Summary
Background
The consequences of aggressive therapy following a myocardial infarction (MI) on ventricular remodeling are not well established. Thus, the objective of this study was to analyze the prevalence, clinical characteristics, and predictors of left ventricular remodeling in the era of modern medical therapy.
Material/Methods
Clinical characteristics and echocardiographic data were analyzed in 66 consecutive patients with anterior infarction at admission and at 6-month follow-up. Ventricular remodeling was defined as an increase of 10% in ventricular end-systolic or end-diastolic diameter.
Results
In our study, 58% of patients presented with ventricular remodeling. Patients with remodeling possessed higher total plasma creatine kinase (CPK), MB-fraction (CPK-MB), heart rate, heart failure, shortness of breath, and reperfusion therapy than patients without remodeling. In contrast, patients with remodeling had a smaller ejection fraction, E-Wave deceleration time (EDT), and early (E′ Wave) and late (A′ Wave) diastolic mitral annulus velocity (average of septal and lateral walls), but a higher E/E′ than patients without remodeling. Patients with remodeling used more diuretics, digoxin, oral anticoagulants and aldosterone antagonists than patients without remodeling. In the multivariate analyses, only E′ Wave was an independent predictor of ventricular remodeling. Each 1 unit increase in the E′ Wave was associated with a 59% increased odds of ventricular remodeling.
Conclusions
In patients with anterior MI, despite contemporary treatment, ventricular remodeling is still a common event. In addition, diastolic function can have an important role as a predictor of remodeling in this scenario.
Keywords: predictors, remodeling, ventricular dilation
Background
Cardiac remodeling may be defined as changes in the size, geometry, shape, composition, and function of the heart [1–4]. After acute myocardial infarction (MI), this process is clinically characterized by an increase in the ventricular cavity. In the acute phase, ventricular dilation is a result of the infarction expansion process, whereas late cavity dilation is the result of the eccentric hypertrophy process [4,5].
Ventricular remodeling is associated with cardiac rupture, ventricular aneurysm, an increased risk for progressive ventricular dysfunction, and cardiovascular death after MI. Therefore, several variables have been used to predict the remodeling process in the acute phase of MI, such as infarct size, infarct location, previous infarct, wall stress, neurohumoral activation, diabetes mellitus, hypertension, decreased ejection fraction, and signs of heart failure [6–9].
In recent years there have been significant advances in the treatment of patients with MI, in particular in the use of anti-remodeling strategies, including reperfusion therapy, angiotensin converting enzyme inhibitors, and beta-blockers [10]. Therefore, the objective of this study was to analyze the prevalence, clinical characteristics, and predictors of left ventricular remodeling in the era of modern medical therapy.
Material and Methods
All procedures were approved by the ethics committee of our institution, and all participants provided their written consent. From January 2008 to November 2009, consecutive patients with anterior myocardial infarction were prospectively recruited.
Acute MI was diagnosed in the presence of 2 of the following criteria: persistent angina pectoris for ≥20 min and ST-segment elevation of ≥2 mm in ≥2 contiguous precordial leads or the presence of a new or presumably new left bundle branch block. Acute MI was later confirmed by the elevation of cardiac enzymes of more than twice the upper limit of the normal range.
Exclusion criteria were active malignancy, infection, end-stage cardiac, pulmonary or hepatic disease, pregnancy, age <18 years, atrial fibrillation, previous myocardial infarction, and valve disease.
At admission, data on patient characteristics, including waist circumference, body mass index, age, sex, heart rate, cardiovascular risk factors, concomitant diseases, adverse events, medical treatment and data regarding symptoms and pre-hospital delay, were recorded. Our definition of diabetes mellitus was based on clinical features and a fasting glucose level of ≥126 mg/dL on 2 separate occasions or ongoing treatment for the disease. Systemic arterial hypertension was considered to be present if the systolic blood pressure was >140 mm Hg and/or diastolic blood pressure was >90 mm Hg or the patient was already maintained on antihypertensive drug therapy. Dyslipidemia was identified according to the National Cholesterol Education Program (NCEP) III guidelines as total cholesterol levels ≥200 mg/dL, or HDL <40 mg/dL for men and <50 mg/dL for women, or a triglycerides level ≥150 mg/dL. Obesity was defined as a body mass index (BMI) ≥30 kg/m2.
