Summary
Today it is known that severe burns can be accompanied by the phenomenon of vasoplegic syndrome (VS), which is manifested by persistent and diffuse vasodilation, hypotension and low vascular resistance, resulting in circulatory and respiratory failure. The decrease in systemic vascular resistance observed in VS is associated with excessive production of nitric oxide (NO). In the last 2 decades, studies have reported promising results from the administration of an NO competitor, methylene blue (MB), which is an inhibitor of the soluble guanylate cyclase (sGC), in the treatment of refractory cases of vasoplegia. This medical hypothesis rationale is focused on the tripod of burns/vasoplegia catecholamine resistant/methylene blue. This article has 3 main objectives: 1) to study the guanylate cyclase inhibition by MB in burns; 2) to suggest MB as a viable, safe and useful co-adjuvant therapeutic tool of fluid resuscitation, and; 3) to suggest MB as burns hypotensive vasoplegia amine-resistant treatment.
Keywords: burn, vasoplegic syndrome, methylene blue, nitric oxide
Background
Today it is known that severe burns can be accompanied by the phenomenon of vasoplegic syndrome (VS), which is manifested by persisted and diffuse vasodilation, hypotension and low vascular resistance, resulting in circulatory and respiratory failure [1]. The decrease in systemic vascular resistance observed in VS is associated with excessive production of nitric oxide (NO) [2]. The plasma NO content is increased during the first hours after burn injury. It seems that the increased concentration of NO, combined with other biochemical phenomena of the systemic inflammatory response, leads to a widespread leakage of protein and intravascular fluid into the interstitial space, resulting in various degrees of edema and hypovolemia [3–5].
In the last 2 decades, studies have reported promising results from the administration of methylene blue (MB), which is an inhibitor of the soluble guanylate cyclase (sGC), in the treatment of refractory cases of vasoplegia [1,2,6,7]. This action of MB results in reduced response of vessels to cyclic guanosine monophosphate (cGMP)-dependent vasodilators such as nitric oxide and carbon monoxide.
This medical hypothesis rationale, focused on the tripod of burns/vasoplegia catecholamine resistant/methylene blue, has 3 main objectives: 1) to study the guanylate cyclase inhibition by MB in burns; 2) to suggest MB as a possible safe and useful co-adjuvant therapeutic tool of fluid resuscitation, and; 3) to suggest MB as burn hypotensive vasoplegia amine-resistant treatment. In an attempt to organize this article according to a logical sequence, we choose the sequence: I – Experimental clinical reasoning (Nitric oxide and burns; Methylene blue and the NO/cGMP pathway); II – Hypothesis, III – Testing the hypothesis, and; IV – Concluding remarks.
The experimental and clinical reasoning
Nitric oxide and burns
Systemic NO production following burn injury
The first investigation to tackle the question of NO and thermal injury was reported in 1993 by Becker et al [8]. In that study, the urinary level of the stable NO metabolite, NO3, was elevated for 1–8 days in rats that had been subjected to a large TBSA (total burned surface area) scald injury. It was also shown that this effect could be prevented by the administration of the non-specific NOS inhibitor, NG-monomethyl-l-arginine (L-NMMA). In the following year, similar findings were reported by Carter et al. (1994) and an attempt was made to identify the major organs that produce NO by measuring tissue NOS activity [9]. Brain, liver, kidney, spleen and the gastrointestinal tract were all seen to have increased levels of NOS activity following heat insult. In addition, thermally injured skin was observed to be more calcium dependent. As in previous reports, the results obtained showed a significant increase in NO/NOS plasma levels in burned patients [10,11].
Nitric oxide is a pivotal mediator of many physiological and pathophysiological events. After thermal injury, an increase of NO in plasma and urinary levels has been observed, but the real importance of this fact is unknown. The stable NO derivatives (NO2-/NO3-) plasma concentrations were determined in 27 burned patients admitted to the Burn Unit at Santa Maria Hospital in Lisbon at days 1, 3, 5, 7, 9, and 15 and their values were compared with healthy controls. A significant increase in the burn patient determinations upon admission was found. The patients with inhalation injury had higher values compared to the other patients, with statistical significance at the 5th day. The patients who died showed an NO increase, with significance at day 5. The determinations in patients with sepsis were higher than in the other patients at day 3. No association with TBSA was found. Considering burned patients, a significant increase in NO was found in patients who died, among patients with inhalation injury, and patients with sepsis. We suggest a possible role of NO determination as an indicator of sepsis and the use of NO synthesis inhibitors in these situations [12].
