Summary
Background
To assess the detection rate of liver lesions in patients with advanced gastroenteropancreatic neuroendocrine carcinomas (GEP-NETs) using echo planar (EP) DWI (diffusion weighted imaging) as compared to standard FSE T2 wi and FFE T1 wi with i.v. (Gd-EOB)-DTPA.
Material/Methods
This prospective single-institution study included 55 patients with liver involvement confirmed by GEP-NETs 1.5T MRI system, using FSE T2, EP DWI and FFE T1 with i.v. (Gd-EOB)-DTPA. The potential differences between detection rates of liver deposits using 3 different MR approaches and between groups of patients were compared.
Results
Mean number of liver deposits: FSE T2=20.7, FFE T1=25.7 and tested EP DWI=24.0. No significant difference was found in overall detection rate of liver deposits seen in 3 different techniques. A significant difference in detection rate of liver deposits was noted between male vs. female and secreting vs. non-secreting cancers. There was nearly perfect agreement between both observers, and each of the tested MRI approaches in regards to number of detected liver lesions (Cohen’s κ=0.848–1).
Conclusions
There were no significant differences among the 3 different MRI approaches in detection rates of liver deposits. Perfect agreement with high detection rate of liver deposits provides a rationale for the use of EP DWI in follow-up studies in GEP-NET patients.
Keywords: MR - DWI, liver deposits, advanced GEP-NET (gastroenteropancreatic neuroendocrine tumors)
Background
Neuroendocrine tumors (NETs) are derived from the diffuse endocrine system and can be found anywhere in the body. The most common group of these tumors is gastroenteropancreatic neuroendocrine tumors (GEP-NETs) [1–3]. This group of tumors is relatively rare, and there is a need for a robust strategy to establish the correct diagnosis and initiate rational treatment regimens [1,2]. These tumors are usually slow growing, but most of them present with great metastatic potential. If the cancer is under control, even in the advanced stages, patients have a relatively good prognosis [1,2,4]; however, some patients have tumors with very rapid growth and aggressive behaviour [1,3,4]. Concerted efforts need to be made to establish a diagnostic imaging approach as a solution for the detection of the tumor and its extent, and to assess the therapeutic efficacy of various regimens [5,6]. This approach should be verified using new technologies, which may potentially give us significantly improved ability to quickly establish the correct diagnosis and extent of disease, and will also be used to test effectiveness of therapy [6–8].
One of the greatest advantages in the management of GEP-NET is the continual development of new imaging technologies. Both functional and anatomical imaging are used routinely in patients with GEP-NETs when detecting the primary tumors and assessing secondary lesions [5,6,9–12]. This approach is currently used as a standard of care in neuroendocrine tumors, as outlined in The European Neuroendocrine Tumour Society Consensus Guidelines (ENETS) [4,6].
MRI seems to be the method of choice in detecting and characterizing liver lesions. Currently, there are still only a limited number of reports concerning the use of MRI techniques in the evaluation of liver deposits in patients with suspected or confirmed liver involvement presented as homogenous neuroendocrine tumour series, including current nomenclature of GEP-NET [9–12].
The initial results of high detection rate using standard MR imaging techniques were based on SE T1-weighted, FSE T2 (fat-suppressed)-weighted and dynamic gadolinium-enhanced T1-weighted images [13]. This led us towards the use of potentially new MRI approaches. The most promising MRI protocol with higher detection rate of liver deposits of neuroendocrine carcinoma used Fast FE T1w images (Ultrafast Gradient Echo Sequence) after i.v. contrast enhancement with hepatotropic agents (Gd-EOB)-DTPA [9]. This was used to determine the early dynamic imaging in arterial and portal venous phases of examination, which are crucial landmarks for GEP-NETs. The potential advantage of these agents could be in the delayed phase of the examination (after 20 minutes) as selective hepatobiliary contrast tracers [14,15].
Another option is using MR diffusion weighted imaging (DWI), which is a very sensitive technique for assessing lesions with high water exchange, potentially within metastatic neuroendocrine carcinomas [16–19]. DWI seems to be an alternative option for standard MR protocols – it is easy and fast to perform and seems to be especially valuable for imaging in detection of liver deposits, particular in patients during their clinical follow-up. EP-DWI could also be performed frequently, particularly in those patients who receive active therapy or in those who have progressive cancer, with a potential need for changes in therapeutic management [9,19–21].
The purpose of the study
The primary objective of this prospective study was to assess the detection rate of liver metastasis in patients with advanced GEP-NET using MR: FSE T2 w images (fat suppressed), echo planar DWI and FFE T1 w after i.v. (Gd-EOB)-DTPA contrast enhancement. The second objective of this study was to confirm the clinical value of EP DWI as a non-inferior imaging approach compared to FSE T2 w or FFE T1 w after i.v. (Gd-EOB)-DTPA. An additional objective was to assess interobserver agreement for detection of liver metastasis in MRI evaluated by both readers, as well as agreement in different MR approaches.