For the adverse events during the follow-up period, stable angina was diagnosed in the presence of cardiac symptoms in a pattern that remained constant in presentation, frequency, character and duration over time, and coronary disease was diagnosed using coronary angiography. Unstable angina was diagnosed in the presence of new cardiac symptoms and positive electrocardiogram (ECG) findings with normal biomarkers or a changing pattern of symptoms and positive ECG findings with normal biomarkers and coronary disease at coronary angiography. All other prespecified definitions utilized in this study were similar to previous clinical trials [11].
The echocardiogram assessment was completed by the same operator during the index hospitalization (approximately 3–5 days after admission) and at the 6-month follow-up. The echocardiograph was an HDI 5000 Sono CT model (Philips Medical Systems, Bothell, Washington, USA) equipped with a 2.0 to 4.0 MHz probe capable of acquiring second harmonic, tissue, pulsed, continuous, and color Doppler, as well as one- and two-dimensional mode images. With individuals positioned in the left lateral decubitus and monitored with an electrocardiographic lead, the following echocardiographic views were obtained: parasternal short-axis to measure the ventricles, aorta and left atrium and the apical 2-, 4- and 5-chambers to evaluate the cavities and the systolic and diastolic functions of the ventricles. All measurements were performed in accordance with the recommendations of the American Society of Echocardiography/European Association of Echocardiography [12]. The average of 3 measurements was calculated for each variable. In the study group, intraobserver and interobserver variabilities were <3% and <5%, respectively.
The left atrium volume was obtained using the Simpson method from the apical 2- and 4-chamber views. LV systolic function was evaluated by measuring the ejection fraction according to the Simpson method. LV diastolic function was evaluated by measuring the early (E-Wave) and late (A-Wave) diastolic mitral inflow velocity, the E- to A-Wave ratio, the E-Wave deceleration time (EDT), the isovolumic relaxation time (IVRT), the early (E′ Wave) and late (A′ Wave) diastolic mitral annulus velocity (the average of the septal and lateral walls) using tissue Doppler, and the E/E′ ratio. Ventricular remodeling was defined as an increase of 10% in the LV end-systolic or end-diastolic diameter at the 6-month follow-up [13].
The comparisons between the groups were completed with Student’s t tests when the data presented a normal distribution. For a non-normal distribution, the comparisons between the groups were completed using Mann-Whitney U tests. The data were expressed as the mean ± standard deviations or the median with the 25th and 75th percentiles. A chi-squared test was used to compare categorical variables. The predictive values were analyzed using a multivariate logistic regression. Data analysis was completed with SigmaStat for Windows v2.03 (SPSS Inc, Chicago, IL). The significance level was considered to be 5%.
Results
Seventy-six consecutive patients were evaluated. Three patients presented with atrial fibrillation, 1 patient had valve disease and 6 patients died. Thus, 66 patients were analyzed at admission and at the 6-month follow-up.
In our study, 58% of patients demonstrated ventricular remodeling. The patients were divided in 2 groups using the clinical and echocardiographic data – patients with remodeling and patients without remodeling.
The clinical characteristics are shown in Table 1. Patients with remodeling presented with higher total plasma creatine kinase (CPK) levels, MB-fraction (CPK-MB), heart rate, incidence of heart failure, shortness of breath, and reperfusion therapy. The remaining variables showed no differences between the groups.
Table 1.
Demographic, clinical and laboratory data.
| Variables | Left ventricular remodeling | P value | |
|---|---|---|---|
| Yes (n=38) | No (n=28) | ||
| Age (yrs) | 57±11 | 61±14 | 0.238 |
| Male (%) | 71 | 82 | 0.454 |
| HP (%) | 60 | 53 | 0.754 |
| DM (%) | 29 | 25 | 0.939 |
| Dyslipidemia (%) | 82 | 89 | 0.498 |
| Smoking (%) | 45 | 29 | 0.280 |
| BMI (kg/m2) | 27±4 | 26±4 | 0.416 |
| CPK (U/L) | 6851 (3963–8734) | 1525 (841–4364) | <0.001 |
| CPK-MB (U/L) | 512 (318–664) | 183 (107–454) | 0.002 |
| HR (beats/min) | 85±14 | 72±16 | 0.001 |
| Heart failure (%) | 64 | 27 | 0.007 |
| SB (%) | 18 | 0 | 0.018 |
| Reperfusion (%) | 94 | 75 | 0.030 |
| TIMI ≥2 (%) | 88 | 96 | 0.384 |
HP – hypertension; DM – diabetes mellitus; BMI – body mass index; SB – shortness of breath; CPK – creatine phosphokinase; CPK-MB – creatine phosphokinase – MB; TIMI – Thrombolysis In Myocardial Infarction grade. Data are expressed as the mean ±SD or the median (including the lower and upper quartiles).
