Summary
Background
Cystatin C (cC) is a cysteine protease inhibitor that may influence immune response. Our aim was to test the effect of a high concentration of cC, characteristic for uremic patients, on neutrophil (PMN) apoptosis and respiratory burst, as well as the cC secretion from PMNs stimulated with proinflammatory cytokines.
Material/Methods
PMNs from 35 healthy volunteers aged 27–61 years were cultured in presence of cC, IL-1β or TNF-α. The percentage of apoptotic cells based on DNA depletion, Fas, FasL and caspase -3 expression were assessed. CC concentrations were determined by ELISA test. The influence of cC on spontaneous, fMLP-, PMA- or OZ-induced burst response of PMNs was tested using chemiluminescence.
Results
PMN cultured in the presence of cC resulted in a significant drop in apoptotic cell percentage (38% [11%; 65%]) compared both to control (70% [29%; 92%], and to the cells cultured with TNF-α (58% [24%; 85%]). These differences were not accompanied by Fas, FasL and caspase-3 expression changes. Spontaneous, fMLP- and PMA-stimulated oxidative burst of PMNs preincubated with cC were significantly downregulated. IL-1β markedly diminished and TNF-α significantly increased cC concentration in culture supernatants.
Conclusions
The presented results suggest that antiapoptotic activity of cC results from its inhibitory effect on ROS production. Thus, the higher concentration of cC characteristic for uremic patients may modulate acute inflammation through maintaining PMN longevity and inhibiting their respiratory burst and proinflammatory cytokine-related changes in cC release from PMNs.
Keywords: cystatin C, inflammation, neutrophil apoptosis, respiratory burst
Background
Cystatin C (cC) is a 120-amino acid, 13-kDa protein which belongs to the family of cysteine protease inhibitors [1]. CC is produced at a constant rate by nucleated cells (eg, cells of the immune system) [2]. CC is freely filtrated in the glomerulus and then reabsorbed and almost completely catabolized in the proximal renal tubule [3]; therefore its concentration in serum reflects the glomerular filtration rate (GRF) [4]. When compared to serum creatine level, cC seems to be less influenced by age, sex, muscle mass, and height [5–7] and it has therefore been proposed as an excellent marker of renal function. However, literature data concerning the impact of endogenous conditions other than renal function on cC concentration are conflicting; some of them indicated that its concentration is not influenced by infection or inflammatory diseases [8]. Others suggested that both inflammation and infection affect cC concentration. CC concentration in elderly persons without chronic kidney disease correlates with inflammatory markers like C-reactive protein (CRP) [9], interleukin-6 (IL-6), tumor necrosis factor α (TNF-α) and TNF-α-soluble receptors [10]. Chronic kidney disease is accompanied by chronic inflammation triggered by uremic toxins and recurrent acute inflammation caused by hemodialysis [11]. Warfel et al showed that LPS and interferon-γ (INF-γ) down-regulate release of cC by monocytes and macrophages [12]. On the other hand, cC inhibits macrophage response to interferon-γ [13]. cC blocks transforming growth factor β (TGF-β) activity [14], and an adverse feedback has also been observed [15]. The aforementioned information indicated that cC can act as a modulator of specific immune response, but there is little data on its connection with unspecific immunity. cC inhibits the growth of bacteria and virus replication [16,17] and interacts with C4, significantly inhibiting the lytic activity of the classic complement pathway and preventing formation of C3 convertase [18]. The modulatory effect of cystatin C on the chemotactic activity of neutrophils has also been shown [19]. In addition, cC influences the immune system and inflammation affects cC release from immune cells.
The aim of our study was to investigate whether a high concentration of cC characteristic for patients with chronic renal failure could influence neutrophil ability to undergo apoptosis and respiratory burst, and also whether proinflammatory cytokines such as IL-1β and TNF-α affect cC secretion from neutrophils.
Material and Methods
Subjects
The study involved 35 healthy volunteers (18 women and 17 men, median age 58, range 27–61) with glomerular filtration rate (GFR) >90 (ml/min/1.73) m2 estimated using a 4- variable modification of diet in renal disease equation (MDRD-4) as neutrophil (PMN) donors. Fifteen end-stage renal disease (ESRD) patients (7 women and 8 men, median age 63, range 24–71, MDRD-4 <15 (ml/min/1.73) m2) before the start of dialysis treatment served as blood serum donors. Inclusion of ESRD patients into this study was intended to evaluate the concentration of cC in uremic condition and used the same concentration of cC in cultured PMNs isolated only from healthy volunteers.
Informed consent was obtained from all donors. The protocol of the study was approved by the Regional Commission for Ethics in Research (RNN 156/05/KB).
Cells isolation
PMNs were isolated from heparinized peripheral blood drawn from healthy donors using density gradient centrifugation on Polymorphprep (Axis-Shield PoC As, Norway) at 450 × g at room temperature for 30 min. The lower phase, containing mainly PMNs and residual erythrocytes, was treated with hypotonic erythrocyte lysis solution. The PMNs were washed twice and resuspended in PBS.
Cell purity and viability were determined by May-Grűnwald-Giemsa staining and Trypan blue exclusion test, respectively. Experiments were conducted on populations of PMNs with purity and viability not lower than 98%.
