Summary
Background
To identify meningioma-specific proteins, cerebrospinal fluid (CSF) from 4 patients with a meningioma and 4 patients with a non-brain tumorous lesion were analyzed.
Material/Methods
Two-dimensional electrophoresis and electrospray quadrupole time-of-flight tandem mass spectrometry analyses revealed 10 unique spots, containing 11 independent proteins (spot #2 and #4 each contained 2 proteins and spot #3 was not identified) were evident in CSF associated with human meningioma: serum albumin precursor (3 different isoforms), Apolipoprotein E (Apo E), Apolipoprotein J precursor (Apo J), Transthyretin precursor (TTR), Prostaglandin D2 synthase 21kDa (PTGDS), proapolipoprotein, Chain D hemoglobin Ypsilanti, alpha-1-antitrypsin (AAT), and beta-2-microglobulin precursor (β2M).
Results
The contents of Apo E, Apo J and AAT were increased, while PTGDS, TTR and β2M were decreased.
Conclusions
The results observed by 2-dimensional electrophoresis were verified by Western blot analysis. The unique proteins may represent possible candidate biomarkers of meningioma.
Keywords: meningioma, human cerebrospinal fluid, proteome, biomarker, brain tumor
Background
Cerebrospinal fluid (CSF) is rich in proteins and, thus, has the potential to be used for diagnostic and prognostic purposes. The present study sought to identify new biodiagnostic markers of meningioma in CSF. Some CSF proteins can be used as tumor markers for the diagnosis of brain tumors. Proteins such as human chorionic gonadotropin (hCG) and α-fetoprotein (α-FP) are secreted from several different CNS structures [1,2], and CSF sampling by lumbar puncture is a well-established procedure for differentiating various germ cell tumors.
Because the flow of proteins from and to the brain is restricted by the blood-brain-barrier, biochemical changes reflected in CSF can indicate the status of the brain parenchyma and, more specifically, the periventricular white matter [3,4]. CSF is the only body fluid in direct contact with the brain and so is a more likely source of biomarkers than any other body fluid [5–7], particularly protein components that result from transcriptional and post-transcriptional control, post-translational modifications, and the transport of proteins between cellular compartments [8].
Meningiomas are the second most frequent type of common primary brain tumor, and account for 26% of all primary brain tumors [9,10]. Although most meningiomas are slow growing benign tumors, huge meningiomas are believed to have surgical risks. However, if a meningioma is diagnosed early, it can be treated non-surgically. It is well-known that benign brain tumors such as meningiomas have a more homogenous genotype than malignant brain tumors. This makes it relatively easy to find candidate marker proteins of meningioma.
Immunolabeling of MIB-1 is useful for predicting the recurrence of benign meningioma [11], but more accurate, non-invasive methods are required. In the era following the description of the human genome, proteomics-based systems provide a convenient means of diagnosing cancer [12]. Furthermore, proteomics has proven to be a powerful means of obtaining insight into biological processes by allowing the screening of whole protein contents at particular time points [13]. In addition, proteomics is compatible with high-throughput analysis and provides high-resolution images.
The present study identified meningioma-specific proteins in the CSF samples of patients with meningioma by using 2-dimensional electrophoresis (2-DE) and mass spectometry. The resulting findings were confirmed by Western blot and suggest that CSF proteomics is a potentially useful diagnostic tool in patients with meningioma.
Material and Methods
Source of CSF and sample preparation
We quantitatively analyzed 11 CSF samples from patients with meningioma and 5 CSF samples from patients with non-brain tumorous lesions, including 4 samples from patients with hydrocephalus and 1 sample from patients with protein infarctions. Four CSF samples with meningioma had enough concentration of proteins to be analyzed by 2-DE. The other 5 CSF samples had low concentration of proteins and only enough concentration to be analyzed by Western blot. We thus selected 8 CSF samples (4 from patients with meningioma [Me1–4], which had a direct exposure to subarachnoid space; and 4 from patients with non-brain tumors [N1–4]) containing many proteins for proteomic analysis, and an additional 5 CSF samples from patients with meningioma, for Western blot analysis for verification (Me5–9). These samples were individually centrifuged at 2000 × g at 4°C to remove circulating cells and debris, and stored in aliquots at −70°C until use. The Institutional Review Board of Ajou University Medical Center (Suwon, Korea) approved this study.
Acetone precipitation
CSF is generally high in salt (>150 mmol/L) and low in protein. Of the available methods for protein precipitation, higher protein recovery from human CSF has been obtained using acetone precipitation [19]. Accordingly, all CSF samples were precipitated with 100% ice-cold acetone in a 1:2 ratio of CSF to acetone, at −20°C for at least 1 h. After centrifugation at 2,000 × g for 15 min at 4°C, the supernatant was discarded and ice-cold acetone was added. The pellets obtained following the same centrifugation conditions were air-dried using a Speedvac (Heto-Holten A/S, Allerod, Denmark) and stored at −20°C.
IEF
IEF was conducted as previously described. Briefly, 500 μL of a rehydration solution composed of 7 M urea, 2M thiourea, 4% w/v CHAPS, 60 mM dithiothreitol, a trace of bromophenol blue, and 0.5% w/v immobilized pH gradient (IPG) buffer (pH 3–10; Bio-Rad Laboratories, Hercules, CA, USA) was prepared. Acetone-precipitated pellets were dissolved in 500 μl rehydration solution for salt removal. Pellets were then disrupted by titration, followed by sonication for 3×30 seconds to eliminate protein aggregates. The solution was centrifuged at 15,000 rpm for 15 min, and the supernatant was taken and placed in an e-tube (Porex, Fairburn, GA, USA). Protein concentrations of samples were measured in triplicate by Bradford method before being loaded to the IEF strip for separation. A precast IPG dry strip was placed gel-side down into the mixture and was covered with mineral oil. After rehydration for 12 h, the first dimension was performed at 200V for 1 h, 500V for 1 h, 1,000V for 1 h, 8,000V over a 30 min gradient, and 8,000V up to 78,000 Vhr (total run=79,700 Vhr). The first dimension was performed using an IPGphor system, using 18 cm Immobiline Dry strips (pH3–10NL; Amersham Biosciences, Franklin Lakes, NJ), and rehydrated overnight with 150 μg of CSF proteins diluted to 350 uL with rehydration buffer. The increasing voltage gradient reduced the salt interference. The electrofocus strip was stored at −70°C until required for the second-dimensional gel electrophoresis.