Nitric oxide and vascular permeability in burn injuries
Some studies have related the production of NO with increased vascular permeability after burn trauma. Sozumi et al. studied the kinetics and role of NO in vascular permeability using an ear thermal injury model. Vascular permeability was suppressed for 3 hours after a thermal injury by the preventive administration of nitric oxide synthase (NOS) inhibitors. The NO content in the injured region was significantly increased compared with the intact region. The authors found that the plasma NO content was significantly increased in a biphasic pattern at 1 and 6 hours post-injury. NOS inhibitors administered as therapeutic treatment suppressed vascular permeability 1 and 6 hours post-burn, concluding that NOS inhibitors might be effective in burn treatment [6].
Inoue et al. (2001) investigated the role of nitric oxide and related synthase in thermal injury using models of experimental burns to assess severity from the aspect of vascular permeability. Thermal injuries were produced in the murine right ear by pinching with a pair of preheated tweezers. Immediately thereafter, Evans blue dye was intravenously administered, and the mice injured with burns were sacrificed at various times. The burned ears were collected and hydrolyzed, and the level of extracted dye was measured as an indicator of inflammation. Vascular hyperpermeability was suppressed by the administration of nitric oxide synthase inhibitors. L-NAME not only suppressed vascular hyperpermeability in thermal injuries in a dose-dependent manner, but was also effective with either prophylactic or therapeutic administration. Although aminoguanidine also suppressed the inflammatory response, it had no effect on the early inflammatory phase. Aminoguanidine, an inhibitor specific to inducible nitric oxide synthase, suppressed the late phase 6 hours after injury, suggesting that inducible nitric oxide synthase is involved in inflammatory responses to thermal injuries. These results also demonstrated that inducible nitric oxide synthase-like protein stained the burned area immunohistochemically. Therefore, both types of enzymes mediating nitric oxide affect inflammatory responses and vascular hyperpermeability, and their regulation may lead to the development of new therapy for thermal injuries [4].
Nitric Oxide and vasoplegia
Excessive NO formation plays important roles in the pathogenesis of shock and multiple organ failure in sepsis and acute lung injury [13]. The NO is synthesized from L-arginine and oxygen by the enzyme nitric oxide synthase (NOS). It is an important diatomic extracellular and intracellular signaling molecule, and acts by inducing sGC, which produces cGMP. The cGMP, among other effects, relaxes smooth muscle, and its best-known biological actions are vasodilatation and bronchodilation. In the systemic inflammatory response syndrome (SIRS) there is an increase in NO synthesis, and often in this situation vasodilatation and hypotension refractory occurs as part of the framework known as VS. Regardless of the initial etiology, VS seems to represent an imbalance between synthesis/release of NO and activation of sGC in vascular smooth muscle cells. Increased inducible nitric oxide synthase (iNOS), and consequent increase in NO production, generate the production of cGMP. This leads to myocardial depression, reduced contractile response to exposure to vasoconstrictors, increased vascular permeability, and circulatory collapse [7]. With respect to the burn trauma, NO synthesis is increased, both locally and systemically, and altered activity of NOS contributes to gastrointestinal, pulmonary and cardiovascular dysfunction [3,14–16].