Culture of neutrophils
PMNs were cultured at 1×106/ml in RPMI 1640 containing 10% fetal calf serum (FCS), 100 U of penicillin and 100 μg/ml of streptomycin in humidified atmosphere with 5% CO2 at 37°C. Then, the above mentioned PMNs suspensions were incubated in the presence of 5 ng/ml of IL-1β (Sigma Chemical Co., St. Louis, MO) or 100 ng/ml of TNF-α (Sigma Chemical Co., St. Louis, MO) or 10 μg/ml of cC (Alexis Biochemicals, San Diego, CA) in 24-well culture plates. The concentration of cC used in experiments was assessed on the basis of its concentration determined in the serum of ESRD patients (cC =9.87±2.51 μg/ml, n=15). Control samples formed PMNs suspensions incubated in the absence of IL-1β, TNF-α and cC. After 18 hours, the culture supernatants were collected and stored at −80°C for further analysis with Elisa tests. PMNs were harvested by washing the wells with cold PBS and then washing twice.
Our results were concordant with literature data concerning cC concentration [20].
Assessment of neutrophil apoptosis
PMNs (106/200 μl) harvested from cultures were resuspended in 200 μl of 0.5% saponin (Sigma Chemical Co., St. Louis, MO) and incubated in the dark at room temperature. After 10 min, PMNs were washed and incubated with ribonuclease A (10 μg/ml) (Sigma Chemical Co., St. Louis, MO) and 20 μg/ml of propidium iodide (PI, Sigma Chemical Co., St. Louis, MO) in the same conditions for the next 20 min. The fluorescence of individual nuclei was measured using a flow cytometer (FACScan, Becton Dickinson, San Diego, CA). The percentage of apoptotic PMNs was calculated as a hypodiploid DNA peak in the FL-2 fluorescence channel.
To confirm that culturing PMNs induced apoptotic but not necrotic changes we have also shown the presence of early apoptotic features in cultured PMNs such as surface phosphatidylserine exposure and nuclear active form of caspase-3 with the use of the Dual Apoptosis Assay Kit (Biotum, Inc., Hayward, USA). The kit contains NucView™ 488 Caspase-3 substrate and sulforhodamine 101-annexin V which enables detection of both apoptotic features in individual cells at the same time. The bright green fluorescence of the nucleus is both generated in response to caspase-3 activity and indicates morphological changes of the nucleus in the cells undergoing apoptosis. PMNs mounted onto a slide were observed under a fluorescence microscope using FITC and Texas-red filters.
Measurement of chemiluminescence (CL)
The measurement of CL was performed with a Luminometer 1251 (BioOrbit linked to an IBM PC AT).
CL response of PMNs to cC (10μg/ml) and its influence on chemiluminescence activated by fMLP (Sigma Chemical Co., St. Louis, MO) (1 nmol/ml), PMA (Sigma Chemical Co., St. Louis, MO) (200 ng/ml) or OZ (Sigma Chemical Co., St. Louis, MO) (0.3 mg/ml) were detected. Cells were preincubated with cC for 30 min and then washed with PBS. The PMNs CL was enhanced with luminol (Sigma Chemical Co., St. Louis, MO) diluted with 0.4% solution of NaOH to the concentration of 5 mg/ml. The test was conducted at 37.0±0.1°C. Each of the studied samples contained: 2×105 PMNs, 20 μl of luminal, and/or 2 μl of cC, 20 μl of fMLP, 30 μl OZ, and 20 μl of PMA. The samples were brought to a final volume of 1 ml with PBS.
The opsonization of zymosan was performed by suspending zymosan in the solution of PBS with autological plasma 1:1 (v/v). Then, the samples were incubated at 37.0°C for 30 min and centrifuged. After washing, zymosan was suspended in PBS and stored at a concentration of 10 mg/ml.
Each measurement was conducted in duplicate during 30 min. The CL was defined as the area under the light emission curve in the function of time (30 min). The area reflects the entire emission of light by the cells during the measurement [21].
Quantification of surface molecules expression
The expression of CD90 (Fas) (Ancell Corporation, Bayport, MN, USA) and CD95L (Fas-L) (Ancell Corporation, Bayport, MN, USA) were estimated using commercial direct monoclonal antibodies according to the manual. PMNs (105/100 μl) harvested from culture were washed and incubated with 80 μl of mouse anti-human CD95/FITC or anti-human CD95L/FITC monoclonal antibodies on ice in the dark. Appropriate isotype control (IgG/FITC, Ancell Corporation, Bayport, MN, USA) was prepared in each experiment.
Median fluorescence intensity (MFI) of the positively stained cells was detected by flow cytometry (FACScan flow cytometer, Becton Dickinson, San Diego, CA).
Assessment of cC concentration
cC concentration were determined in supernatants obtained from cultures carried out in the presence of IL-1β (5 ng/ml) or TNF-α (100 ng/ml). Quantification of cC (human ELISA Kit, Alexis Biochemicals, San Diego, CA) was performed by using immunoenzymatic methods according to the manufacturer’s instruction. Optical density was measured using a Diagnostics Pasteur LP 400 ELISA reader. All assays were conducted in duplicate and the concentrations were determined on the basis of the standard curves.
Statistical analysis
The results are expressed as the median, minimum and maximum in the text, and the lower and upper quartiles are shown in figures. The Mann-Whitney U test was used for comparison of the differences between unpaired groups, and the Wilcoxon test was used to compare different kinds of treatment of the same sample (paired groups). Statistical significance was assumed at P value <0.05.
