA supplementation improves macrophage defenses but has little effect on the epithelium, these factors could explain the results. One example of the possible differential effects of DHA may be its effect on the peroxisome proliferation activator receptor (PPAR) system, which has received considerable attention as an antiinflammatory system. PPAR is a nuclear receptor which, when occupied, can form dimers with the retinoid X receptors, and the heterodimer can act as a transcription factor or as a down regulator of the inflammatory response (4). Both the α and the γ isoforms of PPAR can participate in the down regulation of the inflammatory response. DHA interacts with and activates PPAR-α but not PPAR-γ (3), and the dose of DHA given to the mice is sufficient not only to restore normal DHA levels but also to increase the levels well above normal (12). In epithelial cells, only PPAR-γ is expressed, whereas macrophages express both PPAR-α and PPAR-γ (7, 9, 29). Thus, DHA may down regulate inflammatory responses in macrophages but not in epithelial cells via the PPAR system. Other mechanisms of down regulation of inflammation by DHA are also possible, such as the down regulation of production of arachidonic acid-derived mediators due to reduction in membrane-incorporated arachidonic acid. If this mechanism is more important in macrophages, it could explain the reduced inflammation in response to LPS compared to that in response to P. aeruginosa-containing agarose beads. Therefore, it is possible that DHA correction is more important in the acquisition phase of P. aeruginosa infection, which is circumvented in the agarose bead model, but that when bacterial retention in the lung is established this correction is no longer beneficial.
That Yu and colleagues' (30) results differed from our results is not necessarily surprising. Although we used the same strains of mice, the infection models we used were different. Specifically, the method of administration differed greatly. Yu et al. administered the bacteria by nebulizing the bacteria, whereas in this study the organism was embedded in agarose beads and surgically placed in the lungs of mice. It is likely that diet plays a significant role in innate host defense mechanisms to nonmucoid P. aeruginosa, as demonstrated by Yu et al. However, the model used here does not test the innate host response to mucoid P. aeruginosa and instead tests host defense mechanisms that are initiated once the innate responses are insufficient to clear the bacteria from the lung. In addition, we used a mucoid clinical strain of P. aeruginosa, whereas Yu and colleagues administered a nonmucoid laboratory strain. Whether diet plays a differential role in the response to mucoid versus nonmucoid strains was not determined.
In conclusion, there was no evidence that the diet of the Cftr-knockout mice or their intestinal disease and the accompanying nutritional deficit account for the excessive inflammatory response we and others have documented following challenges from P. aeruginosa-laden agarose beads. Moreover, dietary supplementation with DHA did not improve the clinical outcome of Cftr-knockout mice or wild-type mice in response to bronchopulmonary infection with P. aeruginosa. Other aspects of the cystic fibrosis lesion must explain the excess inflammatory response.
Acknowledgments
This work was supported by Genzyme Corporation, a Research and Development grant from the Cystic Fibrosis Foundation, and NIH grants P30DK27651 and HL60293.
We thank students Kathryn Boland for performing gavage on the mice and Jessica Hoyt for weighing untreated mice and the following members of the CWRU Cystic Fibrosis Animal Core: Alma Genta Wilson, Veronica Peck, and Ebony Boyd for breeding mice and James Poleman and Christiaan van Heeckeren for performing the infection experiments.