Material and Methods
Clinical data
This was a prospective, single-institution, open label study approved by the Ethics Committee of our institution. The study group consisted of 55 patients whose mean age was 56.1 years old (range 35–69 years), with a female-to-male ratio of 30/25. All patients had confirmed, histological proven GEP-NETs with liver involvement. All had clinical stage IV disease (CS) based on recent pTNM classification and ENETS recommendation [2–4].
MRI studies were initiated to evaluate staging, restaging, and/or to evaluate response to therapy. All examinations were performed as routine imaging follow-up of patients with GEP-NETs. All patients had careful clinical evaluation, including history of the disease, and physical examination as routine follow-up. Those patients in whom a confirmed presence of 2 cancers was established (1 neuroendocrine and another malignancy), were excluded from the study. The data was collected in a systematic and comprehensive manner.
Diagnostic imaging
MRI
All patients had liver MRIs performed on a 1.5-T system (Vantage Atlas Z, Toshiba, J) with a SENSE phased array abdominal body coil. Images of the liver were acquired using breath-hold, or respiratory-triggered (with a navigator-echo technique) [17]. In each case, FSE T2 w images were used as a standard approach to detect liver metastases. All parameters of selected sequences of MR examination, including FSE T2 wi fat suppressed, Fast FE T1 wi (Ultrafast Gradient Echo Sequence) after i.v. (Gd-EOB)-DTPA injection and also tested fat suppressed, single shot SE EP DWI performed before i.v. contrast injection are presented in Table 1.
Table 1.
Sequence parameters used for performing FSE T2 weighted image, SE EP DWI and FFE T1 weighted image after i.v. Gd-EOB-DTPA MR imaging of the liver.
| Parameter | FSE T2 wi Fat-Suppressed | Fast FE T1 wi. After i.v. Gd-EOB-DTPA | Single Shot, SE EP DWI |
|---|---|---|---|
| TR/TE | 11000/70 | 3.3–4.5/1.4–1.9 | 4500/70 |
| Flip angle | 90° | 12° | 90° |
| Matrix and interpolated matrix | 224×256 | 128–192 interpolated 256×256 | 112×256 |
| Field of view (cm) | 37×35 | 30–40×30–40 | 34×34 |
| Section thickness; gap (mm) | 7 (0.7) | 7 (0) | 7 (1) |
| Speeder Factor | 1.8 | 1.8 | 2 |
| Phase-encoding direction | Antero-posterior | Antero-posterior | Antero-posterior |
| Number of section | 36 | 36 | 12 |
| Fat suppression | Yes | Yes | Yes |
| b-values | 0, 125, 800 | ||
| Acquisition time (s) | 60 | 60 | 140s |
| Breath hold technique with respiratory-triggered | Yes | Yes | Yes |
Dynamic MRI using FFE T1 w images were acquired after i.v. contrast enhancement with (Gd-EOB)-DTPA (Primovist – Bayer Healthcare, D) at a dose of 0.05 mmol/kg, administered as a bolus (2–3 ml/s) followed by a 20-mL saline flush (2 mL/sec). At least 3 time points were used, centered on arterial, portal venous phase, and late phase. MR images were acquired 20 s and 60 s after contrast injection and equilibrium after 3 min. Additionally, a late phase of (Gd-EOB)-DTPA images were acquired at least 20 min after contrast agent i.v. administration.
Diffusion gradients with 3 b values (0, 125 and 800 s/mm2), recommended by the manufacturer, were applied in 3 directions: phase and frequency encoding, slice select. DWI images were used to compose ADC maps.
Histopathological methods
All patients had presence of GEP-NET cancer within the liver confirmed by histopathology. The biopsy samples or surgical specimens acquired before examination were fixed in 10% buffered formalin and embedded in paraffin. Slides were stained by means of haematoxylin and eosin methods. The immunohistochemical method used analyzed the expression of synaptophysin, chromogranin and the Ki-67 index – MIB1 antibody (Dako; DK). The histopathological diagnosis was established according to WHO criteria described elsewhere [2,3].
Imaging analysis
Due to the prospective design of this study, all images using FSE T2 w, EP-DWI and FFE T1 w, after i.v. (Gd-EOB)-DTPA contrast enhancement were evaluated in a single reading by 2 radiologists independently. The final score of detected liver lesions was made in separate reading sessions by a consensus of both readers (AJS and MLN). The observers recorded all suspicious lesions with a diameter of at least ≥5 mm. Detection of liver lesions less then 5mm were omitted due to limited spatial resolution of DW images. FSE T2, EP-DWI and FFE T1w i after i.v. (Gd-EOB)-DTPA administration were evaluated at a single sitting to minimize recall bias.
As per RECIST, detected lesions were recorded based on liver segmentation, and 5 dominant lesions over 10 mm were noted. Other lesions with diameters above 5 mm were counted without recording their localization. Liver segmentation was based on FFE T1 w images after i.v. (Gd-EOB)-DTPA administration due to very good delineation of liver structures. There was no limit in recording liver lesions with diameters over 5 mm that was counted by each observer.