Results
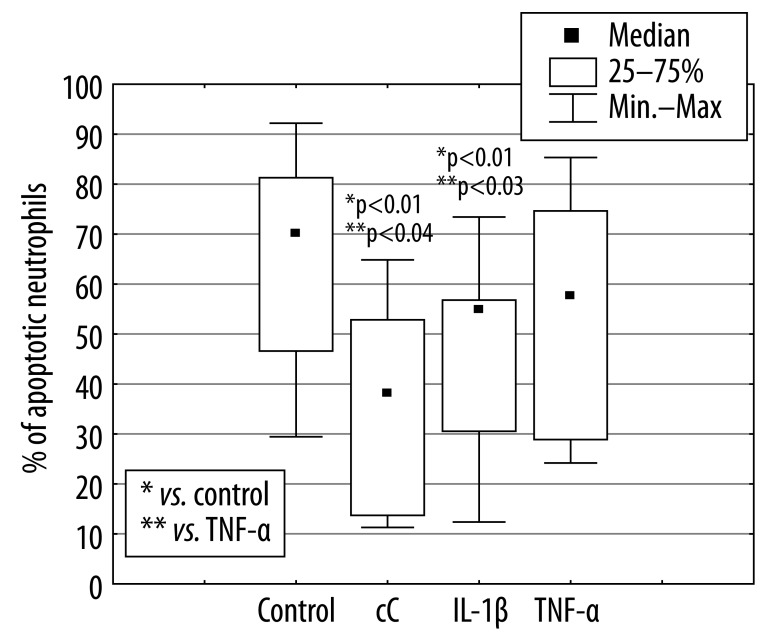
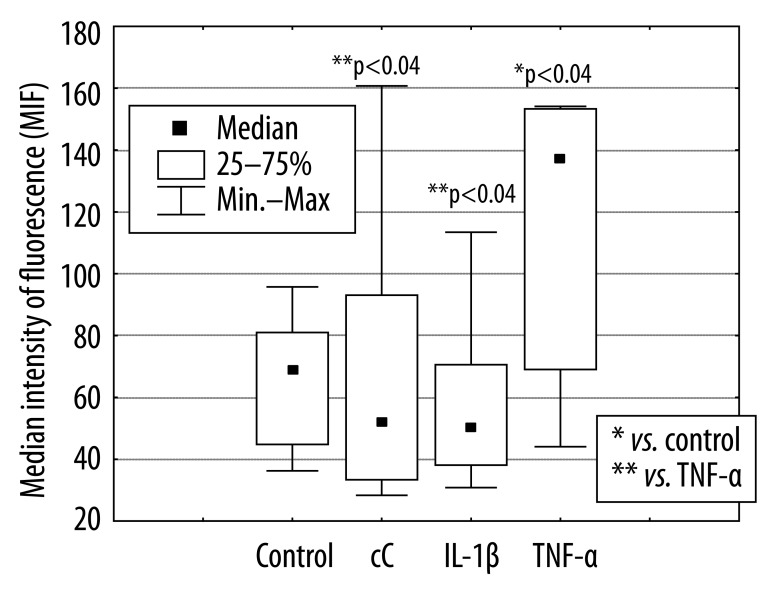
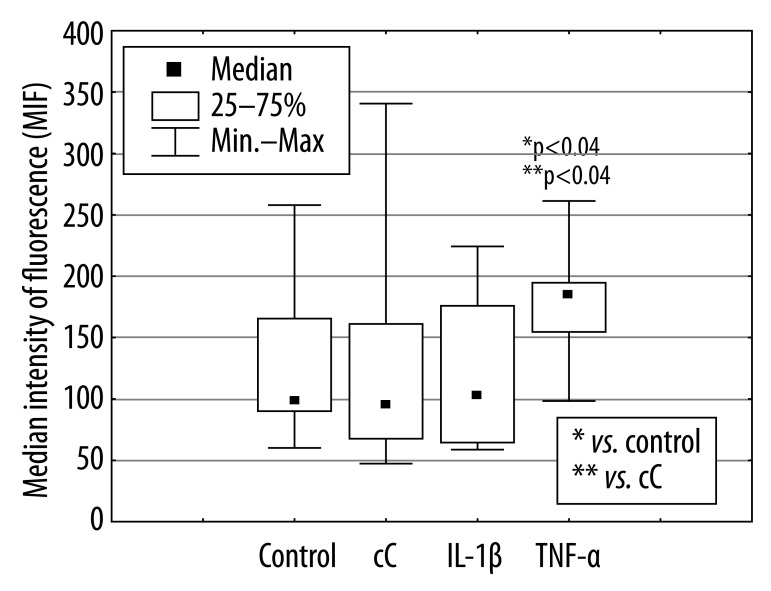
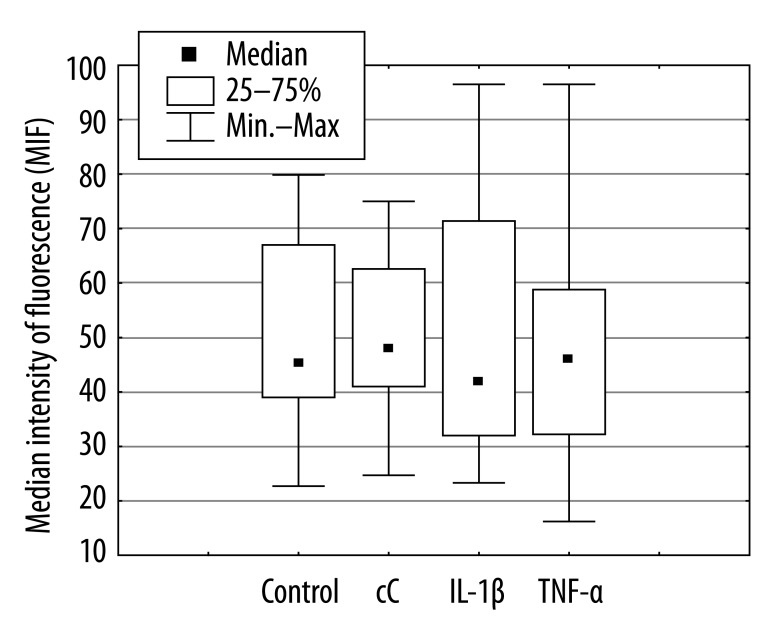
The percentage of apoptotic PMNs after 18 hours of culture in the presence of cC (38% [11%; 65%]) and IL-1β 55% [12%; 73%] were significantly lower in comparison to control cells incubated in RPMI 1640 only (70% [29%; 92%], *p<0.01) or cultured in the presence of TNF-α (58% [24%; 85%], **p<0.03, **p<0.04) (Figure 1). The effect of cC on PMN apoptosis was not accompanied by Fas and FasL expression changes (Figure 2A, B). Expression of Fas and FasL were significantly changed only on cells cultured in the presence of TNF-α. Despite the fact that active form of caspase-3 has been observed (Figure 3) in PMNs with the use of fluorescence microscopy, there was no differences in its expression measured by flow cytometry in different culture conditions (Figure 4), which were as follows: 45MFI [23MFI; 80MFI] in control cells and 48MFI [25MFI; 75MFI], 42MIF [23MFI; 96MFI], 46MFI [16MFI; 96MFI] in cells cultured in the presence of cC, IL-1β and TNF-α, respectively.