2-DE
Frozen CSF samples were thawed at room temperature and treated with lysis buffer containing 10 μL/mL of a protease inhibitor cocktail and 200 mM phenylmethanesulphonylfluoride. The second dimension involved 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a PROTEAN IIXI electrophoresis kit (Bio-Rad). Silver staining was carried out as described previously [36] using a silver stain kit (Amersham Biosciences). Silver staining was used because of its high detection sensitivity, which allows the detection of most proteins down to the nanogram range, which is 100 times more sensitive than using Coomassie brilliant blue. The method is reliable and reproducible, and gives essentially colorless backgrounds in most gel electrophoresis systems. 2-DE was carried out at least 3 times independently.
Image acquisition analysis
Silver-stained 2-DE gels were scanned with a digital scanner and spot detection was performed qualitatively and quantitatively using a Progenesis Workstation 2005 (Nonlinear Dynamics, Newcastle-upon-Tyne, UK) as described previously. A 2-fold increase in the differential expression of protein spots was evaluated using statistical analysis tools. All data obtained were statistically assessed using the Mann-Whitney and the unpaired Student’s t test. Data are expressed as mean±standard deviation (SD), and p values of <0.05 were considered significant.
In-gel digestion
In-gel digestion of protein spots on silver-stained gels was performed as previously described [38]. Excised gel pieces were destained in 30 mM potassium ferricyanide and 100 mM sodium thiosulfate, and then rinsed several times with 150 μL of distilled water until the yellow color of the ferricyanide completely disappeared. Each gel piece was dehydrated in 100% ACN acetonitrile (ACN) until it became opaque and was rehydrated with 100 mM ammonium bicarbonate (NH4HCO3) until transparent. This dehydration and rehydration process was repeated 3 times, and was followed by a single dehydration in 100% ACN. The gel pieces were then dried in a vacuum centrifuge and rehydrated at 4°C for 45 min in digestion buffer containing modified porcine trypsin in 50 mM NH4HCO3 at a concentration of 0.01 μg/μL (Promega, Madison, WI, USA). Excess supernatant was removed and gel pieces were soaked in 30 μL of 50 mM NH4HCO3 overnight (16 h) at 37°C. The resulting tryptic fragments were eluted from the excised gels by diffusion into 0.5% trifluoroacetic acid, 50% ACN and 100% ACN, and transferred into new tubes.
Protein identification and database searching
Liquid chromatography tandem mass spectrometry (LC-MS/MS) was performed using a QTOF II mass spectrometer (Micromass, Manchester, UK). Samples were loaded onto a 300 μM (internal diameter) × 5 mm (length) (C18, 100 Å pore size, 5 μm diameter) guard column using a FAMOS autosampler (LC Packings, Sunnyvale, CA, USA). Peptide separation was carried out on a 75 μm × 10 cm fused silica microcapillary reverse phase column using an Ultimate apparatus (LC Packings). The A and B mobile phases were composed of 0% and 80% ACN, respectively; each also contained 0.5% acetic acid and 0.02% formic acid. The gradient was started at 5% B for 20 min, and then ramped to 20% B for 3 min, 60% for 30 min, 100% for 2 min, held at 100% for 7 min, and finally at 5% for 2 min. The column was equilibrated with 5% B for 9 min before the next run. During gradient elution, 3 tandem mass spectrometry (MS/MS) spectra were acquired per data-dependent cycle from the MS master spectrum. A threshold of 10 (counts/s) was set to trigger an MS/MS event. The electrospray was set at 2.5 kV, and specific m/z values of the peptide fragmented by collision-induced dissociation (CID) were excluded from reanalysis for 1 min. The spectra were used to search the IPI human database (IPI.HUMAN.v.3.27) to identify proteins via Mascot Daemon (Matrix Science, London, UK). In general, a peptide tolerance of 1.5 Da, MS/MS tolerance of 0.8 Da, 1 missed trypsin cleavage, oxidation of Met, and fixed modification of carbamidomethyl cysteine were selected as matching parameters for the search program. We initially used an MS tolerance of 0.8 Da. However, for the peptides with large molecular weight (of more than about 2,000 MW), the next monoisotope ion can be selected for the MS/MS fragmentation in data-dependant MS/MS mode because of their isotope distribution. In this case, it was crucial that M+1 ion was selected as the precursor ion. They showed mass tolerance of 1Da, compared to theoretical peptide monoisotope MW. In order to include this case, we used a peptide tolerance of 1.5 Da in mascot search. However, Q-TOF MS provides good resolution, and identified peptides with M or M+1 isotope ion showed mass tolerance less than 0.5 Da compared to its theoretical M or M+1 ion, respectively. The spectra were used to search the IPI human database (IPI.HUMAN.v.3.27) to identify proteins via Mascot Daemon (version 2.0, Matrix Science, London, UK). In general, a peptide tolerance of 1.5 Da, MS/MS tolerance of 0.8 Da, 1 missed trypsin cleavage, oxidation of Met, and fixed modification of carbamidomethyl cysteine were selected as matching parameters for the search program. Protein identification was carried out using at least 2 peptides from the protein database search.
Western Blot Analysis
Electrophoresis and blotting were performed using the BioRad Criterion system to analyze acetone precipitated proteins from meningioma CSF samples treated with protease inhibitor cocktail and phosphatase inhibitor. Samples containing equal amounts of CSF protein (10 μg) were subjected to 12.5% SDS-PAGE (Sigma-Aldrich, St. Louis, MO, USA) in a Bio-Rad mini gel electrophoretic unit at a constant voltage of 60~80 mA until the dye front was near the end of the gel. The resolved proteins were then transferred to a polyvinylidene fluoride membrane at 300 mV for 90 min at 4°C using a wet transfer system (Bio-Rad). Each membrane was blocked with 5% bovine albumin serum (BSA) (Sigma-Aldrich) in 0.1% TBS (1 mL/L Tween-20 in 0.01 mmol/L phosphate-buffered saline, pH 7.4 [PBS]) for 30 min. Each membrane was then incubated overnight at 4°C with mouse monoclonal antibodies against apolipoprotein E (ApoE; Abcam, Cambridge Science Park, UK; 1:5,000 dilution), apolipoprotein J precursor (ApoJ; Abcam; 1:2,000 dilution), AAT (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:5,000 dilution), beta-2-microglobulin (β2M; Abcam; 1:1,000 dilution), prostaglandin D2 synthase 21kDa (PTGDS; ProteinTech, Chicago, IL, USA; 1:2,000 dilution), or transthyretin precursor (TTR; ProteinTech; 1:1,000 dilution) in blocking buffer (5% bovine serum albumin in TBS), independently. Membranes were then washed with 1 mL/L Tween-20 in 0.01 mmol/L PBS and incubated for 1 h at room temperature with horseradish peroxidase (HRP) conjugated-goat anti-mouse and HRP conjugated-anti-rabbit IgG (Zymed Laboratories, San Francisco, CA, USA) diluted 1:2,000 in blocking buffer. Immunoblots were detected using enhanced chemiluminescence Western blotting detection reagents and Amersham ECL Western Blotting Systems (Amersham International, Little Chalfont, UK) according to the manufacturer’s instructions. Chemiluminescence was measured using AGFA medical X-ray film blue.