Methylene Blue and the NO/cGMP Pathway
Methylene blue and vasoplegia
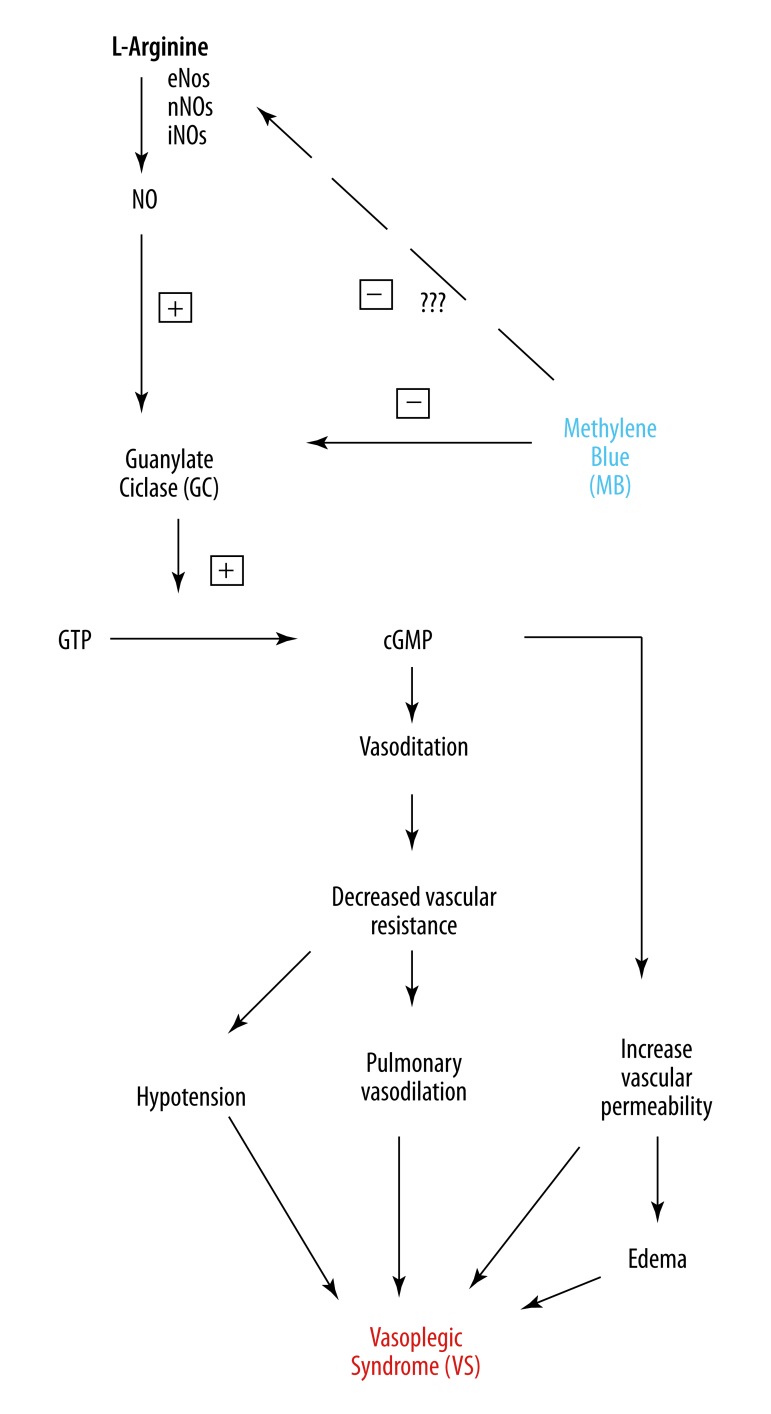
Methylene blue may be a valuable adjunct in the treatment of anaphylaxis and other causes of refractory hypotension [17–19]. The VS has multifactorial genesis, and in the case of patients undergoing cardiac surgery is mainly due to exposure of the body to non-physiological materials and the use of heparin/protamine [20], triggering the SIRS. Evora et al. published several reports of reversal of vasoplegic syndrome with the administration of MB in cardiac surgery and anaphylactic shock [2,6,18,21–23]. Recently, MB was also used with success in a patient with poor hemodynamic status, who underwent aortic aneurism repair [24]. Moreover, MB is an effective therapy in the prevention of dialysis hypotension [25]. Figure 1 shows the relationship between the pathophysiology of VS and the reversing action of MB.
Figure 1.
Possible mechanism of action of methylene blue (MB) competing with nitric oxide (NO) in the development of vasoplegic syndrome (VS).
Based on “15 years of questions, answers, doubts and certainties,” Evora et al. (2009) made some observations about the use of MB to treat vasoplegia: 1) Heparin and angiotensin-converting enzyme inhibitors are risk factors; 2) in the recommended doses it is safe (the lethal dose is 40 mg/kg); 3) the use of MB does not cause endothelial dysfunction; 4) the MB effect appears in cases of NO upregulation; 5) MB is not a vasoconstrictor (by blocking of the cGMP system it releases the cAMP system, facilitating the norepinephrine vasoconstrictor effect); 6) the most commonly used dosage is 2 mg/kg as i.v. bolus followed by the same continuous infusion because plasmatic concentrations strongly decays in the first 40 minutes; 7) there is a possible “window of opportunity” for MB’s effectiveness. These data took into account sepses and cardiac surgery vasoplegia, but can be considered as general concepts. Although there are no definitive multicentre studies, MB used to treat heart surgery VS, at the present time, is the best, safest and cheapest option [2].