Figure 1.
Influence of cC (10 μg/ml), IL-1β (5 ng/ml) and TNF-α (100 ng/ml) on the percentage of apoptotic PMNs after 18 hours of culture.
Figure 2A.
Influence of cC (10 μg/ml), IL-1β (5 ng/ml) and TNF-α (100 ng/ml) on Fas expression on surface of PMNs after 18 hours of culture.
Figure 2B.
Influence of cC (10 μg/ml), IL-1β (5 ng/ml) and TNF-α (100 ng/ml) on Fas-L expression on PMNs after 18 hours of culture.
Figure 3.
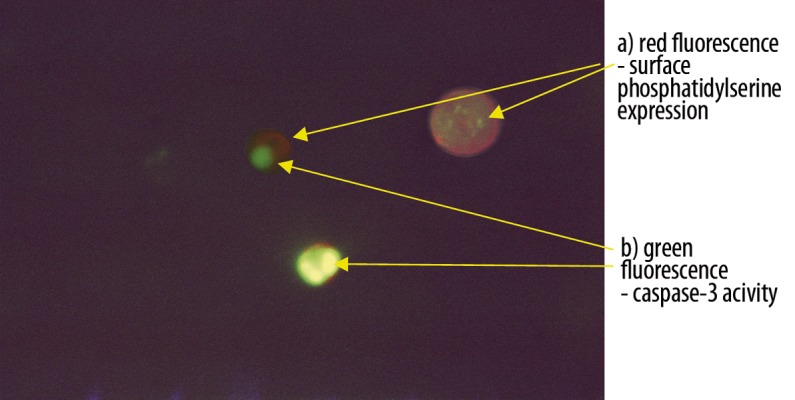
Dual staining of PMNs with NucView488 Caspase-3 and sulforhodamine101-annexinV after 18 hours of culture. The image shows 3 features of apoptosis: phosphatidylserine translocation (red fluorescence), active form of caspase -3 and chromatin condensation (green fluorescence) (magnification ×200). The photo was taken using Jenalumar fluorescent microscope (Carl Zeiss, Jena).
Figure 4.
Caspase-3 expression in PMNs cultured for 18 hours with cC (10 μg/ml), IL-1β (5 ng/ml) or TNF-α (100 ng/ml).
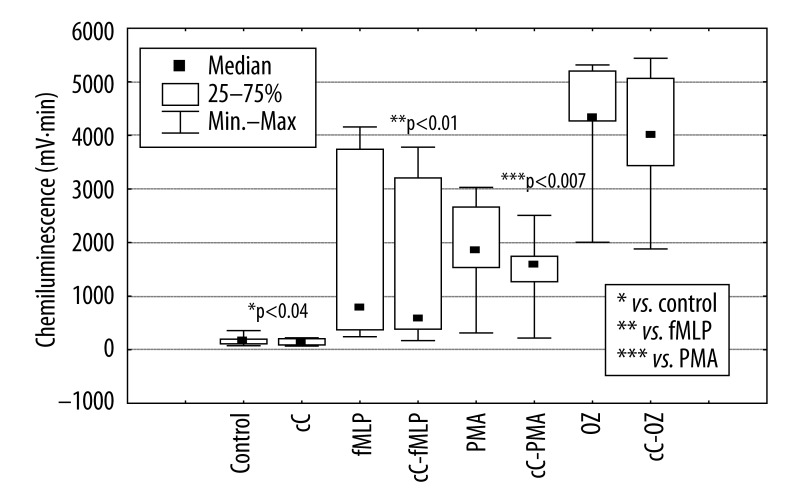
Thirty min preincubation of PMNs with cC (Figure 5) significantly (*p<0.04) diminished their spontaneous burst response (from 166 mV·min to 148 mV·min), also in comparison to that stimulated by fMLP (from 777 mV·min to 582 mV·min,** p<0.01) and PMA (from 1857 mV·min to 1580 mV·min, ***p<0.007). cC did not significantly change chemiluminescence of PMNs stimulated by OZ.
Figure 5.
The influence of cC (10 μg/ml) on respiratory response of PMNs stimulated by fMLP, PMA and OZ.