The medications utilized during the hospitalization are shown in Table 2. Patients with remodeling used more diuretics, digoxin, oral anticoagulants and aldosterone antagonist than patients without remodeling. The remaining variables showed no differences between the groups. After 6 months, the rates of patients using aspirin, angiotensin-converting enzyme inhibitors, and beta-blockers were 95%, 86% and 82%, respectively. Importantly, after 6 months, considering angiotensin-converting enzyme inhibitors, 76% of patients with remodeling and 78% of patients without remodeling continued with medication. Considering beta blockers, 76% of patients with remodeling and 93% of patients without remodeling continued with medication.
Table 2.
Medication data.
| Variables | Left ventricular remodeling | P value | |
|---|---|---|---|
| Yes (n=38) | No (n=28) | ||
| FT (%) | 18 | 17 | 0.792 |
| ASA (%) | 100 | 100 | 1.00 |
| Clopidogrel (%) | 100 | 100 | 1.00 |
| Heparin (%) | 95 | 93 | 1.00 |
| ACE i (%) | 95 | 93 | 1.00 |
| Beta-blockers (%) | 97 | 100 | 1.00 |
| Nitrates (%) | 37 | 30 | 0.282 |
| Digoxin (%) | 63 | 36 | 0.051 |
| Spironolactone (%) | 40 | 14 | 0.050 |
| Diuretics (%) | 66 | 36 | 0.030 |
| Statins (%) | 100 | 93 | 0.176 |
FT – fibrinolytic therapy; ASA – acetylsalicylic acid; ACE i – angiotensin converting enzyme inhibitor.
The initial echocardiographic data are shown in Table 3. Patients with remodeling presented with smaller ejection fractions, EDTs, E′ Waves, and A′ Waves than patients without remodeling. In contrast, patients with remodeling presented with higher E/E′ ratios than patients without remodeling. The remaining variables showed no differences between the groups. The main echocardiographic data after 6 months are shown in Table 4.
Table 3.
Initial echocardiographic data.
| Variables | Left ventricular remodeling | P value | |
|---|---|---|---|
| Yes (n=38) | No (n=28) | ||
| LA (mm) | 41.0 (37–46) | 40.0 (38–44) | 0.668 |
| LVDD (mm) | 48.0 (45–53) | 50.0 (49–52) | 0.114 |
| LVSD (mm) | 32.0 (29–37) | 33.0 (31–36) | 0.508 |
| E′ wave (cm/s) | 8.3 (5.5–9.4) | 10.5 (9.5–11.7) | 0.002 |
| A′ wave (cm/s) | 11.6 (10.0–13.9) | 14.8 (13.8–16.0) | 0.001 |
| E/E′ | 8.5 (5.8–11.2) | 6.1 (5.1–6.8) | 0.001 |
| E/A | 0.79 (0.65–1.00) | 0.78 (0.69–0.89) | 0.791 |
| IVRT (ms) | 111±21 | 115±16 | 0.350 |
| EDT (ms) | 170±56 | 238±51 | <0.001 |
| EF (%) | 37.0 (35–50) | 48.0 (43–58) | <0.001 |
LV – left ventricle; LA – left atrium; LVDD – LV end-diastolic dimension; LVSD – LV systolic dimension; E′ wave – early diastolic mitral annulus velocity (average of septal and lateral walls); A′ wave – late diastolic mitral annulus velocity (average of septal and lateral walls); IVRT – isovolumetric relaxation time; EDT – E-Wave deceleration time; EF – ejection fraction. Data are expressed as the mean ±SD or the median (including the lower and upper quartiles).
Table 4.
Echocardiographic data after 6 months.
| Variables | Left ventricular remodeling | P value | |
|---|---|---|---|
| Yes (n=38) | No (n=28) | ||
| LA (mm) | 43.0 (40.0–47.0) | 40.8 (39.0–42.5) | 0.027 |
| LVDD (mm) | 52.6 (49.1–57.0) | 50.0 (48.0–51.0) | 0.007 |
| LVSD (mm) | 36.6 (34.1–44.0) | 32.0 (30.2–35.7) | <0.001 |
| E′ wave (cm/s) | 9.0 (8.0–11.0) | 11.0 (9.3–11.0) | 0.013 |
| EF (%) | 45.0 (40–48) | 53.0 (49–61) | <0.001 |
LV – left ventricle; LA – left atrium; LVDD – LV end-diastolic dimension; LVSD – LV systolic dimension; E′ wave – early diastolic mitral annulus velocity (average of septal and lateral walls); EF – ejection fraction. Data are expressed as the mean ±SD or the median (including the lower and upper quartiles).