Editor: J. N. Weiser
REFERENCES
- 1.Beaulac, C., S. Clement-Major, J. Hawari, and J. Lagace. 1996. Eradication of mucoid Pseudomonas aeruginosa with fluid liposome-encapsulated tobramycin in an animal model of chronic pulmonary infection. Antimicrob. Agents Chemother. 40:665-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaulac, C., S. Sachetelli, and J. Lagace. 1999. Aerosolization of low phase transition temperature liposomal tobramycin as a dry powder in an animal model of chronic pulmonary infection caused by Pseudomonas aeruginosa. J. Drug Target. 7:33-41. [DOI] [PubMed] [Google Scholar]
- 3.Berger, J., and D. E. Moller. 2002. The mechanisms of action of PPARs. Annu. Rev. Med. 53:409-435. [DOI] [PubMed] [Google Scholar]
- 4.Bordji, K., J. P. Grillasca, J. N. Gouze, J. Magdalou, H. Schohn, J. M. Keller, A. Bianchi, M. Dauca, P. Netter, and B. Terlain. 2000. Evidence for the presence of peroxisome proliferator-activated receptor (PPAR) α and γ and retinoid Z receptor in cartilage. PPARγ activation modulates the effects of interleukin-1β on rat chondrocytes. J. Biol. Chem. 275:12243-12250. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, P. W., III, and J. A. Phillips III. 1992. The cystic fibrosis gene and relationships to clinical status. Semin. Respir. Infect. 7:150-157. [PubMed] [Google Scholar]
- 6.Cash, H. A., D. E. Woods, B. McCullough, W. G. Johanson, Jr., and J. A. Bass. 1979. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 119:453-459. [DOI] [PubMed] [Google Scholar]
- 7.Chinetti, G., S. Griglio, M. Antonucci, I. P. Torra, P. Delerive, Z. Majd, J. C. Fruchart, J. Chapman, J. Najib, and B. Staels. 1998. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J. Biol. Chem. 273:25573-25580. [DOI] [PubMed] [Google Scholar]
- 8.Ciesielski, C. J., E. Andreakos, B. M. Foxwell, and M. Feldmann. 2002. TNFα-induced macrophage chemokine secretion is more dependent on NF-κB expression than lipopolysaccharides-induced macrophage chemokine secretion. Eur. J. Immunol. 32:2037-2045. [DOI] [PubMed] [Google Scholar]
- 9.Clark, R. B., D. Bishop-Bailey, T. Estrada-Hernandez, T. Hla, L. Puddington, and S. J. Padula. 2000. The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses. J. Immunol. 164:1364-1371. [DOI] [PubMed] [Google Scholar]
- 10.Eckman, E. A., C. U. Cotton, D. M. Kube, and P. B. Davis. 1995. Dietary changes improve survival of CFTR S489X homozygous mutant mouse. Am. J. Physiol. 269:L625-L630. [DOI] [PubMed] [Google Scholar]
- 11.Ehringer, W., D. Belcher, S. R. Wassall, and W. Stillwell. 1990. A comparison of the effects of linolenic (18:3 omega 3) and docosahexaenoic (22:6 omega 3) acids on phospholipid bilayers. Chem. Phys. Lipids 54:79-88. [DOI] [PubMed] [Google Scholar]
- 12.Freedman, S. D., M. H. Katz, E. M. Parker, M. Laposata, M. Y. Urman, and J. G. Alvarez. 1999. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftr−/− mice. Proc. Natl. Acad. Sci. USA 96:13995-14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gosselin, D., M. M. Stevenson, E. A. Cowley, U. Griesenbach, D. H. Eidelman, M. Boule, M. F. Tam, G. Kent, E. Skamene, L. C. Tsui, and D. Radzioch. 1998. Impaired ability of Cftr knockout mice to control lung infection with Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 157:1253-1262. [DOI] [PubMed] [Google Scholar]
- 14.Heeckeren, A., R. Walenga, M. W. Konstan, T. Bonfield, P. B. Davis, and T. Ferkol. 1997. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J. Clin. Investig. 100:2810-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansen, H. K., H. P. Hougen, S. J. Cryz, Jr., J. Rygaard, and N. Hoiby. 1995. Vaccination promotes TH1-like inflammation and survival in chronic Pseudomonas aeruginosa pneumonia in rats. Am. J. Respir. Crit. Care Med. 152:1337-1346. [DOI] [PubMed] [Google Scholar]