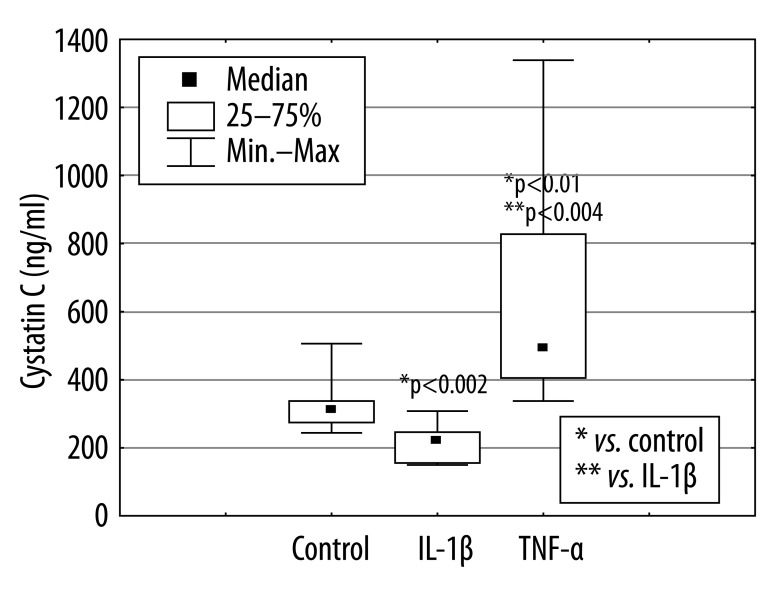
The presence of IL-1β in culture of PMNs significantly (*p<0.002) diminished cC concentration in supernatants from 312.3 ng/ml [244 ng/ml; 506 ng/ml] to 220 ng/ml [149.5 ng/ml; 307.8 ng/ml] and TNF-α significantly (*p<.0.01) increased cC concentration to the value of 493.5 ng/ml [337 ng/ml; 1340 ng/ml] (Figure 6).
Figure 6.
Cystatin C production by PMNs cultured in the presence of IL-1β or TNF-α for 18 hours.
Discussion
End-stage renal disease patients exhibit abnormally high incidence of bacterial and viral infections, which are the main cause of their morbidity and mortality [22,23], as a result of paradoxical coexistence of immune deficiency with activation a major part of the immune system [24]. It is believed that uremic toxins, including a new one – cC [25], are responsible for this dysregulation.
PMNs are short-lived cells that are cleared from the bloodstream by apoptosis. Tight regulation of PMNs life span is a crucial mechanism maintaining immune system homeostasis. Extended PMNs life span may lead to chronic inflammation, but shortened PMNs life span may enhance susceptibility to infections [26].
In the present study we have shown that cC in the concentration of 10 μg/ml decreased the apoptosis rate of PMNs. There are no data in the literature describing the influence of cC on the ability of PMN to undergo apoptosis, but a growing body of evidence supports the notion that uremia modulates this ability [27]. The final effect exerted by uremic plasma on PMN apoptosis seems to be dependent on the composition and concentrations of a wide range of compounds recognized as uremic toxins accumulated in the blood. In vitro studies have shown that PMN from uremic patients undergo accelerated apoptosis [28–30] and uremic plasma obtained from dialyzed patients accelerates apoptosis of PMNs from healthy donors [31].
Hampering effects of cC on PMN apoptosis can result from its inhibitory influence on cathepsin activity. Increasing evidence suggests that lysosomal enzymes are important mediators of apoptosis. Cathepsins can trigger apoptosis by numerous pathways – they can directly induce cytochrome c release, formation of mitochondrial reactive oxygen species (ROS) [32], activation of caspase-3 [33] and pro-apoptotic proteins [34,35].
PMN apoptosis can be induced through 2 pathways – the intrinsic mitochondrial pathway activated by intracellular signals such as ROS or DNA damage, which is a normal turnover of aging cells in tissues; and the extrinsic pathway activated by Fas/FasL interaction or TNF-α ligation to its receptors.
Jaber et al. showed that uremic serum increased expression of Fas and FasL and accelerated PMN apoptosis, suggesting that uremic toxins were responsible for those effects [36]. Our results showed that cC downregulated PMN apoptosis without direct influence on the Fas/FasL pathway. There were no differences in Fas and FasL expression on PMNs cultured with the presence of cC compared to expression in control cells.
PMNs die even in the absence of any extracellular stimuli (in our study the control cells reached 70% of apoptotic PMNs), but regardless of apoptotic intrinsic or extrinsic pathways (both of which converge to a common execution phase of apoptosis) it requires the activation of caspase-3 as an early event of this process. Although, in our study we showed the expression of the active form of caspase-3 in apoptotic PMNs, we did not find differences in its expression in PMNs cultured in the presence or absence of cC, IL-1β and TNF-α.
In contrast to cC, there is much data describing the mechanisms by which IL-1β prevents and TNF-α induces neutrophil apoptosis. Results of our study are consistent with the available literature reports, indicating that the percentage of apoptotic PMNs treated with IL-1β was significantly lower in comparison to control PMNs or PMNs cultured in the presence of TNF-α. Inhibition of apoptosis by IL-1β is a complex process and is dependent both on the favorable influence of IL-1β on the expression of anti-apoptotic Bcl2 family proteins or Toll-like receptor (TLR) and caspase-3 downregulation [37]. In our study, PMNs apoptosis stimulated by TNF-α was significantly higher in comparison to PMNs cultured in the presence of cC or IL-1β. However, PMNs apoptosis in the control group (70%) was even greater than in PMNs treated with TNF-α (58%), which is the major extrinsic mediator of the apoptosis. An explanation of this fact derives from the dual role of TNF-α, which may exert both pro-apoptotic and anti-apoptotic effects, depending on the neutrophils’ in vitro assay condition [38].