Results
Proteomic Analysis of human CSF
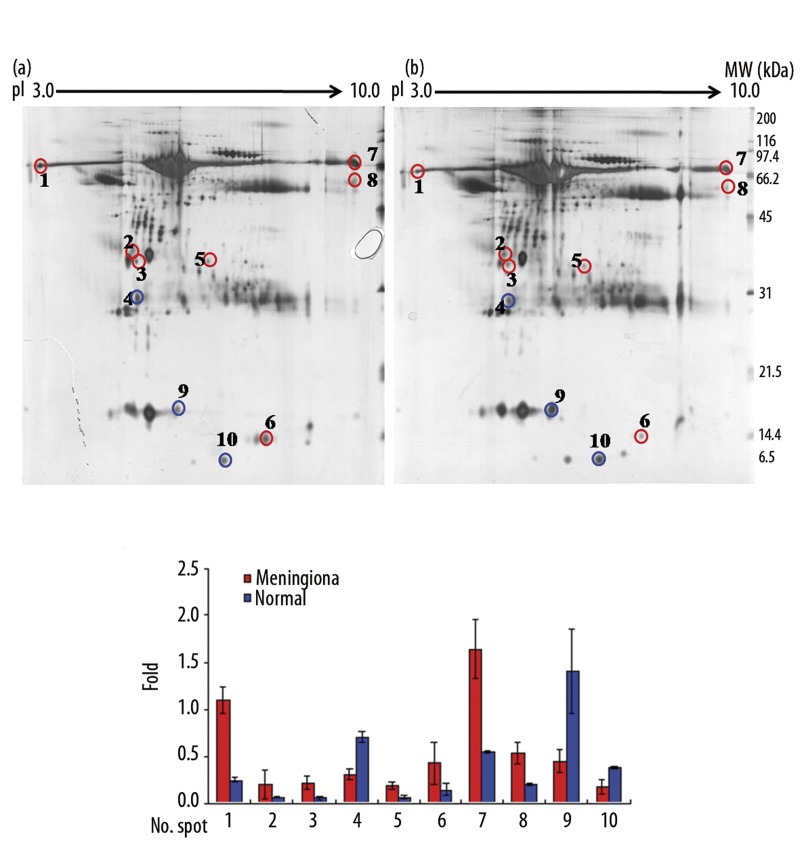
To establish protein profiles in CSF, we performed 2-DE on 10 CSF samples (5 benign meningiomas and 5 non-tumorous lesions) over a pH range 3–10 (Figure 1). The available clinical information is included in Table 1. Consistently expressed protein patterns and individual proteins were selected using the t test (p<0.05). The 2 groups were analyzed in 3 independent gels, and protein spots of interest were identified by electrospray quadrupole time-of-flight tandem mass spectrometry (ESI-Q-TOF MS/MS). Consistent differences between the expression profiles of CSF from patients with a meningioma or a non-brain tumor were elucidated.
Figure 1.
2-D gel electrophoretic separation of a human CSF proteome. Approximately 150 μg of protein was used. (A) Acetone-precipitated CSF samples from patients with (a) meningioma, and (b) from patients with a non-brain tumor were analyzed by 2-DE analysis using IEF strips, pH 3–10, and 12.5% SDS-PAGE. Spots were visualized by silver staining and spot patterns were analyzed using Progenesis workstation version 2005 software. Samples were run in triplicate. (B) Representative quantifications of CSF proteins in meningioma and non-brain tumor patients. The gels shown represent one of triplicate samples.
Table 1.
Clinical characteristics of analyzed patientsa.
| Sample code | Pathlogic finding | Location | Age | Sex | Conc (μg/ml) |
|---|---|---|---|---|---|
| Me1 | Menigotheliomatous | Tuberculum sellar meningioma, midline | 64 | F | 0.66424 |
| Me2 | Transitional, WHO G1 | Meningioma Rt optic sheath | 54 | F | 0.66393 |
| Me3 | Menigotheliomatous | Olfactory groove meningioma, midline | 62 | F | 0.5388 |
| Me4 | Angiomatous | Convexity meningioma, Lt. F-T | 37 | M | 0.49706 |
| Me5 | Menigotheliomatous | Falx | 34 | M | 0.36897 |
| Me6 | Psammomatous | Left frontal | 67 | F | 0.33285 |
| Me7 | Fibrous | Left frontal | 72 | F | 0.29396 |
| Me8 | Transitional | Right frontal | 62 | F | 0.24383 |
| Me9 | Meningotheliomatous | Left side tentorium | 64 | F | 0.22579 |
| Me10 | Atypical | Intraventricle | 59 | F | 0.13212 |
| Me11 | Fibrous type | Intraventricle | 44 | F | 0.02865 |
| N1 | Hydrocephalus | – | 71 | M | 1.07824 |
| N2 | Hydrocephalus | – | 68 | M | 0.78305 |
| N3 | Hydrocephalus | – | 46 | M | 0.50279 |
| N4 | Cerebral infarction, rt temporal | – | 55 | M | 0.33872 |
| N5 | Hydrocephalus | – | 61 | F | 0.23315 |
Me – Meningioma CSF; N – control CSF; F – female; M – male; Conc – concentration.
Ten protein spots differently expressed in CSF samples of meningioma patients
To compare protein expression profiles in the CSF samples of meningioma and non-brain tumor patients, 150 μg protein samples from each group were resolved on IEF strips and separated by 12.5% SDS-PAGE in the second dimension (Figure 1A). Of the more than 1,000 protein spots detected on each gel, 10 distinguishing protein spots differentially expressed by more than 2-fold in either the meningioma or non-brain tumor group were identified (Figure 1B). Seven of these 10 proteins were overexpressed in the CSF of meningiomas and the other 3 were underexpressed (Table 2).