Lesion characteristics were assessed in each case, which accounted for the potential difference between the benign and malignant nature of the counted lesions. Discrimination between benign and malignant lesions was performed and classified lesions as cystic, solid or mixed. Simple cysts were excluded from the count of liver lesions. More specifically, the following criteria were used: a lesion was considered benign (cyst) if the lesion was hyperintense on FSE T2 w images and on DWI in b=125 sec/mm2, [16,17], with a strong signal intensity decrease at b=800 sec/mm2 and an ADC map that was significantly higher than the surrounding liver [18]. A lesion was considered as metastatic if the lesion was strong, or moderately hyperintense on FSE T2 w images [19] and on DWI at b=125 sec/mm2 and remained hyperintense compared to surrounding normal liver parenchyma at b=800 sec/mm2, with an ADC lower or almost the same as that of the surrounding liver [19]. A lesion was considered indeterminate if the above criteria were not met (eg, if there was a partial signal intensity decrease or isointense ADC).
Consensus evaluation and reference standard
The detection of liver deposits and their characterization was done by the consensus reading of the 2 readers (A.J.S. and M.L.N.), which was performed 4 weeks after the initial interpretation of EP-DWI, FSE T2 wi and FFE T1 wi after i.v. Gd-EOB-DTPA administration. All lesions detected by the 2 observers were reviewed by comparing results of their search. The final decision was made by consensus if any doubt of the nature of the lesions existed. Solid, benign lesions like hemangiomas, focal nodular hyperplasia and adenomas were diagnosed using well known validated criteria. As stated above, all other benign lesions such as cysts, or in case of any doubt existed as to the nature of the lesion, were not counted. Other solid lesions were considered as highly probable malignancy, based on MR findings: enhancement characteristics, clinical history and previous examination. The presence of benign lesions like hemangiomas, which could overestimate the disease extent, was not recorded, due to the high probability of liver involvement in the natural history of GEP-NET carcinomas.
Statistical analysis
Statistica version 7.0 (StatSoft, Tulsa, OK) was used for statistical analysis. A separate analysis was performed for each of the end points – comparison of detection rate in liver deposits using different approaches of MRI was performed using Wilcoxon Matched Pair-test (dependent samples). Potential differences between groups of patients: sex, origin of primary malignancy (foregut, midgut, hindgut or unknown origin), hormone overproduction (secreting vs. non-secreting tumors), differentiation of cancer cell (G), somatostatin analogue therapy or previous chemotherapy, were analyzed using Mann Whitney U test (independent samples). The final analysis was performed for FES T2 wi, EP-DWI and FFE T1 w images after i.v. Gd-EOB-DTPA, including separate results for each reader and final consensus results with mean detection rate for both readers. All focal liver lesions over 5 mm in diameter were counted.
Cohen’s Kappa Statistics (κ coefficients) were used to assess interobserver agreement for liver lesion detection (0.00–0.20 indicated slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–1.00, almost perfect agreement) [22]. Additional interobserver analysis was performed for different counted number of liver deposits. The stratification included patients with n<5; 5≤n<20, 20≤n<50 and those at least with 50 lesions. P<0.05 was considered statistically significant.
Results
The distribution of the primary tumor localization base on surgery and/or imaging follow-up was as follows: 18 patients with foregut tumors (33%), 25 subjects with midgut tumors (45%), 6 patients had hindgut tumors (11%), and the same number with cancers of unknown origin (11%). Primary tumors were still present in 25 patients. All patients had histological confirmation of neuroendocrine carcinoma primary and liver deposits. Most patients had WHO group 2 cancers (51 cases), while 4 patients WHO group 3 cancers. Regarding differentiation of tumor cells, 16 cases were well-differentiated cancers (G1), 35 patients had moderately-differentiated (G2) tumors, and the remainder (4 patients) had poorly-differentiated carcinomas (G3). The remainder of the patients had an initial pathological confirmation of neuroendocrine carcinoma as their primary tumor with further spread of the disease based on both clinical grounds and imaging techniques. Other clinical details such as hormone overproduction in secreting tumors, previous radionuclide target therapy (90Y DOTATAE), chemotherapy, and “cold” analogue therapy are presented in Table 2.
Table 2.
Patients clinical characteristics (N=55) with confirmed GEP-NET cancer, all patients with clinical stage IV (CS) of disease.
| Number | Percent | |
|---|---|---|
| Age (mean and range (years) | 56.2 (35–69; SD 9.9)) | |
| Female/male | 30/25 | 64/36 |
| Primary tumour localization Foregut/midgut/hindgut/UNO* | 18/25/6/6 | 33/45/11/11 |
| Presence of primary tumour | 25 | 45 |
| Grading of cancer cell (G1/G2/G3) | 16/35/4 | 29/64/7 |
| Secretor vs. non-secretor cancer | 24/31 | 44/66 |
| Previous PRRT [90Y DOTATATE] | 51 | 93 |
| Previous SST analogue therapy | 35 | 64 |
| Previous chemotherapy | 24 | 44 |
UNO – unknown origin.
The rate of patients with liver deposits counted on standard FSE T2 w fat-suppressed images were as follows: less than 5 deposits 11% (6 cases), 6–19 deposits 40% (22 patients), 21–49 lesions were 38% (21 cases), patients with at least 50 liver lesions were 11% (6 cases), examples of patients with different number of liver deposits are presented in Figures 1, 2.