There are strong suggestions that neutrophil longevity depends more on redox status of the cells and ROS accumulation than on death receptor ligation [39]. The high frequency of bacterial infections in patients with chronic renal disease suggests that their ability to generate ROS and to kill bacteria may by impaired by uremia. Conflicting results demonstrating increase [40], lack of relevant changes [41] or inhibition of PMN respiratory burst [31] suggests that intensity of ROS production in uremic patients is the result of the influence of huge number of diverse uremic toxins on PMN activity. There is only 1 paper, by Leung-Tack et al., describing the inhibitory effect of cC on PMN respiratory burst in response to opsonized zymosan [42]. We revealed that cC reduced not only fMLP- and PMA-induced ROS production, but also the spontaneous one. Recent publications have shown that ROS overproduction by neutrophils in response to fMLP and PMA is accompanied by activation of protein kinase Cδ [43,44] mediated by caspase-3 [45]. Other authors also stated that lysosomes and cathepsins B [46], whose activity is inhibited by cC, are involved in this process. Zhao et al. proved that lysosomal rupture and cathepsins release stimulated mitochondrial ROS production, membrane permeability and cytochrome c release followed by apoptosis [47]. The role of lysosomes and cathepsins in neutrophil apoptosis was supported by recently published papers [48–50].
Conclusions
Recapitulating all the above-mentioned facts, we suggest that cC may diminish spontaneous as well as fMLP- and PMA-induced ROS production by inhibition of cathepsins activity. The same mechanism may be responsible for the decreased apoptotic rate of neutrophils cultured in the presence of cC.
We also examined whether the main proinflammatory cytokines, such as IL-1β and TNF-α, influenced cC release from PMNs. We found that IL-1β and TNF-α exerted opposite effects on cC release from cultured PMNs. These results suggest that, in spite of the fact that cC gene is referred as a “housekeeping gene”, PMNs synthesize cC with constant rate and, inflammatory cytokines can influence only its release from PMNs to extracellular milieu.
Taken together, inflammatory cytokines influence the ability of PMNs to release cC, and cC can modulate acute inflammation through up-regulation of PMN longevity and inhibition of their respiratory burst.
We hypothesize that the inhibition of PMN apoptosis and ROS production observed in our experiments results from the inhibitory activity of cC towards cathepsins, and that high concentration of cC in patients with chronic renal disease is the effect of kidney failure and of inflammation coexisting with this state.
Footnotes
Conflicts of interest statement
None to declare.
Source of support: This work was supported by grant 2P05B 082 30 from the Ministry of Scientific Research and Information Technology
References
- 1.Barrett AJ. The cystatins: a diverse superfamily of cysteine peptidase inhibitors. Biomed Biochim Acta. 1986;45:1363–74. [PubMed] [Google Scholar]
- 2.Abrahamson M, Olafsson I, Palsdottir A, et al. Structure and expression of the human cystatin C gene. Biochem J. 1990;268:287–94. doi: 10.1042/bj2680287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thielemans N, Lauwerys R, Bernard A. Competition between albumin and low-molecular weight proteins for renal tubular uptake in experimental nephropaties. Nephron. 1994;66:453–58. doi: 10.1159/000187863. [DOI] [PubMed] [Google Scholar]
- 4.Grubb A, Simonsen O, Sturfelt G, et al. Serum concentration of cystatin C, Factor D and 2-microglobulin as a measure of glomerular filtration rate. Acta Med Scand. 1985;218:499–503. doi: 10.1111/j.0954-6820.1985.tb08880.x. [DOI] [PubMed] [Google Scholar]
- 5.Bökenkamp A, Domanetzki MD, Zinck R, et al. Cystatin C – a new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101:875–81. doi: 10.1542/peds.101.5.875. [DOI] [PubMed] [Google Scholar]
- 6.Vinge E, Lindergärd B, Nilsson-Ehle P, Grubb A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scan J Clin Lab Invest. 1999;59:587–92. doi: 10.1080/00365519950185076. [DOI] [PubMed] [Google Scholar]
- 7.Finney H, Newman DJ, Price CP. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem. 2000;37:49–59. doi: 10.1258/0004563001901524. [DOI] [PubMed] [Google Scholar]
- 8.Simonsen O, Grubb A, Thysell H. The blood serum concentration of cystatin C (gamma-trace) as a measure of the glomerular filtration rate. Scand J Clin Lab Invest. 1985;45:97–101. doi: 10.3109/00365518509160980. [DOI] [PubMed] [Google Scholar]
- 9.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–21. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 10.Keller CR, Odden MC, Fried LF, et al. Kidney function and markers of inflammation in elderly persons without chronic kidney disease: the health, aging, and body composition study. Kidney Int. 2007;71:239–44. doi: 10.1038/sj.ki.5002042. [DOI] [PubMed] [Google Scholar]