Table 2.
Identification of differentially expressed protein spots in the CSF of meningioma and non-brain tumor patients.
| Spot noa | Protein name (Gene symbol) | IPI number | Mowse scoreb | Mw (Da)/pIc | Sequence Determined by TOF-TOF | No of peptide matched | Coverage (%)d | Intensity averagee | Fold changef |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Serum albumin precursor | IPI00384697 | 210 | 69180/5.91 | K.LVAASQAALGL.- K.SLHTLFGDK.L K.AVMDDFAAFVEK.C R.RHPDYSVVLLLR.L K.VPQVSTPTLVEVSR.N K.DVFLGMFLYEYAR.R + Oxidation (M) R.HPYFYAPELLFFAK.R K.VFDEFKPLVEEPQNLIK.Q |
8 | 16 | Men: 1.10±0.14 Con: 0.24±0.03 |
+4.51 |
| 2 | Apolipoprotein E | IPI00383266 | 510 | 36185/5.81 | R.WELALGR.F R.FWDYLR.W R.QWAGLVEK.V R.LAVYQAGAR.E R.LGPLVEQGR.V R.LQAEAFQAR.L K.LEEQAQQIR.L R.QQTEWQSGQR.W + Pyro-glu (N-term Q) R.AKLEEQAQQIR.L K.SWFEPLVEDMQR.Q + Oxidation (M) K.VQAAVGTSAAPVPSDNH.- K.VQAAVGTSAAPVPSDNH.- R.GEVQAMLGQSTEELR.V R.GEVQAMLGQSTEELR.V K.SELEEQLTPVAEETR.A K.AYKSELEEQLTPVAEETR.A R.WVQTLSEQVQEELLSSQVTQELR.A |
17 | 48 | Men: 0.20±0.15 Con: 0.07±0.00 |
+2.87 |
| 2 | Apolipoprotein J precursor | IPI00795363 | 167 | 48772/6.27 | K.TLLSNLEEAK.K R.ELDESLQVAER.L R.ASSIIDELFQDR.F K.LFDSDPITVTVPVEVER.K K.LFDSDPITVTVPVEVSR.K |
5 | 12 | Men: 0.20±0.15 Con: 0.07±0.00 |
+2.87 |
| 3 | Unidentified | – | – | – | – | – | – | – | – |
| 4 | Prostaglandin D2 synthase 21kDa | IPI00644952 | 93 | 21015/7.66 | R.TMLLQPAGSLGSYSYR.S R.TMLLQPAGSLGSYSYR.S R.TMLLQPAGSLGSYSYR.S R.TMLLQPAGSLGSYSYR.S + Oxidation (M) R.TMLLQPAGSLGSYSYR.S + Oxidation (M) K.AQGFTEDTIVFLPQTDK.C K.AQGFTEDTIVFLPQTDK.C |
7 | 17 | Men: 0.31± 0.06 Con: 0.70±0.05 |
−2.29 |
| 4 | Proapolipoprotein | IPI00021841 | 112 | 28944/5.45 | R.QGLLPVLESFK.V K.DLATVYVDVLK.D K.VSFLSALEEYTK.K R.DYVSQFEGSALGK.Q |
4 | 18 | Men: 0.31± 0.06 Con: 0.70±0.05 |
−2.29 |
| 5 | Uncharacterized protein albumin | IPI00022434 | 190 | 71530/6.33 | K.YLYEIAR.R K.LVNEVTEFAK.T K.AAFTECCQAADK.A+2 Carbamidomethyl (c) K.YICENQDSISSK.L+ Carbamidomethyl (c) K.QEPERNECFLQHK.D+ Carbamidomethyl (c);pyro-glu (N-term Q) R.RHPYFYAPELLFFAK.R |
6 | 11 | Men: 0.19±0.04 Con: 0.07±0.02 |
+2.85 |
| 6 | Chain D, Hemoglobin Ypsilanti | IPI00796636 | 212 | 15905/7.26 | K.SAVTALWGK.V R.LLVVYPWTQR.F R.LLVVYPWTQR.F K.VNVDEVGGEALGR.L K.EFTPPVQAAYQK.V K.EFTPPVQAAYQK.V K.VLGAFSDGLAHLDNLK.G K.VLGAFSDGLAHLDNLK.G K.VLGAFSDGLAHLDNLK.G R.FFESFGDLSTPDAVMGNPK |
10 | 54 | Men: 0.43±0.23 Con: 0.15±0.06 |
+2.92 |
| 7 | Serum albumin precursor | IPI00384697 | 489 | 69180/5.91 | K.YLYEIAR.R K.FQNALLVR.Y K.LVAASQAALGL.- K.SLHTLFGDK.L K.KQTALVELVK.H K.LVNEVTEFAK.T K.AVMDDFAAFVEK.C K.AVMDDFAAFVEK.C + Oxidation (M) R.RHPDYSVVLLLR.L K.VPQVSTPTLVEVSR.N K.VPQVSTPTLVEVSR.N K.DVFLGMFLYEYAR.R + Oxidation (M) K.DVFLGMFLYEYAR.R + Oxidation (M) K.KVPQVSTPTLVEVSR.N K.AEFAEVSKLVTDLTK.V R.HPYFYAPELLFFAK.R R.RHPYFYAPELLFFAK.R K.VFDEFKPLVEEPQNLIK.Q |
18 | 25 | Men: 1.65±0.31 Con: 0.55±0.02 |
+3.02 |
| 8 | Alpha-1-antitrypsin | IPI00553177 | 146 | 46677/5.43 | K.LSITGTYDLK.S K.FNKPFVFLMIEQNTK.S K.DTEEEDFHVDQVTTVK.V R.TLNQPDSQLQLTTGNGLFLSEGLK. |
4 | 15 | Men: 0.53±0.12 Con: 0.20±0.01 |
+2.73 |
| 9 | Transthyretin precursor | IPI00022432 | 262 | 15877/5.52 | K.ALGISPFHEHAEVVFTANDSGPR.R K.TSESGELHGLTTEEEFVEGIYK.V R.YTIAALLSPYSYSTTAVVTNPKE.- R.RYTIAALLSPYSYSTTAVVTNPK.E K.TSESGELHGLTTEEEFVEGIYKVEIDTK.S K.TSESGELHGLTTEEEFVEGIYKVEIDTK.S |
6 | 51 | Men: 0.45±0.12 Con: 1.41±0.45 |
−3.10 |
| 10 | Beta-2-microglobulin precursor | IPI00004656 | 112 | 13706/6.06 | R.VNHVTLSQPK.I R.VNHVTLSQPK.I K.VEHSDLSFSK.D K.SNFLNCYVSGFHPSDIEVDLLK.N + Carbamidomethyl (C) |
4 | 35 | Men: 0.18±0.08 Con: 0.38±0.01 |
−2.17 |
Spot numbers were defined according to position in 2-DE gels;
Individual ions scores >40 indicate identity or extensive homology (p<0.05);
Theoretical Mr (Da)/pI are based on the amino acid sequence of the identified protein;
Amino acid sequence coverage of the identified proteins;
Average ratio for levels in the CSFs of meningioma and non-brain tumor patients. Values are the averages of at least three results. (Men – meningioma, Con – non-brain tumor);
Spot intensity was increased (+) or decreased (−) in CSFs of meningioma patients vs. non-brain tumor patients.