Figure 1.
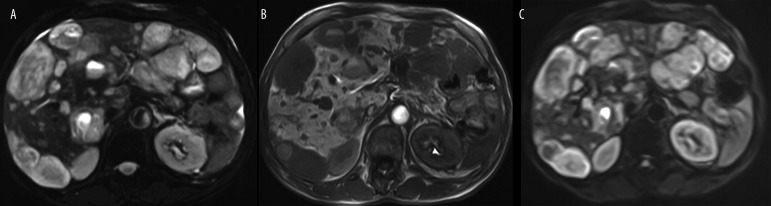
An example of multiple liver deposits (N=33) in a 58 year old male with midgut (small bowel), neuroendocrine carcinoma, well differentiated – G2 (WHO group 2), pT3N1M1, initially CS IV. (A) standard axial FSE T2 w fat-suppressed images, (B) Axial SE T1 w images after i.v. (Gd-EOB)-DTPA late image after 20 min. and (C) Axial SE Single Shot EP-DWI (b=125 sec/mm2).
Figure 2.
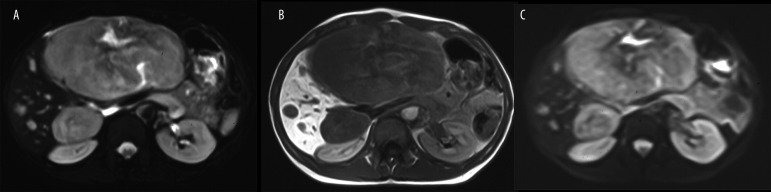
An example of few liver deposits (N=12) in a 45 year old female with hindgut (rectum), neuroendocrine carcinoma, well differentiated – G2 (WHO group 2), pT2N1M1, initially CS IV. (A) standard axial FSE T2 w fat-suppressed images, (B) Axial SE T1 w images after i.v. (Gd-EOB)-DTPA late image after 20 min. and (C) Axial SE Single Shot EP-DWI (b=125 sec/mm2).
The mean number of liver deposits in all patients (averaged for 2 observers) using standard FSE T2 w fat-suppressed images was 20.7, using EP-DWI was 24.0 and FFE T1 w images after i.v. (Gd-EOB)-DTPA was 25.7, all details presented in Table 3. There was no significant difference in overall detection rate of liver deposits seen in EP-DWI, FSE T2 w images and FFE T1 w images after i.v. (Gd-EOB)-DTPA with both readers; data sets of both radiologists were used (intraobserver and interobserver, P>0.05).
Table 3.
Number of detected liver lesions by two observers in different MRI approaches for all patients and also in different group of subjects like gender, primary tumor localization or hormone overproduction.
| Subjects | Number of detected liver lesions by two observers | ||
|---|---|---|---|
| FSE T2 wi Mean; SD, CI ±95% | EP-DWI Mean; SD, CI ±95% | SE T1 i.v. (Gd-EOB)-DTPA Mean; SD, CI ±95% | |
| Female (N=30) | 16.6 (14.6; 11–22.0) | 19.6 (16.9; 13–25.0) | 20.4 (16.8; 14–26.0) |
| Male (N=25) | 26.5 (18.4; 18.9–34.1) | 29.9 (20.3; 21–38.0) | 32.6 (21.6; 23.7–41.5) |
| Foregut (N=18) | 20.8 (19.9; 10.9–30.7) | 24.2 (22.5; 13–35.0) | 25.4 (23,6; 13.6–37,10 |
| Midgut (N=25) | 23.9 (13.1; 18–29.0) | 26.9 (14.2; 21–32.0) | 29.0 (15.6; 22.7–35.6) |
| Hindgut (N=6) | 10.0 (12.9; 3–23.0) | 12.5 (14.8; 3–28.0) | 14.5 (14.3; 3 3–29.0) |
| UNO (N=6) | 21.5 (24.6; 4–47.0) | 25.3 (28.5; 4–55.0) | 25.7 (28; 4–55.5) |
| Secretor (N=24) | 25.3 (17.4; 10.8–23.5) | 29.2 (20; 12.8–27.5) | 31.2 (20.6; 13.8–28.9) |
| Non-Secretor (N=31) | 17.2 (15.3; 18.9–31.8) | 20.1 (16.8; 21.9–36.1) | 21.3 (18.1; 23.6–38.9) |
| All (N=55) | 20.7 (16.7; 17.6–23.9) | 24.0 (18.9; 20.4–27.6) | 25. 7 (19.9.9; 21.9–29.5) |