- 11.Descamps-Latscha B, Goldfarb B, Nguyen AT, et al. Establishing the relationship between complement activation and stimulation of phagocyte oxidative metabolism in hemodialyzed patients: a randomized prospective study. Nephron. 1991;59:279–85. doi: 10.1159/000186565. [DOI] [PubMed] [Google Scholar]
- 12.Warfel AH, Zucker-Franklin D, Frangione B, Ghiso J. Constitutive secretion of cystatin c (gamma-trace) by monocytes and macrophages and its downregulation after stimulation. J Exp Med. 1987;166:1912–97. doi: 10.1084/jem.166.6.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frendeus KH, Wallin H, Janciauskiene S, Abrahamson M. Macrophage responses to interferon-gamma are dependent on cystatin C levels. Int J Biochem Cell Bioll. 2009;41:2262–69. doi: 10.1016/j.biocel.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Sokol JP, Schiemann WP. Cystatin c antagonizes transforming growth factor β in normal and cancer cells. Mol Cancer Res. 2004;2:183–95. [PubMed] [Google Scholar]
- 15.Solem M, Rawson C, Lindburg K, Barnes D. Transforming growth factor beta regulates cystatin C in serum-free mouse embryo (SFME) cells. Biochem Biophys Res Commun. 1990;172:945–51. doi: 10.1016/0006-291x(90)90767-h. [DOI] [PubMed] [Google Scholar]
- 16.Björck L, Åkesson P, Bohus M, et al. Bacterial growth blocked by a synthetic peptide based on the structure of a human proteinase inhibitor. Nature. 1989;337:385–86. doi: 10.1038/337385a0. [DOI] [PubMed] [Google Scholar]
- 17.Peri P, Hukkanen V, Nuutila K, et al. The cysteine protease inhibitors cystatins inhibit herpes simplex virus type 1-induced apoptosis and virus yield in HEp-2 cells. J Gen Virol. 2007;88:2101–5. doi: 10.1099/vir.0.82990-0. [DOI] [PubMed] [Google Scholar]
- 18.Ghiso J, Saball E, Leoni J, et al. Binding of cystatin C to C4: The importance of sense – antisense peptides in their interaction. Proc Natl Acad Sci USA. 1990;87:1288–91. doi: 10.1073/pnas.87.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung-Tack J, Tavera C, Martinez J, Colle A. Neutrophil chemotactic activity is modulated by human cystatin C, an inhibitor of cysteine proteases. Inflammation. 1990;14:247–58. doi: 10.1007/BF00915809. [DOI] [PubMed] [Google Scholar]
- 20.Tian S, Kusano E, Ohara T, et al. Cystatin C measurement and its practical use in patients with various renal diseases. Clin Nephrol. 1997;48:104–8. [PubMed] [Google Scholar]
- 21.Kantorski J, Tchórzewski H. The effect of serine and thiol protease inhibitors on the chemiluminescence of human neutrophils in investigations in vitro. J Biolumin Chemilumin. 1992;7:37–45. doi: 10.1002/bio.1170070106. [DOI] [PubMed] [Google Scholar]
- 22.Mailloux LU, Bellucci AG, Wilkes BM, et al. Mortality in dialysis patients: analysis of the causes of death. Am J Kidney Dis. 1991;18:326–35. doi: 10.1016/s0272-6386(12)80091-6. [DOI] [PubMed] [Google Scholar]
- 23.Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with general population. Kidney Int. 2000;58:1758–64. doi: 10.1111/j.1523-1755.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 24.Descamps-Latscha B, Jungers P, Witko-Sarsat V. Immune system dysregulation in uremia: Role of oxidative stress. Blood Purif. 2002;20:481–84. doi: 10.1159/000063558. [DOI] [PubMed] [Google Scholar]
- 25.Vanholder R, De Smet R, Glorieux G, et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–43. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 26.Whyte MK, Meagher LC, MacDermont J, Hasslet C. Impairment of function in aging neutrophils is associated with apoptosis. J Immunol. 1993;150:5124–34. [PubMed] [Google Scholar]
- 27.Cohen G, Rudnicki M, Hőorl HW. Uremic toxins modulate the spontaneous apoptotic cell death and essential functions of neutrophils. Kidney Int Suppl. 2001;78:S48–52. doi: 10.1046/j.1523-1755.2001.59780048.x. [DOI] [PubMed] [Google Scholar]
- 28.Cendoroglo M, Jaber BL, Balakrishnan V, et al. Neutrophil apoptosis and dysfunction in uremia. J Am Soc Nephrol. 1999;10:93–100. doi: 10.1681/ASN.V10193. [DOI] [PubMed] [Google Scholar]
- 29.Jaber BL, Balakrishnan V, Cendoroglo M, et al. Modulation of neutrophil apoptosis by uremic plasma during hemodialysis. Blood Purif. 1998;16:325–35. doi: 10.1159/000014352. [DOI] [PubMed] [Google Scholar]
- 30.Majewska E, Baj Z, Sulowska Z, et al. Effects of uraemia and haemodialysis on neutrophil apoptosis and expression of apoptosis-related proteins. Nephrol Dial Transplant. 2003;18:2582–88. doi: 10.1093/ndt/gfg441. [DOI] [PubMed] [Google Scholar]
- 31.Sardenberg C, Suassuna P, Andreoli MC. Effect of uraemia and dialysis modality on polymorphonuclear cell apoptosis and function. Nephrol Dial Transplant. 2006;21:160–65. doi: 10.1093/ndt/gfi095. [DOI] [PubMed] [Google Scholar]