Identification of meningioma associated proteins
We performed discriminatory analysis based on the expressions of the 10 protein spots. The selected spots were excised and analyzed by ESI-Q-TOF MS/MS (Supplemental data). The 10 protein spots were identified by positive gel matching of protein expression patterns with existing 2-DE gel databases (Figure. 1A). Eleven proteins were identified in the 9 protein spots (spots #2 and #4 contained 2 proteins each (Table 2) using Mowse scores (Individual ions scores >39, identity or extensive homology p<0.05). The other protein spot (spot #3 in Figure 1) was not identified. Albumin was identified in multiple spots (spot #1, #5 and #7, in Figure 1 and Table 2). Multiple spots on 2-D gels are presumably due to post-transcriptional modification, expression of differential isoforms derived from different genes, or proteolytic degradation of proteins in vivo and in vitro. It is well known that single genes can give rise to several isoforms, modified forms, and sliced forms of proteins [14,15].
Gene ontology analysis
The 10 proteins were analyzed using FatiGO (http://fatigo.bioinfo.cnio.es) and AmiGO (http://amigo.geneontology.org). Based on this analysis, proteins were divided into groups by biological process. The largest group was composed of 4 proteins involved in cellular metabolic processes: Apo J, Proapolipoprotein, Chain D Hemoglobin Ypsilanti, and TTR. The largest molecular function category was protein binding, which contained 6 proteins. This was followed by high-density lipoprotein binding, endopeptidase inhibitor activity, and receptor binding. In terms of cellular component classification, the major class was the extracellular region, which was composed of 4 proteins.
Western blot and immunohistochemical analyses for the verification of biomarkers identified from meningioma patients
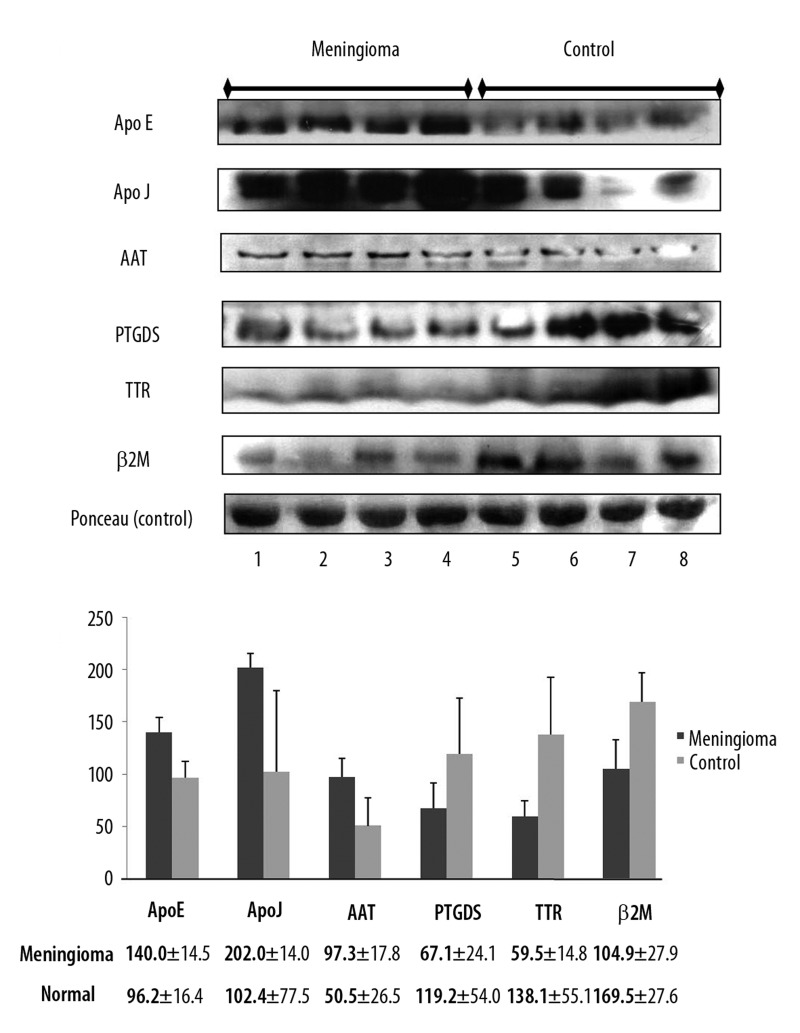
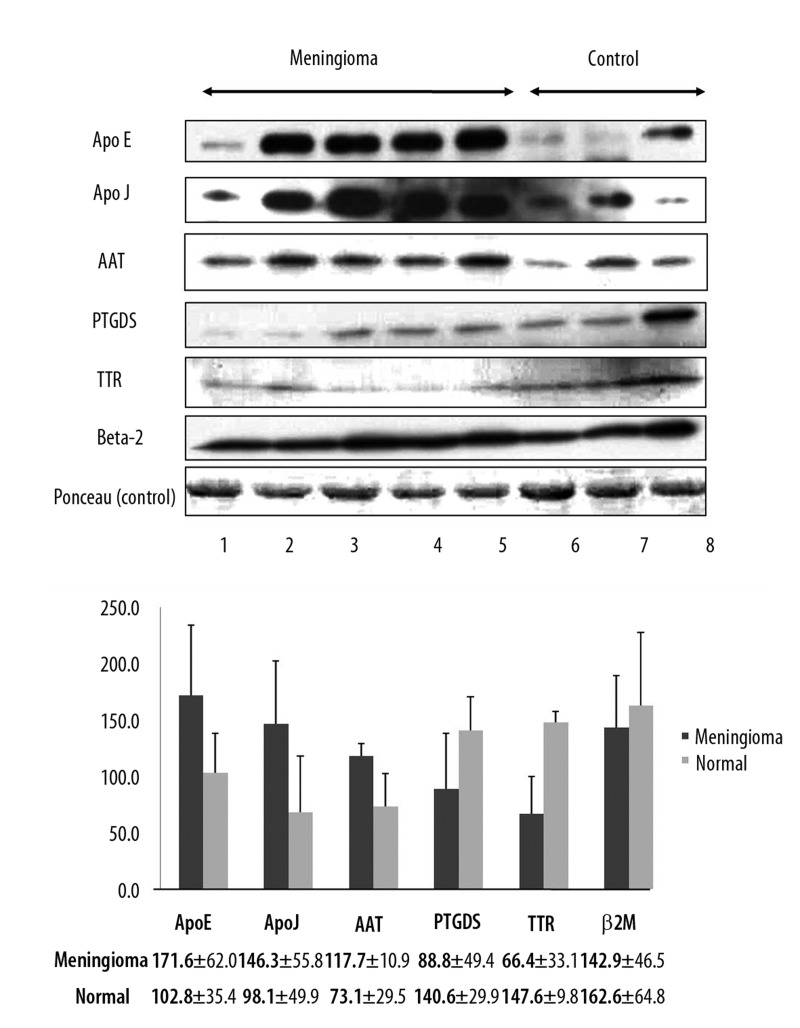
CSF proteins found to be differentially expressed by 2-DE were confirmed by Western blot and immunohistochemical analyses. All the CSF samples (5 benign meningiomas [Me1–5], and 5 non-tumorous lesions [N1–5]) used in 2-DE experiments were also used in Western blot and immunohistochemical analyses. We performed Western blot analysis of an additional 4 CSF samples from patients with meningioma for verification (Me6–9, Table 1, and Figure 2). Western blot analysis was performed using 6 selected CSF proteins for which antibodies are commercially available: Apo E, Apo J, AAT, PTGDS, TTR, and β2M. For Western blot experiment, ponceau red staining was used as an internal control. Because CSF does not have cytoskeleton proteins such as beta-actin, which is usually used as an internal control marker, we performed semi-quantitative analysis for Western blot by using the image J software program, which shows band intensity. Protein expression levels as determined by Western blot corresponded to the results obtained by 2-DE (Figure 2A) and band quantification was confirmed by Image Gauge version 4.0 (FUJI PHOTO FILM CO., LTD) (Figure 2B). For additional verification, we also performed Western blot analysis with 4 additional CSF samples (Me6–9 in Table 1) from patients with meningioma in which protein concentration was not high enough to run 2-DE, but was high enough to be analyzed by Western blot. The other 2 CSF samples (Me10–11 in Table 1) from patients with meningioma had insufficient protein concentration to be analyzed by Western blot. Protein expression pattern from additional samples were similar to those of Figure 2 (Figure 3A, B).
Figure 2.