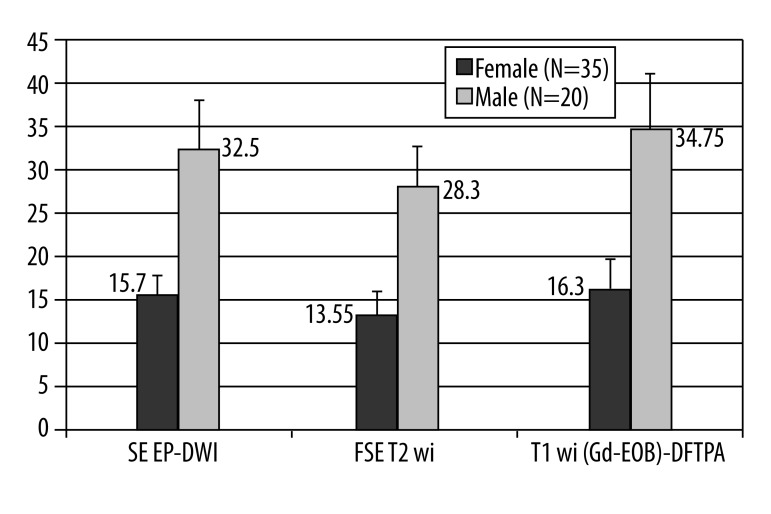
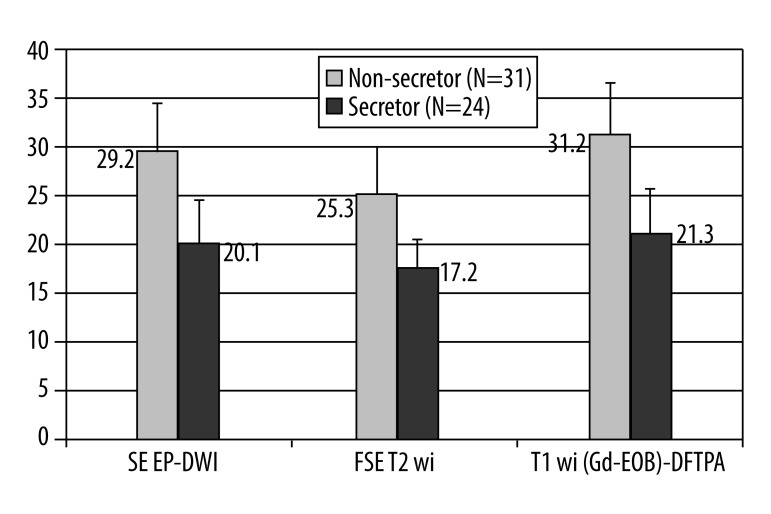
There was a significant difference in the detection rate of lesions in male patients compare to number of lesions found in females. Both readers, as well as their consensus reading, confirmed that male patients had a significantly higher number of liver deposits than their female counterparts, including all MRI approaches (Mann-Whitney U test P=0.03–0.04) (graphical illustration presented in Figure 3). Both readers noted a significant difference in detection rates between patients with secreting tumors (hormone overproduction) and those patients with non-secreting tumors (Mann-Whitney U test P=0.02–0.03) (Figure 4). There was also a significant difference in liver deposits between patients who were treated with cold analogues compared to those without such therapy (Mann-Whitney U test P=0.005–0.001).
Figure 3.
A graphical presentation of differences between detection rate of liver deposits in male and female patients with GEP-NET carcinomas, including all tested MRI approaches (Mann-Whitney U test P=0.03–0.04).
Figure 4.
A graphical presentation of differences between detection rate of liver deposits between patients with secreting tumours (hormone overproduction) and those patients with non-secreting tumours (Mann-Whitney U test P=0.02–0.03).
Other potential prognostic factors such as origin of primary cancer, differentiation of cancers cells (G), or previous chemotherapy had no significant impact on the number of liver deposits found in these patients. The mean liver metastasis detection rate of both readers for all patients, including separate groups: sex, primary tumor localization or hormone overproduction (using FSE T2 w images, EP-DWI and FFE T1 w images after i.v. (Gd-EOB)-DTPA), are additionally presented in Table 3.
Interobserver agreement
There was nearly perfect agreement of both readers in liver metastasis detection rate using each of the tested MRI techniques. The agreement was excellent in separate groups of patients – those with less than 5 lesions, more than 5 but less than 20 lesions, those who had over 20 lesions but less than 50, and those who had over 50 deposits (Cohen’s κ=0.848–1). All details of Cohen κ statistics are presented in Table 4.
Table 4.
Cohen κ statistics: Both readers in separate group of patients those with less than 5 lesions, those with more than 5 and less 20 lesions, those who had over 20, but less than 50, and last group over 50 deposits including each of tested MRI approach.