Methylene blue (MB) guanylate cyclase inhibition and pulmonary edema
Due to its ability to reduce vascular permeability, MB seems to also act in resolution of lung edema [26]. Evora and Rodrigues (2006) wrote a letter describing MB use in a patient with a history of drug addiction who underwent placement of a bileaflet aortic valve prosthesis for native aortic valve endocarditis. The patient required high dose norepinephrine infusion intra-operatively and remained hypotensive after weaning from cardiopulmonary bypass. He experienced persistent increase in cardiac output, low systemic vascular resistance, and pulmonary edema associated with hypoxemia. MB was started as a continuous infusion, followed by a bolus of 3 mg/kg twice daily. Despite the lack of increase in mean arterial pressure, even with norepinephrine, the cardiac output gradually decreased and the systemic vascular resistance increased following MB administration. The patient also experienced rapid resolution of pulmonary edema and improvement in oxygenation after MB was given [22].
Methylene blue and volume resuscitation
Jeroukhimov et al. (2001) carried out an interesting investigation to compare pre-hospital hypotensive resuscitation with volume resuscitation, and to discover whether reagents that inhibit free-oxygen radical formation, such as MB, can improve resuscitation and survival. After 30 minutes of controlled hemorrhage, rats were subjected to 60 minutes of uncontrolled hemorrhage with simultaneous resuscitation. Hartmann’s solution alone, or blood, or a bolus of MB, were infused to maintain the mean arterial pressure (MAP) at 80 or 40 mmHg. Then hemorrhaging was stopped, and Hartmann’s solution plus whole blood were infused to obtain a MAP that was within normal limits. During uncontrolled hemorrhage, a MAP of 80 mmHg could not be reached in animals resuscitated with Hartmann’s solution alone, and all died. All the rats that received Hartmann’s solution or whole blood associated to a bolus of MB achieved a higher MAP. MAP of 40 mmHg was attained in all animals regardless of the resuscitation fluid. Only 15 of 24 animals resuscitated to a MAP of 80 mmHg survived, compared with 22 survivors of the 24 rats resuscitated to a MAP of 40 mmHg. MB or whole blood drastically reduced the volumes of shed blood and fluids and moderated the reduction in packed cell volume, particularly during hypotensive resuscitation. The authors concluded that hypotensive protocols should be used to increase survival. MB given with the electrolyte solutions could negate their detrimental effects during resuscitation [27].
MB has been used to treat hypovolemic states. Ghiassi et al. (2004) performed a study in dogs to evaluate pre-hospital resuscitation after refractory hemorrhagic shock with a combination of MB and limited-volume lactated Ringer’s solution. After blood loss to a MAP of 50 mm Hg in canines, refractory hemorrhagic shock was defined as minimal hemodynamic response to phenylephrine. The protocols included no treatment (control), MB bolus, limited-volume lactated Ringer’s solution, and combined MB/lactated Ringer’s solution. Hemodynamic parameters were collected at baseline, during shock, during refractory hemorrhagic shock, and 30, 60, 90, and 120 minutes after treatment. Radiolabeled microspheres were used to measure end-organ perfusion and oxygen delivery. MB/lactate Ringer’s resuscitation improved pre-hospital survival, MAP and cardiac output, vital end-organ blood flow and oxygen delivery, and decreased serum lactate levels, as compared with the MB and lactated Ringer’s single therapies. The investigators concluded that resuscitation after refractory hemorrhagic shock, using a combination of MB and limited-volume lactated Ringer’s solution, improves pre-hospital survival and hemodynamic stability and reduces ischemic damage in an acute setting. This form of treatment has proven useful as a temporizing measure for resuscitation after refractory hemorrhagic shock in a canine model, and warrants further study for its application to hemorrhagic trauma patients [28].