Verification of the expression of six identified proteins by Western blotting. (A) Lanes 1–4 contain meningioma CSF; lanes 5–8 contain non-brain tumor CSF. Ponceau red staining of total protein levels served as an internal control. (B) Comparison of the quantifications of six proteins identified in the CSF samples of meningioma and non-brain tumor patients.
Figure 3.
Western blot for verification of additional CSF sample from meningioma. (A) Lanes 1–5 contain meningioma CSF; lanes 6–8 contain non-brain tumor CSF. Ponceau red staining of total protein levels served as an internal control. (B) Comparison of the quantification of six proteins identified in the CSF samples of meningioma and non-brain tumor patients.
Discussion
Proteomics is a broad protein profile screening approach that permits the direct analysis of proteins expressed differentially or uniquely by cells or tissues. Proteomic research has been developed after the definition of the human genome. Using a combination of 2-DE, mass spectrometry, and bioinformatics, a particular cell type can be readily scanned and its protein expression pattern identified. Furthermore, recent studies have shown that specific proteomic patterns and proteins can differentiate subtypes or grades of human brain tumors [11,12,16]. In this study, we identified biologically relevant protein markers secreted into the CSF [11] to determine if proteins or protein patterns can be used as diagnostic biomarkers of meningiomas.
Genotypes of benign brain tumors are more homogenous than those of malignant brain tumors; therefore, we investigated more homogenous and common meningiomas. CSF is well-suited for sampling, and its accessibility by lumbar puncture is relatively non-invasive [6]. Furthermore, human CSF has been used as an important source for protein biomarker studies [17].
The CSF circulates within the ventricles of the brain, and surrounds the brain and bone marrow in the subarachnoid space [18]; more than 65% of its total protein content is accounted for by albumin and immunoglobulin. Furthermore, CSF has a high salt concentration and a low protein concentration [19]. We did not remove these abundant proteins, because the proteins could also be differentially expressed in the CSF of the patients with or without a meningioma. In this study, we used acetone precipitation to obtain an appropriate sample preparation and increase protein recovery.
Our study, similar to others in the literature [20–23], had a small sample size, because it was difficult to obtain a good quality of CSF samples from the patients with brain tumors. We verified 3 replications in each sample to overcome the problem of small sample size.
Proteomics combines high-resolution 2-DE, high-sensitivity MS, and continuously expanding protein databases [24]. To identify specific proteins that might be correlated with morphological changes, we generated 2-DE maps for proteome analysis. Using 2-DE, we found that 7 secreted protein spots were expressed at high levels in the majority of CSF of samples from patients with meningioma, and that 3 protein spots were expressed at lower levels. Furthermore, these protein expressions identified by 2DE were also confirmed by Western blot using meningioma tissues. In a previous study, Apolipoprotein A-II was found to be highly expressed in the CSF of a pediatric brain tumor patient, and this protein was associated with a disrupted blood-brain barrier [25]. It was recently reported that ApoE has been found in normal human brain tissue and in human intracranial neoplasm; it is a 34 kDa lipophilic protein and circulates in plasma as a constituent of lipoproteins [26]. On the other hand, ApoJ is a major carrier protein of soluble circulating amyloid β in body fluids, and may keep the peptide in a soluble form and is thus considered to have an anti-amyloidogenic effect [27]. However, our results show that ApoE and ApoJ are possible biomarkers of meningioma.