| Number of Lesions Detected (N) | FSE T2 wi Cohen κ range | EP-DWI Cohen κ range | SE T1 i.v. (Gd-EOB)-DTPA Cohen κ range |
|---|---|---|---|
| N≤5 | 0.936 | 0.945 | 1.000 |
| 5<N≤20 | 0.917 | 0.919 | 1.000 |
| 20<N≤50 | 0.924 | 0.922 | 0.961 |
| N>50 | 0.899 | 0.847 | 0.912 |
| Overall | 0.921 | 0.920 | 0.974 |
Discussion
Accurate detection of liver deposits in neuroendocrine tumors is an important issue for planning further treatment, especially if it considers radical surgery, debulking or palliation [1,4,10,11]. The presented data indicates a slightly higher detection rate of liver deposits in patients with neuroendocrine carcinoma when using EP-DWI and FFE T1 w images after i.v. (Gd-EOB)-DTPA as compared to the standard FSE T2 w fat-suppressed MRI approach. Based on our results, this was not statistically significant, but using both new MR approaches we were able to detect more lesions in each of the tested groups of patients. Another great advantage of using MR to assess liver involvement in neuroendocrine carcinomas is its high detection rate of small (5–10 mm) lesions. These results agree with other published data indicating an improved detection rate of EP DW MRI over standard FSE T2 in different tumors [6,20,21,23]. This is particularly important in detection of small lesions, and poses a particular challenge to most imaging diagnostic modalities available today. Advances in current standard techniques like MDCT, or US with contrast enhancement, as well as functional imaging using 99mTc or 68Ga labelled somatostatin analogues are helpful, but fall short, and usually underestimate liver involvement of patients with this particular disease [12]. Heterogeneous expression of type 2 somatostatin receptors is a common problem in both functional imaging methods using 99mTc SRS or 68Ga DOTATATE PET, which can lead to low or no expression of these receptors at all [24]. Even well differentiated tumors (WHO group 2) could potentially have minimal expression of these receptors, again underestimating liver burden [10,12,13].
Early detection of even the smallest lesions is crucial in further patient management [2,4,6,11]. With early detection some patients may benefit from potentially curative liver surgery or focal ablative therapy [5]. In case of significant underestimation, surgery can have detrimental effects, and further deterioration of the patient can occur. We recommend the use of EP-DWI sequences to improve detection rates as well as possibly improve the characterization of small lesions, as reported before by Parikh [16] and Ichikawa [19]. Small solid foci identified within the liver were highly likely to be metastatic deposits due to the natural course of GEP-NETs. In those patients, the high metastatic potential of these tumors is well known [1–3]. Further lesion characterization was not the main objective in our study. Due to the natural course of neuroendocrine carcinomas and their high metastatic potential, we expect a very high positive predictive value in this group of patients [1–4,11–13].
The present MR algorithm for patients with GEP-NETs consists of SE T1 and currently more useful refocused GRE sequences like Fast Field Echo (FFE) T1w and FSE T2 w sequences with fat-suppression followed by dynamic imaging GRE FFE T1 wi after i.v. contrast enhancement usually with Gd-DTPA [6,12,13,21]. These are done with breath-hold, with respiratory-triggered techniques as to eliminate potential movement artefacts and continue examination after the next breath-hold. Another advantage of MRI approaches to improve detection rate of liver metastasis is high agreement between both readers in detection rates using different MR sequences; these findings seem to match those reported by Parikh [16]. It seems that this single technique has a high detection rate, independent of interobserver bias.
Our routine use of the breath-hold technique shortened the examination time on average by approximately 5 minutes. In about 10% of the patients we used the breath-triggered technique due to the clinical status of the patient or insufficient patient cooperation. The breath-triggered technique could potentially improve image quality, but the prolonged examination time can cause patient fatigue and in turn translate to patient movement, which deteriorates image quality. Some reports insist on the sole use of the breath-triggered technique. In particular, measurements of ADC maps based on DWI seem to be more reproducible using the breath-triggered technique. In clinical practice the breath-hold technique can be a sufficient tool for good image acquisition without the need for pure research settings [20,21].
Slightly better detection rates were obtained using liver-specific (Gd-EOB)-DTPA contrast media, but this was not statistically significant when compared to the results obtained with conventional FSE T2 and tested EP-DWI sequences. Twenty minutes after contrast administration, an increase in the liver-to-lesion contrast, which allows for easier and more precise lesion localization, can be observed [9,14,15]. Significant improvement of detection rate using (Gd-EOB)-DTPA was noted in several reports, which indicates the high diagnostic value of this approach [9,15,25]. Standard, dynamic acquisitions offer the possibility to explore the arterial supply and portal blood flow, which is very abundant in neuroendocrine metastasis within the liver [10–13].
More lesions were found during the late phase of the examination, most likely due to the liver-specific nature of (Gd-EOB)-DTPA that does not allow for intracellular accumulation in these lesions. Our experience is similar to other reports of the potential clinical use of this agent [9,15,25]. Great image quality, perfect delineation of lesions, and good visualization of liver anatomy are all characteristics of SE T1 w images. These attributes make SE T1 w images indispensible when planning surgical treatment. Some reports state that the surgical approach will be changed in up to 15% of the patients, based on the very sensitive information that MR studies can provide [26].