Nitric oxide synthase inhibitors versus guanylate cyclase inhibitors
Nitric oxide stimulates soluble guanylyl cyclase to increase cGMP production, leading to smooth muscle relaxation. This most important vasodilatation mechanism occurring in sepsis, cardiac surgery vasoplegic syndrome, anaphylaxis, transplanted liver reperfusion and cardiogenic shock secondary to myocardium injury is not reversed by vasoconstrictor amines. NOS inhibitors, on the other hand, are not currently in clinical use due to their lack of specificity, with consequent risk of generalized tissue necrosis. For these reasons, it seems more appropriate to use MB as a therapeutic agent in the aforementioned shock-related vasoplegic states. Methylene blue does not interfere with NOS and has played a longstanding beneficial role in many other clinical conditions. As a potent guanylyl cyclase inhibitor, it blocks the increase in cyclic GMP levels, and, consequently, prevents vascular smooth muscle NO endothelium-dependent relaxation [23].
Hypothesis
MB inhibition of guanylate cyclase: a) has been proven, in basic and clinical studies, as an adjuvant treatment option in cases of catecholamines-resistant vasoplegia; 2) its use is safe and often lifesaving; and 3) nitric oxide pharmacological inhibition interferes, not only in reversing vasoplegia, but also in vascular permeability, often caused by the systemic inflammatory response associated with burns. This review article has 3 main objectives: 1) to study the guanylate cyclase inhibition by MB in burns; 2) to suggest MB as a viable, safe and useful co-adjuvant therapeutic tool of fluid resuscitation; and 3) to suggest MB as a burns hypotensive vasoplegia amine-resistant treatment.
Testing the Hypothesis
Jaskille et al. (from the Department of Surgery, and Burn Center, Washington Hospital Center, Washington, DC) (2008) reported the first cases of MB infusion in 2 burn patients refractory to norepinephrine. The patients had severe burns, 95% and 80% TBSA, not responding to conventional treatment. Fluid requirements were estimated according to Parkland formula, and then to maintain a urinary output of 30–50 mL/hr. Patient #1 had 95% TBSA, had adrenal insufficiency and was receiving steroids according to the Annane protocol, as well as vasopressin at 0.2 U/min. His norepinephrine requirements were 55 mcg/kg/min. Patient #2 had 80% TBSA and was receiving 20 mcg/kg/min of norepinephrine. Circulatory failure was defined as inability to maintain MAP >70 mmHg. Hemodynamic and physiologic parameters were measured before and after infusion of a single dose of 2 mg/kg of MB. Both patients showed dramatic improvements in their shock after MB. Patient #1 had an initial response within 30 minutes and reached maximum effect at 1 hour. His norepinephrine requirements decreased to 0.2 mcg/kg/min and vasopressin decreased to 0.04 U/min. Patient #2 showed effects within 15 minutes of the infusion, and by 2 hours the norepinephrine was stopped. No adverse effects were noted in either of these 2 patients [1].
Conclusions
Vasoplegia resulting from severe burns may persist despite adequate fluid resuscitation and treatment with norepinephrine, vasopressin and steroids. Methylene blue, currently used to treat methemoglobinemia in burn patients, has been used to treat vasoplegia after cardiopulmonary bypass. The fact that MB successfully reversed refractory vasoplegia after severe burns in the 2 patients reported by Jaskille et al. suggests a new tool for treating a small subgroup of patients who exhibit persistent vasoplegia from their burn injuries. We suggest that a controlled randomized trial is needed to assess its effects on a large number of patients to assess graft survival and MB safety used in the milieu of catecholamine-resistant vasoplegia.
This review supports the therapeutic approach based on our clinical experience and critically reviews the specialized literature, on the assumption that the cGMP system seems to still be underestimated. What can we do when circulatory shock becomes refractory to the traditional therapeutic measures, including fluid administration, inotropes and vasoconstrictors? Responses to this question are presently limited to the accumulated evidence regarding 3 vasoconstrictive cAMP-independent mechanisms herein mentioned as ‘known mechanisms’: (1) cGMP/NO-dependent vasoconstriction; (2) vasopressin administration; and (3) hyperpolarization-dependent vasoconstriction (Figure 1). Another frequently asked question is: ”Why don’t these therapeutic alternatives always work?” We believe that there are at least 6 aspects pertaining to this inquiry: (1) no consideration of the existing ‘guidelines’ or ‘evidence-based medicine’ regarding the accepted available treatment options; (2) lack of knowledge of different vasodilatation mechanisms; (3) the possibility of ensuing crosstalk among the different vasodilatation mechanisms; (4) the soluble guanylyl cyclase enzymatic dynamics; (5) the common use of MB administration as a ‘rescue or ultimate’ therapeutic effort; and 6) the few considered MB action over vascular permeability.