- 16.Konstan, M. W., S. M. Butler, M. E. Wohl, M. Stoddard, R. Matousek, J. S. Wagener, C. A. Johnson, and W. J. Morgan. 2003. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J. Pediatr. 142:624-630. [DOI] [PubMed] [Google Scholar]
- 17.Konstan, M. W., K. M. Vargo, and P. B. Davis. 1990. Ibuprofen attenuates the inflammatory response to Pseudomonas aeruginosa in a rat model of chronic pulmonary infection. Implications for antiinflammatory therapy in cystic fibrosis. Am. Rev. Respir. Dis. 141:186-192. [DOI] [PubMed] [Google Scholar]
- 18.Kuehl, F. A., Jr., and R. W. Egan. 1980. Prostaglandins, arachidonic acid, and inflammation. Science 210:978-984. [DOI] [PubMed] [Google Scholar]
- 19.Palmberg, L., B. M. Larsson, P. Malmberg, and K. Larsson. 1998. Induction of IL-8 production in human alveolar macrophages and human bronchial epithelial cells in vitro by swine dust. Thorax 53:260-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rennard, S. I., G. Basset, D. Lecossier, K. M. O'Donnell, P. Pinkston, P. G. Martin, and R. G. Crystal. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532-538. [DOI] [PubMed] [Google Scholar]
- 21.Starke, J. R., M. S. Edwards, C. Langston, and C. J. Baker. 1987. A mouse model of chronic pulmonary infection with Pseudomonas aeruginosa and Pseudomonas cepacia. Pediatr. Res. 22:698-702. [DOI] [PubMed] [Google Scholar]
- 22.Stillwell, W., W. Ehringer, and L. J. Jenski. 1993. Docosahexaenoic acid increases permeability of lipid vesicles and tumor cells. Lipids 28:103-108. [DOI] [PubMed] [Google Scholar]
- 23.Stillwell, W., L. J. Jenski, F. T. Crump, and W. Ehringer. 1997. Effect of docosahexaenoic acid on mouse mitochondrial membrane properties. Lipids 32:497-506. [DOI] [PubMed] [Google Scholar]
- 24.Taylor, C. J., and N. Aswani. 2002. The pancreas in cystic fibrosis. Paediatr. Respir. Rev. 3:77-81. [DOI] [PubMed] [Google Scholar]
- 25.van Heeckeren, A. M., A. Scaria, M. D. Schluchter, T. W. Ferkol, S. Wadsworth, and P. B. Davis. 2004. Delivery of CFTR by adenoviral vector to cystic fibrosis mouse lung in a model of chronic Pseudomonas aeruginosa lung infection. Am. J. Physiol. Lung Cell Mol. Physiol. 286 [Online.] [DOI] [PubMed]
- 26.van Heeckeren, A. M., and M. D. Schluchter. 2002. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab. Anim. 36:291-312. [DOI] [PubMed] [Google Scholar]
- 27.van Heeckeren, A. M., J. Tscheikuna, R. W. Walenga, M. W. Konstan, P. B. Davis, B. Erokwu, M. A. Haxhiu, and T. W. Ferkol. 2000. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am. J. Respir. Crit. Care Med. 161:271-279. [DOI] [PubMed] [Google Scholar]
- 28.Wesley, A., K. Dawson, C. Hewitt, and A. Kerr. 1993. Clinical features of individuals with cystic fibrosis in New Zealand. N. Z. Med. J. 106:28-30. [PubMed] [Google Scholar]
- 29.Yang, X. Y., L. H. Wang, T. Chen, D. R. Hodge, J. H. Resau, L. DaSilva, and W. L. Farrar. 2000. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor γ (PPARγ) agonists. PPARγ co-association with transcription factor NFAT. J. Biol. Chem. 275:4541-4544. [DOI] [PubMed] [Google Scholar]
- 30.Yu, H., S. Z. Nasr, and V. Deretic. 2000. Innate lung defenses and compromised Pseudomonas aeruginosa clearance in the malnourished mouse model of respiratory infections in cystic fibrosis. Infect. Immun. 68:2142-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]