Despite this, the SE EP-DWI sequence has shown clinical utility in improving the detection rate of GEP-NET metastases. [9,25]. In our experience, the entire examination should not take more then 20–25 minutes, which is an acceptable time frame in patients with advanced liver metastases. In our opinion, SE T1 w, along with EP-DWI sequences, are indicated in the assessment of GEP-NET patients and should be a part of the standard protocol. In our experience, any additional delayed acquisitions after i.v. (Gd-EOB)-DTPA are uncomfortable for patients and usually cause image degradation due to movement artefacts and lead to repeat scanning. Due to the high definition of those images with highest detection rate, we suggest starting with an initial (Gd-EOB)-DTPA examination, in order to confirm liver involvement. Follow-up study could be then performed using non-contrast examination based on FSE T2 and EP DW MRI. Over 88% of our patents had multiple lesions, which eliminated the possibility for a curative surgical procedure. In a few cases where conventional imaging indicated the presence of 5 or fewer lesions, further surgical planning was based on MR findings. In some of these cases, MR revealed more lesions (data not shown), which disqualified the patients from surgical intervention; this emphasizes the importance of small lesion detection (5–10 mm), without which the stage of the disease is often grossly underestimated. This underestimation can place an unresectable patient at unnecessary risk of a surgical procedure. All MR approaches used in our study seem to be efficient enough in detecting lesions between 5 and 10 mm, which affects proper patient management and prognosis. The high metastatic potential of GEP-NETs give high probability that the lesions detected are indeed metastatic; this characteristic allowed us to abandon potential false-positive detection analysis [1,2,4,7–12].
The potential resolution of the problem with the long duration of (Gd-EOB)-DTPA examination is use of an extra analgesia in case of pain due to prolonged immobilization of the patient [25]. The duration of (Gd-EOB)-DTPA-enhanced scans is the main limitation of their clinical use in routine diagnostic imaging follow-up; such scans should be restricted to a selected patient population [27,28]. The additional cost of the contrast agent could also prove to be a limiting factor when used as a screening test in GEP-NET patients with widespread metastases to the liver.
Conclusions
In this study, all of the tested MR approaches – standard FSE T2 fat suppressed, EP-DWI and also FEE T1 w after i.v. (Gd-EOB)-DTPA – were characterized by a high detection rate of liver deposits in patients with advance GEP-NET.
The slightly higher detection rate of FFE T1 wi (not statistically significant) after i.v. Gd-EOB-DTPA is offset by the added time of examination, which could be difficult to perform in patients whose disease is in an advanced clinical stage, or those who suffer pain during examinations. Another limitation of routine use of (Gd-EOB)-DTPA in patients with GEP-NET is its high cost.
EP-DWI should be used together with other sequences of standard MRI examination to describe the precise anatomical localization of the liver deposits in case any doubt exists.
Footnotes
Source of support: This work has been partly supported by research grant Nr N518 413 438 of the Polish Ministry of Science and Higher Education and also by Polish Patients Neuroendocrine Support Group
References
- 1.Caplin ME, Buscombe JR, Hilson AJ, et al. Carcinoid tumour. Lancet. 1998;352:799–805. doi: 10.1016/S0140-6736(98)02286-7. [DOI] [PubMed] [Google Scholar]
- 2.Plöckinger U, Rindi R, Arnold R, et al. Guidelines for the Diagnosis and Treatment of Neuroendocrine Gastrointestinal Tumours. Neuroendocrinology. 2004;80:394–424. doi: 10.1159/000085237. [DOI] [PubMed] [Google Scholar]
- 3.Kloeppel G, Rindi G, Anlauf M, et al. Site-specific biology and pathology of gastroenteropancreatic neuroendocrine tumours. Virchows Archive. 2007;451:S9–27. doi: 10.1007/s00428-007-0461-0. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson B, Kloeppel G, Krenning EP, et al. Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Tumours. Well Differentiated Jejunal – Ileal Tumor/Carcinoma Neuroendocrinology. 2008;87:8–19. doi: 10.1159/000111034. [DOI] [PubMed] [Google Scholar]
- 5.Ćwikla JB, Nasierowska-Guttmejer A, Jeziorski KG, et al. Algorytm diagnostyczny guzów neuroendokrynnych układu pokarmowego (GEP – NET) i oskrzela. Pol J Radiol. 2005;70:85–92. [in Polish] [Google Scholar]
- 6.Sundin A, Vullierme MP, Kaltsas G, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumours: radiological examinations. Neuroendocrinology. 2009;20:167–83. doi: 10.1159/000184855. [DOI] [PubMed] [Google Scholar]
- 7.Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the Radiolabelled Somatostatine Analog [177Lu-DOTA0Tyr3]octreotate: Toxicity, Efficacy and Survival. J Clin Oncol. 2008;26:2124–30. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 8.Ćwikła JB, Sankowski AJ, Seklecka N, et al. Efficacy of radionuclide treatment 90Y-DOTATATE in patients with progressive metastatic gastroenteropancreatic neuroendocrine carcinomas (GEP-NET). A phase II study. Annals Oncology. 2010;21:787–94. doi: 10.1093/annonc/mdp372. [DOI] [PubMed] [Google Scholar]