List of abbreviations
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine
- DHR 123
dihydrorhodamine 123
- eNOS
endothelial nitric oxide synthase
- GC
guanylate cyclase
- iNOS
inducible nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- L-NAME
L-Nitro-Arginine Methyl Ester
- L-NMMA
NG-monomethyl-L-arginine
- MAP
mean arterial pressure
- MB
methylene blue
- MODS
multiple organ dysfunction syndrome
- MPO
myeloperoxidase
- NO
nitric oxide
- NOS
nitric oxide synthase
- ODQ
1H-[1,2,4] oxadiazolo [4,3-alpha] quinoxalin-1-one
- SIRS
systemic inflammatory response syndrome
- sGP
soluble guanylate cyclase
- SNP
sodium nitroprusside
- TBSA
total burned surface area
- VS
vasoplegic syndrome
Footnotes
Conflict of interest statement
There is no conflict of interest.
Source of support: Departmental sources
References
- 1.Jaskille AD, Jeng JC, Jordan MH. Methylene blue in the treatment of vasoplegia following severe burns. J Burn Care Res. 2008;29:408–10. doi: 10.1097/BCR.0b013e31816677b5. [DOI] [PubMed] [Google Scholar]
- 2.Evora PR, Ribeiro PJ, Vicente WV, et al. Methylene blue for vasoplegic syndrome treatment in heart surgery: Fifteen years of questions, answers, doubts and certainties. Rev Bras Cir Cardiovasc. 2009;24:279–88. doi: 10.1590/s0102-76382009000400005. [DOI] [PubMed] [Google Scholar]
- 3.Chen LW, Wang JS, Hwang B, et al. Reversal of the effect of albumin on gut barrier function in burn by the inhibition of inducible isoform of nitric oxide synthase. Arch Surg. 2003;138:1219–25. doi: 10.1001/archsurg.138.11.1219. [DOI] [PubMed] [Google Scholar]
- 4.Inoue H, Ando K, Wakisaka N, et al. Effects of nitric oxide synthase inhibitors on vascular hyperpermeability with thermal injury in mice. Nitric Oxide. 2001;5:334–42. doi: 10.1006/niox.2001.0350. [DOI] [PubMed] [Google Scholar]
- 5.Sozumi T. The role of nitric oxide in vascular permeability after a thermal injury. Anna Plasti Surg. 1997;39:272–77. doi: 10.1097/00000637-199709000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Evora PR, Levin RL. Methylene blue as drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypass. J Thoraci Cardiovasc Surg. 2004;127:895–96. doi: 10.1016/j.jtcvs.2003.09.046. author reply 896. [DOI] [PubMed] [Google Scholar]
- 7.Stawicki SP, Sims C, Sarani B, et al. Methylene blue and vasoplegia: Who, when, and how? Mini Rev Med Chem. 2008;8:472–90. doi: 10.2174/138955708784223477. [DOI] [PubMed] [Google Scholar]
- 8.Becker WK, Shippee RL, McManus AT, et al. Kinetics of nitrogen oxide production following experimental thermal injury in rats. J Trauma. 1993;34:855–62. doi: 10.1097/00005373-199306000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Carter EA, Derojas-Walker T, et al. Nitric oxide production is intensely and persistently increased in tissue by thermal injury. Biochem J. 1994;304( Pt 1):201–4. doi: 10.1042/bj3040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gamelli RL, George M, Sharp-Pucci M, et al. Burn induced nitric oxide release in humans. J Trauma. 1995;39:869–77. doi: 10.1097/00005373-199511000-00010. discussion 877–78. [DOI] [PubMed] [Google Scholar]
- 11.Preiser JC, Reper P, Vlasselaer D, et al. Nitric oxide production is increased in patients after burn injury. J Trauma. 1996;40:368–71. doi: 10.1097/00005373-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 12.do Rosario Caneira da Silva M, Mota Filipe H, Pinto RM, et al. Nitric oxide and human thermal injury short term outcome. Burns. 1998;24:207–12. doi: 10.1016/s0305-4179(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 13.Lange M, Enkhbaatar P, Nakano Y, et al. Role of nitric oxide in shock: the large animal perspective. Front Biosci. 2009;14:1979–89. doi: 10.2741/3357. [DOI] [PubMed] [Google Scholar]