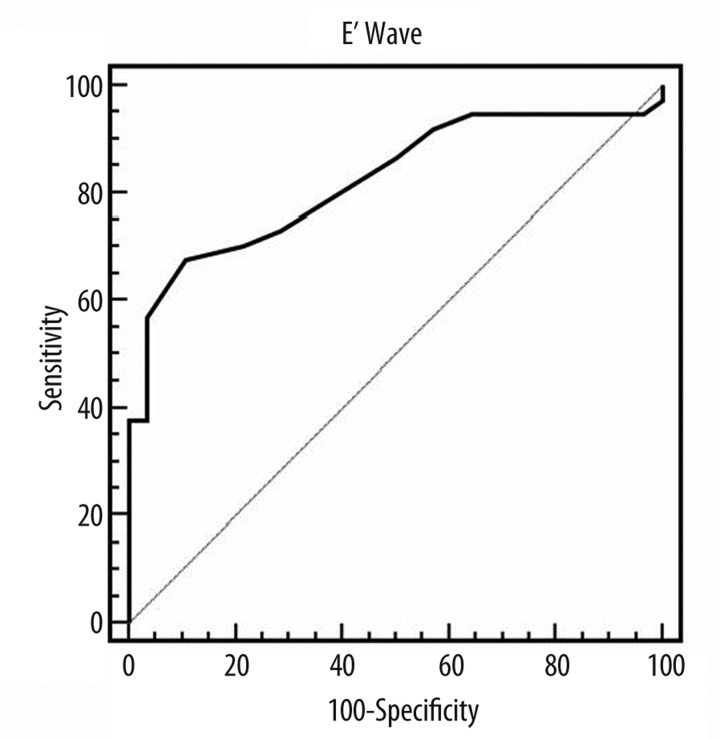
In the multivariate analyses, only the E′ Wave was an independent predictor of ventricular remodeling (Table 5). Each 1 unit increase in the E′ Wave was associated with a 59% increased odds of ventricular remodeling. In addition, Figure 1 shows the ROC curve for ventricular remodeling with cutoff ≤9; sensitivity=67.6%; specificity=89.3% (AUC=0.822; 95% CI=0.708–0.906; p=0.0001).
Table 5.
Multiple logistic regression for ventricular remodeling prediction.
| Variables | Odds ratio 95% CI | P value | |
|---|---|---|---|
| E′ wave (cm/s) | 0.629 | 0.473–0.836 | 0.001 |
| HR (beats/min) | 1.019 | 0.981–1.058 | 0.340 |
| CPK/100 (U/L) | 1.014 | 0.992–1.036 | 0.210 |
| EF (%) | 0.035 | 0.001–12.613 | 0.265 |
E′ wave – early diastolic mitral annulus velocity (average of septal and lateral walls); HR – heart rate; CPK – creatine phosphokinase, EF – ejection fraction.
Figure 1.
ROC curve for ventricular remodeling. Cutoff ≤9; sensitivity=67.6%; specificity=89.3% (AUC=0.822; 95% CI=0.708–0.906; p=0.0001).
Discussion
The goal of this study was to analyze the prevalence, clinical characteristics, and predictors of left ventricular remodeling after coronary occlusion in the era of modern medical therapy. Despite aggressive treatment, including a high percentage of reperfusion and anti-remodeling strategies, ventricular enlargement is common in patients with anterior MI. E′ Wave assessed using tissue Doppler is an independent predictor of remodeling at 6-month follow-up.
Classically, experimental and clinical evidence suggest that ventricular remodeling is a frequent event after MI. Indeed, studies completed in dogs and rats following coronary occlusion found an expansion of 81% and 65%, respectively [14,15]. Likewise, left ventricular enlargement was present, ranging from 40% to 50%, in patients after MI [6,16–19]. Importantly, another study demonstrated that 61% of patients with anterior myocardial infarction dilated compared with 33% of patients with inferior infarction [6].