In a previous study, transthyretin was found to be expressed by most benign and malignant choroids plexus tumors; however, gliomas and meningiomas do not express transthyretin [28]. In our study, transthyretin was found to be more highly expressed in the CSF of patients with a non-brain tumor. Prostaglandin D synthase is regarded as a useful biomarker of early diabetic nephropathy and the early phase of gentamicin-induced renal impairment, and has been considered a specific cell marker of meningioma [29–31]. However, our study shows that prostaglandin D synthase expression levels in meningioma patients were lower than in the non-brain tumor patients. Furthermore, our study indicates that β2M was down-regulated in patients with meningioma, whereas it has been reported to be up-regulated in prostate cancer growth-stimulating factor [32]. All of the proteins identified in the present study are detailed in Table 2. Four of the 11 identified proteins were found to be related to cellular metabolism, and 6 were found to be related to protein binding, primarily in the extracellular region.
Briefly, we used human CSF to obtain pure proteins from patients with meningioma and subjected these to 2-DE analysis and definitive ESI-Q-TOF protein sequencing to identify proteomic profiles and proteins that differentiate meningioma and non-brain tumors. The proteomic experimental methods used were found to be simple, accurate, and reliable. These possible biomarker proteins that we identified in the patients with meningiomas are proportionally high in human CSF [33].
Conclusions
In conclusion, we found 11 independent candidate proteins in 10 protein spots, differentially expressed by more than 2-fold in the CSF samples of patients with or without meningioma. Seven protein spots were up-regulated and 3 were down-regulated in patients with meningioma. Proteome-based clustering findings were found to be significantly correlated with Western blot findings using excised tissues and CSF samples. Our observations and findings suggest that this proteomics study of CSF might be used in diagnosis of brain tumor without surgical resection.
Proteins identification with mass spectrum by ESI Q TOF.
Acknowledgments
We sincerely thank the mass spectrometry team at the Korea Basic Science Institute for the protein identification. YMP was partly supported by a grant (2009K001266) from the Brain Research Center, The 21st Century Frontier Research Program of the Ministry of Education, Science and Technology, Republic of Korea; by a grant from the National Research Foundation of Korea Grant funded by the Korean Government (MEST)” (2012, University-Institute cooperation program); and by a grant from the Korea Basic Science Institute project “Operation of the Advanced Multi-purpose Mass Spectrometers” (G30124). All authors participated in interpretation of findings. YMP and KGC were responsible for conception and design of the study. JHK performed molecular biology experiments and protein identification, and also drafted the manuscripts. YMP and KGC modified and commented on the draft. SKL: Analyzed data. YCY: Analyzed data. NHP: Performed experiments. DBP: Performed experiments. JSY: Designed experiments. HJA: Wrote the manuscript. All authors read and approved the final version of the paper. All authors confirm that the content has not been published elsewhere and does not duplicate their published work.
Footnotes
Source of support: YMP was partly supported by a grant (2009K001266) from the Brain Research Center, The 21st Century Frontier Research Program of the Ministry of Education, Science and Technology, Republic of Korea, by a grant from the National Research Foundation of Korea Grant funded by the Korean Government (MEST) (2012, University-Institute cooperation program), and by a grant from the Korea Basic Science Institute project “Operation of the Advanced Multi-purpose Mass Spectrometers” (G30124)
References
- 1.Khwaja FW, Reed MS, Olson JJ, et al. Proteomic identification of biomarkers in the cerebrospinal fluid (CSF) of astrocytoma patients. J Proteome Res. 2007;6:559–70. doi: 10.1021/pr060240z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan X, Desiderio DM. Proteomics analysis of human cerebrospinal fluid. J Chromatogr. 2005;815:179–89. doi: 10.1016/j.jchromb.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 3.Le Bastard N, Martin JJ, Vanmechelen E, et al. Added diagnostic value of CSF biomarkers in differential dementia diagnosis. Neurobiol Aging. 2010;31(11):1867–76. doi: 10.1016/j.neurobiolaging.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Tarnaris A, Watkins LD, Kitchen ND. Biomarkers in chronic adult hydrocephalus. Cerebrospinal Fluid Res. 2006;3:11. doi: 10.1186/1743-8454-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zougman A, Pilch B, Podtelejnikov A, et al. Integrated analysis of the cerebrospinal fluid peptidome and proteome. J Proteome Res. 2008;7:386–99. doi: 10.1021/pr070501k. [DOI] [PubMed] [Google Scholar]
- 6.Khwaja FW, Duke-Cohan JS, Brat DJ, Van Meir EG. Attractin is elevated in the cerebrospinal fluid of patients with malignant astrocytoma and mediates glioma cell migration. Clin Cancer Res. 2006;12:6331–36. doi: 10.1158/1078-0432.CCR-06-1296. [DOI] [PubMed] [Google Scholar]
- 7.Roy S, Josephson SA, Fridlyand J, et al. Protein biomarker identification in the CSF of patients with CNS lymphoma. J Clin Oncol. 2008;26:96–105. doi: 10.1200/JCO.2007.12.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwadate Y, Sakaida T, Hiwasa T, et al. Molecular classification and survival prediction in human gliomas based on proteome analysis. Cancer Res. 2004;64:2496–501. doi: 10.1158/0008-5472.can-03-1254. [DOI] [PubMed] [Google Scholar]
- 9.Ko KW, Nam DH, Kong DS, et al. Relationship between malignant subtypes of meningioma and clinical outcome. J Clin Neurosci. 2007;14:747–53. doi: 10.1016/j.jocn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Bruna J, Brell M, Ferrer I, et al. Ki-67 proliferative index predicts clinical outcome in patients with atypical or anaplastic meningioma. Neuropathology. 2007;27:114–20. doi: 10.1111/j.1440-1789.2007.00750.x. [DOI] [PubMed] [Google Scholar]