- 9.Sankowski AJ, Ćwikła JB, Nowicki ML, et al. Comparison of MRI DWI vs. MRI T1 contrast enhancement using (Gd)-DTPA and (Gd-EOB)-DTPA in detection rate of liver metastasis in patients with advance gastroenteropancreatic-neuroendocrine tumours (GEP-NET) Eur Radiol. 2010;(Suppl) Abstract. [Google Scholar]
- 10.Chrzan R, Sowa-Staszczak A, Tomaszuk M, et al. Usefulness of SPECT/CT fusion in the verification of lesions detected in somatostatin receptor scintigraphy and in the evaluation of therapy efficiency in patients with gastroenteropancreatic neuroendocrine tumors. Pol J Radiol. 2009;74:29–33. [Google Scholar]
- 11.Cwikła JB, Buscombe JR, Caplin ME, et al. Diagnostic imaging of carcinoid metastases to the abdomen and pelvis. Med Sci Monit. 2004;10(Suppl 3):9–16. [PubMed] [Google Scholar]
- 12.Dromain C, de Baere T, Lumbroso J, et al. Detection of liver metastases from endocrine tumours: a prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J Clin Oncol. 2005;23:70–78. doi: 10.1200/JCO.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Cwikła JB, Nasierowska-Guttmejer A, Jeziorski K, et al. Diagnostic imaging approach to gastroentero-pancreatic carcinomas of neuroendocrine origin – single NET center experience in Poland. Neuroendocrinol Letters. 2007;28:789–800. [PubMed] [Google Scholar]
- 14.Schuhmann-Giampieri G, Schmitt-Willich H, Press WR, et al. Preclinical evaluation of Gd-EOB-DTPA as a contrast agent in MR imaging of the hepatobiliary system. Radiology. 1992;183:59–64. doi: 10.1148/radiology.183.1.1549695. [DOI] [PubMed] [Google Scholar]
- 15.Vogl TJ, Kuemmel S, Hammerstingl R, et al. Liver tumours: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology. 1996;200:59–67. doi: 10.1148/radiology.200.1.8657946. [DOI] [PubMed] [Google Scholar]
- 16.Parikh T, Drew SJ, Lee VS, et al. Focal liver lesions detection and characterization with Diffusion MR Imaging: Comparison with breath hold T2 weighted imaging. Radiology. 2008;246:812–22. doi: 10.1148/radiol.2463070432. [DOI] [PubMed] [Google Scholar]
- 17.Taouli B, Vilgrain V, Dumont E, et al. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology. 2003;226:71–78. doi: 10.1148/radiol.2261011904. [DOI] [PubMed] [Google Scholar]
- 18.Danet IM, Semelka RC, Leonardou P, et al. Spectrum of MRI appearances of untreated metastases of the liver. AJR. 2003;181:809–17. doi: 10.2214/ajr.181.3.1810809. [DOI] [PubMed] [Google Scholar]
- 19.Ichikawa T, Haradome H, Hachiya J, et al. Diffusion-weighted MR imaging with single-shot echo-planar imaging in the upper abdomen: preliminary clinical experience in 61 patients. Abdom Imaging. 1999;24:456–61. doi: 10.1007/s002619900539. [DOI] [PubMed] [Google Scholar]
- 20.Touli B, Koh D-M. Diffusion-Weighted MR Imaging of the liver. Radiology. 2010;254:47–66. doi: 10.1148/radiol.09090021. [DOI] [PubMed] [Google Scholar]
- 21.Bruegel M, Gaa J, Waldt S, et al. Diagnosis of hepatic metastasis: comparison of respirationrespiration- triggered diffusion-weighted echoplanar MRI and five t2-weighted turbo spinecho sequences. AJR. 2008;191:1421–29. doi: 10.2214/AJR.07.3279. [DOI] [PubMed] [Google Scholar]
- 22.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363–74. [PubMed] [Google Scholar]
- 23.Coenegrachts K, Delanote J, Ter Beek L, et al. Improved focal liver lesion detection: comparison of single-shot diffusion-weighted echoplanar and single-shot T2 weighted turbo spin echo techniques. Br J Radiol. 2007;80:524–31. doi: 10.1259/bjr/33156643. [DOI] [PubMed] [Google Scholar]
- 24.Reubi JC, Schar JC, Waser B, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273–82. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 25.Huppertz A, Balzer T, Blakeborough A, et al. Improved detection of focal liver lesions at MR Imaging: Multicenter Comparison of Gadoxetic Acid-enhanced MR Images with Intraoperative Findings. Radiology. 2004;230:266–75. doi: 10.1148/radiol.2301020269. [DOI] [PubMed] [Google Scholar]
- 26.Denecke T, Rühl R, Hildebrandt B, et al. Planning transarterial radioembolization of colorectal liver metastases with Yttrium 90 microspheres: evaluation of a sequential diagnostic approach using radiologic and nuclear medicine imaging techniques. Eur Radiol. 2008;18:892–902. doi: 10.1007/s00330-007-0836-2. [DOI] [PubMed] [Google Scholar]
- 27.Wiggermann Ph, Sieron D, Brosche Ch, et al. Transarterial Chemoembolization of Child-A hepatocellular carcinoma: Drug eluting bead TACE (DEB TACE) vs. TACE with Cisplatin/Lipidol (cTACE) Med Sci Monit. 2011;17(4):CR189–95. doi: 10.12659/MSM.881714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarząbek M, Jargiełło T, Wolski A, et al. Drug-eluting microspheres transarterial chemoembolization (DEM TACE) in patients with liver metastases. Pilot study. Pol J Radiol. 2011;76(3):26–32. [PMC free article] [PubMed] [Google Scholar]