Recent improvements in medical therapy and the management of acute MI could impact the incidence and extent of left ventricular remodeling post-MI, including reperfusion therapy [20], angiotensin-converting enzyme inhibitors [21], and beta-blockers [22]. In fact, in the 17 patients with anterior MI, there was a significant increase in left ventricular end-diastolic volume from 2 weeks to 1 month; however, no significant change occurred thereafter [23]. Likewise, progressive left ventricular dilation occurred in 24% of patients after MI in the 86 patients treated with primary percutaneous coronary intervention [24]. In contrast, for patients with anterior MI treated with reperfusion and medications to prevent remodeling, approximately 32% presented with left ventricular dilation after 1 year of follow-up [9]. In 82 patients with MI reperfused within 12 hours of symptoms, 32% of patients developed significant left ventricular dilation, which is defined as a ≥20% increase in left ventricular end-diastolic volume from hospitalization to the 6-month follow-up. In this study, patients with both anterior and inferior infarction were included [25]. In another study of consecutive patients ≥70 years old with MI, the 6-month prevalence of remodeling was 34% [26]. Therefore, the prevalence of ventricular remodeling after anterior myocardial infarction in the era of modern medical therapy is still unclear.
In this study, 58% of patients presented with ventricular remodeling. An important finding was that more than 86% of patients were submitted to reperfusion therapy, and more than 83% of patients presented a thrombolysis in myocardial infarction (TIMI) grade ≥2. In addition, the majority of patients were treated with angiotensin-converting enzyme inhibitors and beta-blockers at hospitalization and after 6 months. Therefore, despite contemporary treatment, our data suggest that ventricular remodeling is still a frequent event, at least in patients with anterior MI. It is important to consider that adequate reperfusion in patients with acute myocardial infarction salvages myocardium and reduces mortality. However, successful restoration of epicardial coronary artery patency does not always lead to adequate reperfusion at the microvascular level (phenomenon of no-reflow). In addition, there is a relationship between no-reflow and ventricular remodeling after MI. In our study, despite more reperfusion, the remodeling group presented bigger infarct size than the group without remodeling. Therefore, we conclude that patients with remodeling might present more no-reflow phenomenon than the group without remodeling.
Another important issue is the remodeling prediction. The importance of identifying patients at risk for progressive dilation is well known. If left ventricular dilation were diagnosed at an early stage, a more aggressive therapeutic approach could be undertaken to potentially improve the prognosis after MI.
Previous studies have established that infarct size, anterior location, coronary patency and some anti-remodeling medications are independent predictors of progressive left ventricular dilation [4–8]. However, the role of systolic function variables as predictors of remodeling is less clear [6,8,9]. Recently, a restrictive pattern of diastolic dysfunction was found to be a predictor of remodeling after MI in some [27] but not all [22] studies. In this study, the ejection fraction was smaller in patients with remodeling compared to patients without remodeling. However, in the multivariate analysis, ejection fraction did not predict remodeling. In agreement with our results, 20% of patients in the HEART study demonstrated complete recovery of function during the first 2 weeks; this suggests that the predictive value of an early assessment of left ventricular function may be limited [28]. In contrast, infarct size and early diastolic mitral annulus velocity were independent predictors of ventricular remodeling. Therefore, our data suggest that diastolic function may be a stronger predictor of remodeling than is systolic function.
Finally, we should considerer the major limitations of this study. Our study included a small sample size and patients from a single medical center. In addition, we did not study the phenomenon of no-reflow. Despite that, we believe that our study adds important data about ventricular remodeling after anterior myocardial infarction in the era of modern medical therapy.
Conclusions
In conclusion, our data demonstrate that in patients with anterior MI, despite contemporary treatment, ventricular remodeling is still a common event. In addition, diastolic function can have an important role as a predictor of remodeling in this scenario.