- 32.Zhao M, Antunes F, Eaton JW, Brunk UT. Lysosomal enzymes promote mitochondrial oxidant production, cytochrome c release and apoptosis. Eur J Biochem. 2003;270:3778–86. doi: 10.1046/j.1432-1033.2003.03765.x. [DOI] [PubMed] [Google Scholar]
- 33.Vancompernolle K, Van Herreweghe F, Pynaert G, et al. Atractyloside-induced release of cathepsin B, a protease with caspase-processing activity. FEBS Lett. 1998;438:150–58. doi: 10.1016/s0014-5793(98)01275-7. [DOI] [PubMed] [Google Scholar]
- 34.Stoka V, Turk B, Schendel SL, et al. Lysosomal protease pathways to apoptosis. Cleavage of bid, not pro-caspases, is the most likely route. J Biol Chem. 2001;276:3149–57. doi: 10.1074/jbc.M008944200. [DOI] [PubMed] [Google Scholar]
- 35.Bidere N, Lorenzo HK, Carmona S, et al. Cathepsin D triggers Bax activation, resulting in selective apoptosis-inducing factor (AIF) relocation in T lymphocytes entering the early commitment phase to apoptosis. J Biol Chem. 2003;278:31401–11. doi: 10.1074/jbc.M301911200. [DOI] [PubMed] [Google Scholar]
- 36.Jaber BL, Perianayagam MC, Balakrishnan VS, et al. Mechanisms of neutrophil apoptosis in uremia and relevance of the Fas (Apo-1, CD95)/Fas ligand system. J Leukoc Biol. 2001;69:1006–12. [PubMed] [Google Scholar]
- 37.Mica L, Harter L, Trentz O, Keel M. Endotoxin reduces CD95-induced neutrophil apoptosis by cIAP-2 mediated caspase-3 degradation. J Am Coll Surg. 2004;199:595–602. doi: 10.1016/j.jamcollsurg.2004.05.272. [DOI] [PubMed] [Google Scholar]
- 38.Van den Berg JM, Weyer S, Weening JJ, et al. Divergent effects of tumor necrosis factor α on apoptosis of human neutrophils. J Leukoc Biol. 2001;69:467–73. [PubMed] [Google Scholar]
- 39.Scheel-Toellner D, Wang K, Craddock R, et al. Reactive oxygen species limit neutrophil life span by activating death receptor signaling. Blood. 2004;104:2557–64. doi: 10.1182/blood-2004-01-0191. [DOI] [PubMed] [Google Scholar]
- 40.Rysz J, Potargowicz E, Banach M, et al. Increased whole blood chemiluminescence in patients with chronic renal failure independent of hemodialysis treatment. Arch Immunol Ther Exp. 2006;54:347–55. doi: 10.1007/s00005-006-0040-0. [DOI] [PubMed] [Google Scholar]
- 41.Gastaldello K, Husson C, Wens R, et al. Role of complement and platelet-activating factor in the stimulation of phagocytosis and reactive oxygen species production during haemodialysis. Nephrol Dial Transplant. 2000;15:1638–46. doi: 10.1093/ndt/15.10.1638. [DOI] [PubMed] [Google Scholar]
- 42.Leung-Tack J, Tavera C, Gensac MC, et al. Modulation of phagocytosis-associated respiratory burst by human cystatin C: role of the N-terminal tetrapeptide Lys-Pro-Pro-Arg. Exp Cell Res. 1990;188:16–22. doi: 10.1016/0014-4827(90)90272-c. [DOI] [PubMed] [Google Scholar]
- 43.Brown GE, Steward MQ, Liu H, et al. A novel assay system implicates PtdIns(3,4)P(2), PtdIns(3)P, and PKC delta in intracellular production of reactive oxygen species by the NADPH oxidase. Mol Cell. 2003;11:35–47. doi: 10.1016/s1097-2765(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 44.Matron C, Chakravarti A, Allaeys I, Poubelle PE. PKC-delta controls the fMLF-induced overproduction of superoxide by neutrophils. Free Radic Biol Med. 2010;48:207–15. doi: 10.1016/j.freeradbiomed.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 45.Pongracz J, Webb P, Wang K, et al. Spontaneous neutrophil apoptosis involves caspase-3 mediated activation of protein kinase C-δ. J Biol Chem. 1999;274:37329–34. doi: 10.1074/jbc.274.52.37329. [DOI] [PubMed] [Google Scholar]
- 46.Jones BA, Rao YP, Stravitz RT, Gores GJ. Bile salt-induced apoptosis of hepatocytes involves activation of protein kinase C. Am J Physiol. 1997;272:G1109–15. doi: 10.1152/ajpgi.1997.272.5.G1109. [DOI] [PubMed] [Google Scholar]
- 47.Zhao M, Antunes F, Eaton JW, Brunk UT. Lysosomal enzymes promote mitochondrial oxidant production and cytochrome c release and apoptosis. Eur J Biochem. 2003;270:3778–86. doi: 10.1046/j.1432-1033.2003.03765.x. [DOI] [PubMed] [Google Scholar]
- 48.Droga-Mazovec G, Bojič L, Petelin A, et al. Cysteine cathepsins trigger caspase-dependent cell death through cleavage of Bid and antiapoptotic bcl-2 homologues. J Biol Chem. 2008;283:19140–50. doi: 10.1074/jbc.M802513200. [DOI] [PubMed] [Google Scholar]
- 49.Prince LR, Bianchi SM, Vaughan KM, et al. Subversion of lysosomal pathway regulating neutrophil apoptosis by a major bacterial toxin, pyocyanin. J Immunol. 2008;180:3502–11. doi: 10.4049/jimmunol.180.5.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blomgran R, Zheng L, Stendahl O. Cathepsin-cleaved Bid promotes apoptosis in human neutrophils via oxidative stress-induced lysosomal membrane permeabilization. J Leukoc Biol. 2007;81:1213–23. doi: 10.1189/jlb.0506359. [DOI] [PubMed] [Google Scholar]