- 11.Vankalakunti M, Vasishta RK, Das Radotra B, Khosla VK. MIB-1 immunolabeling: a valuable marker in prediction of benign recurring meningiomas. Neuropathology. 2007;27:407–12. doi: 10.1111/j.1440-1789.2007.00801.x. [DOI] [PubMed] [Google Scholar]
- 12.Wulfkuhle JD, Paweletz CP, Steeg PS, et al. Proteomic approaches to the diagnosis, treatment, and monitoring of cancer. Adv Exp Med Biol. 2003;532:59–68. doi: 10.1007/978-1-4615-0081-0_7. [DOI] [PubMed] [Google Scholar]
- 13.Hanrieder J, Wetterhall M, Enblad P, et al. Temporally resolved differential proteomic analysis of human ventricular CSF for monitoring traumatic brain injury biomarker candidates. J Neurosci Methods. 2009;177:469–78. doi: 10.1016/j.jneumeth.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 14.Porubleva L, Vander Velden K, Kothari S, et al. The proteome of maize leaves: use of gene sequences and expressed sequence tag data for identification of proteins with peptide mass fingerprints. Electrophoresis. 2001;22:1724–38. doi: 10.1002/1522-2683(200105)22:9<1724::AID-ELPS1724>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Nam MH, Heo EJ, Kim JY, et al. Proteome analysis of the responses of Panax ginseng C. A. Meyer leaves to high light: use of electrospray ionization quadrupole-time of flight mass spectrometry and expressed sequence tag data. Proteomics. 2003;3:2351–67. doi: 10.1002/pmic.200300509. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto H, Li J, Vortmeyer AO, et al. Comparative proteomic profiles of meningioma subtypes. Cancer Res. 2006;66:10199–204. doi: 10.1158/0008-5472.CAN-06-0955. [DOI] [PubMed] [Google Scholar]
- 17.Pan S, Zhu D, Quinn JF, et al. A combined dataset of human cerebrospinal fluid proteins identified by multi-dimensional chromatography and tandem mass spectrometry. Proteomics. 2007;7:469–73. doi: 10.1002/pmic.200600756. [DOI] [PubMed] [Google Scholar]
- 18.Shintaku M, Hashimoto K, Okamoto S. Intraventricular meningioma with anaplastic transformation and metastasis via the cerebrospinal fluid. Neuropathology. 2007;27:448–52. doi: 10.1111/j.1440-1789.2007.00786.x. [DOI] [PubMed] [Google Scholar]
- 19.Yuan X, Russell T, Wood G, Desiderio DM. Analysis of the human lumbar cerebrospinal fluid proteome. Electrophoresis. 2002;23:1185–96. doi: 10.1002/1522-2683(200204)23:7/8<1185::AID-ELPS1185>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Tendulkar RD, Pai Panandiker AS, Wu S, et al. Irradiation of Pediatric High-Grade Spinal Cord Tumors. Int J Radiat Oncol Biol Phys. 2010;78(5):1451–56. doi: 10.1016/j.ijrobp.2009.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soga N, Kise H, Arima K, Sugimura Y. Third-line gemcitabine monotherapy for platinum-resistant advanced urothelial cancer. Int J Clin Oncol. 2010;15(4):376–81. doi: 10.1007/s10147-010-0071-8. [DOI] [PubMed] [Google Scholar]
- 22.Jin T, Hu LS, Chang M, et al. Proteomic identification of potential protein markers in cerebrospinal fluid of GBS patients. Eur J Neurol. 2007;14:563–68. doi: 10.1111/j.1468-1331.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- 23.van der Ham M, de Koning TJ, Lefeber D, et al. Liquid chromatography-tandem mass spectrometry assay for the quantification of free and total sialic acid in human cerebrospinal fluid. J Chromatogr. 2010;878(15–16):1098–102. doi: 10.1016/j.jchromb.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Yuan X, Desiderio DM. Protein identification with Teflon as matrix-assisted laser desorption/ionization sample support. J Mass Spectrom. 2002;37:512–24. doi: 10.1002/jms.307. [DOI] [PubMed] [Google Scholar]
- 25.de Bont JM, den Boer ML, Reddingius RE, et al. Identification of apolipoprotein A-II in cerebrospinal fluid of pediatric brain tumor patients by protein expression profiling. Clin Chem. 2006;52:1501–9. doi: 10.1373/clinchem.2006.069294. [DOI] [PubMed] [Google Scholar]
- 26.Murakami M, Ushio Y, Morino Y, et al. Immunohistochemical localization of apolipoprotein E in human glial neoplasms. J Clin Invest. 1988;82:177–88. doi: 10.1172/JCI113568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zlokovic BV. Cerebrovascular transport of Alzheimer’s amyloid beta and apolipoproteins J and E: possible anti-amyloidogenic role of the blood-brain barrier. Life Sci. 1996;59:1483–97. doi: 10.1016/0024-3205(96)00310-4. [DOI] [PubMed] [Google Scholar]
- 28.Albrecht S, Bayer TA, Kraus JA, Pietsch T. Transthyretin expression in medulloblastomas and medulloblastoma cell lines. Neuropathol Appl Neurobiol. 1995;21:399–409. doi: 10.1111/j.1365-2990.1995.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 29.Kawashima M, Suzuki SO, Yamashima T, et al. Prostaglandin D synthase (beta-trace) in meningeal hemangiopericytoma. Mod Pathol. 2001;14:197–201. doi: 10.1038/modpathol.3880285. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama H, Echizen H, Gomi T, et al. Urinary lipocalin-type prostaglandin D synthase: a potential marker for early gentamicin-induced renal damage? Ther Drug Monit. 2009;31:126–30. doi: 10.1097/FTD.0b013e31819566f1. [DOI] [PubMed] [Google Scholar]
- 31.Yamashima T, Sakuda K, Tohma Y, et al. Prostaglandin D synthase (beta-trace) in human arachnoid and meningioma cells: roles as a cell marker or in cerebrospinal fluid absorption, tumorigenesis, and calcification process. J Neurosci. 1997;17:2376–82. doi: 10.1523/JNEUROSCI.17-07-02376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi C, Zhu Y, Su Y, Chung LW, Cheng T. Beta2-microglobulin: emerging as a promising cancer therapeutic target. Drug Discov Today. 2009;14:25–30. doi: 10.1016/j.drudis.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Shores KS, Udugamasooriya DG, Kodadek T, Knapp DR. Use of peptide analogue diversity library beads for increased depth of proteomic analysis: application to cerebrospinal fluid. J Proteome Res. 2008;7:1922–31. doi: 10.1021/pr7006889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proteins identification with mass spectrum by ESI Q TOF.