- 14.Chen LW, Hwang YC, Chen CJ, et al. Burn-induced lung damage in rat is mediated by a nitric oxide/cgmp system. Shock (Augusta, Ga) 2003;20:369–74. doi: 10.1097/01.shk.0000086520.18735.df. [DOI] [PubMed] [Google Scholar]
- 15.Chen LW, Hwang YC, Wang JS, et al. Inhibition of nitric oxide synthase reverses the effect of albumin on lung damage in burn. J Am Coll Surg. 2005;200:574–83. doi: 10.1016/j.jamcollsurg.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Rawlingson A. Nitric oxide, inflammation and acute burn injury. Burns. 2003;29:631–40. doi: 10.1016/s0305-4179(03)00079-2. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira Neto AM, Duarte NM, Vicente WV, et al. Methylene blue: an effective treatment for contrast medium-induced anaphylaxis. Med Sci Monit. 2003;9(11):CS102–6. [PubMed] [Google Scholar]
- 18.Rodrigues JM, Pazin Filho A, Rodrigues AJ, et al. Methylene blue for clinical anaphylaxis treatment: a case report. Sao Paulo Med J. 2007;125:60–62. doi: 10.1590/S1516-31802007000100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weissgerber AJ. Methylene blue for refractory hypotension: a case report. AANA J. 2008;76:271–74. [PubMed] [Google Scholar]
- 20.Viaro F, Dalio MB, Evora PR. Catastrophic cardiovascular adverse reactions to protamine are nitric oxide/cyclic guanosine monophosphate dependent and endothelium mediated: should methylene blue be the treatment of choice? Chest. 2002;122:1061–66. doi: 10.1378/chest.122.3.1061. [DOI] [PubMed] [Google Scholar]
- 21.Evora PR. Should methylene blue be the drug of choice to treat vasoplegias caused by cardiopulmonary bypass and anaphylactic shock? J Thorac Cardiovasc Surg. 2000;119:632–34. doi: 10.1016/s0022-5223(00)70152-8. [DOI] [PubMed] [Google Scholar]
- 22.Evora PR, Rodrigues AJ. Methylene blue revised. J Thorac Cardiovasc Surg. 2006;131:250–51. doi: 10.1016/j.jtcvs.2005.09.012. author reply 251. [DOI] [PubMed] [Google Scholar]
- 23.Evora PR, Rodrigues AJ, Vicente WV, et al. Is the cyclic GMP system underestimated by intensive care and emergency teams? Med Hypotheses. 2007;69:564–67. doi: 10.1016/j.mehy.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 24.Piraccini E, Agnoletti V, Corso R, et al. The use of methylene blue in the abdominal aortic surgery: a case report. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia. 2010;2:215–18. [PMC free article] [PubMed] [Google Scholar]
- 25.Peer G, Itzhakov E, Wollman Y, et al. Methylene blue, a nitric oxide inhibitor, prevents haemodialysis hypotension. Nephrol Dial Transplant. 2001;16:1436–41. doi: 10.1093/ndt/16.7.1436. [DOI] [PubMed] [Google Scholar]
- 26.Kirov MY, Evgenov OV, Evgenov NV, et al. Infusion of methylene blue in human septic shock: A pilot, randomized, controlled study. Criti Care Med. 2001;29:1860–67. doi: 10.1097/00003246-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Jeroukhimov I, Weinbroum A, Ben-Avraham R, et al. Effect of methylene blue on resuscitation after haemorrhagic shock. Eur J Surg. 2001;167:742–47. doi: 10.1080/11024150152707716. [DOI] [PubMed] [Google Scholar]
- 28.Ghiassi S, Sun YS, Kim VB. Methylene blue enhancement of resuscitation after refractory hemorrhagic shock. J Trauma. 2004;57:515–21. doi: 10.1097/01.ta.0000136159.22721.3d. [DOI] [PubMed] [Google Scholar]