Footnotes
Source of support: This work was supported by the Botucatu Medical School
References
- 1.Duarte DR, Minicucci MF, Azevedo PS, et al. Influence of lisinopril on cardiac remodeling induced by tobacco smoke exposure. Med Sci Monit. 2010;16(8):BR255–59. [PubMed] [Google Scholar]
- 2.Oliveira SA, Junior, Dal Pai-Silva M, Martinez PF, et al. Diet-induced obesity causes metabolic, endocrine and cardiac alterations in spontaneously hypertensive rats. Med Sci Monit. 2010;16(12):BR367–73. [PubMed] [Google Scholar]
- 3.Oliveira SA, Junior, Dal Pai-Silva M, Martinez PF, et al. Differential nutritional, endocrine, and cardiovascular effects in obesity-prone and obesity-resistant rats fed standard and hypercaloric diets. Med Sci Monit. 2010;16(7):BR208–17. [PubMed] [Google Scholar]
- 4.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling-concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–82. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 5.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 6.Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation. 1993;87:755–63. doi: 10.1161/01.cir.87.3.755. [DOI] [PubMed] [Google Scholar]
- 7.Zornoff LAM, Paiva SAR, Duarte DR, Sparado J. Ventricular remodeling after myocardial infarction: concepts and clinical implications. Arq Bras Cardiol. 2009;92:157–64. doi: 10.1590/s0066-782x2009000200013. [DOI] [PubMed] [Google Scholar]
- 8.Bolognese L, Cerisano G. Early predictors of left ventricular remodeling after acute myocardial infarction. Am Heart J. 1999;138:S79–83. doi: 10.1016/s0002-8703(99)70325-x. [DOI] [PubMed] [Google Scholar]
- 9.Savoye C, Equine O, Tricot O, et al. Left ventricular after anterior wall acute myocardial infarction in modern clinical practice. Am J Cardiol. 2006;98:1144–49. doi: 10.1016/j.amjcard.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Kushner FG, Hand M, Smith SC, Jr, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271–306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereaux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Merlo M, Pyxaras SA, Pinamonti B, et al. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol. 2011;57:1468–76. doi: 10.1016/j.jacc.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Eaton LW, Bulkley BH. Expansion of acute myocardial infarction: its relationship to infarct morphology in a canine model. Circ Res. 1981;49:80–88. doi: 10.1161/01.res.49.1.80. [DOI] [PubMed] [Google Scholar]
- 15.Hochman LS, Bulkley BH. Expansion of acute myocardial infarction: an experimental study. Circulation. 1982;65:1446–50. doi: 10.1161/01.cir.65.7.1446. [DOI] [PubMed] [Google Scholar]
- 16.Schuster EH, Bulkley BH. Expansion of transmural myocardial infarction: a pathophysiologic factor in cardiac rupture. Circulation. 1979;60:1532–38. doi: 10.1161/01.cir.60.7.1532. [DOI] [PubMed] [Google Scholar]
- 17.Pirolo JS, Hutchins GM, Moore GW. Infarct expansion. Pathologic analysis of 204 patients with a single myocardial infarct. J Am Coll Cardiol. 1986;7:349–54. doi: 10.1016/s0735-1097(86)80504-6. [DOI] [PubMed] [Google Scholar]
- 18.Jeremy RW, Hackworthy RA, Bautovich G, et al. Infarct artery perfusion and changes in left ventricular volume in the month after acute myocardial infarction. J Am Coll Cardiol. 1987;9:989–95. doi: 10.1016/s0735-1097(87)80298-x. [DOI] [PubMed] [Google Scholar]
- 19.Jeremy RW, Allman KC, Bautovitch G, Harris PJ. Patterns of left ventricular dilation during the six months after myocardial infarction. J Am Coll Cardiol. 1989;13:304–10. doi: 10.1016/0735-1097(89)90503-2. [DOI] [PubMed] [Google Scholar]
- 20.Solomon SD, Pfeffer MA. The decreasing incidence of left ventricular remodeling following myocardial infarction. Basic Res Cardiol. 1997;92:61–65. doi: 10.1007/BF00805561. [DOI] [PubMed] [Google Scholar]
- 21.Pfeffer MA, Lamas GA, Vaughan DE, et al. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med. 1988;319:80–86. doi: 10.1056/NEJM198807143190204. [DOI] [PubMed] [Google Scholar]
- 22.Senior R, Basu S, Kinsey C, et al. Carvedilol prevents remodeling in patients with left ventricular dysfunction after after acute myocardial infarction. Am Heart J. 1999;137:646–52. doi: 10.1016/s0002-8703(99)70217-6. [DOI] [PubMed] [Google Scholar]
- 23.Bellenger NG, Swinburn JMA, Rajappan K, et al. Cardiac remodeling in the era of aggressive medical therapy: does it still exist? Int J Cardiol. 2002;83:217–25. doi: 10.1016/s0167-5273(02)00034-7. [DOI] [PubMed] [Google Scholar]
- 24.Loboz-Grudzien K, Kowalska A, Brzezinska B, et al. Early predictors of adverse left ventricular remodelling after myocardial infarction treated by primary angioplasty. Cardiol J. 2007;14:238–425. [PubMed] [Google Scholar]
- 25.Garcia-Alvarez A, Sitges M, Delgado V, et al. Relation of plasma brain natriuretic peptide levels on admission for ST-elevation myocardial infarction to left ventricular end-diastolic volume six months later measured by both echocardiography and cardiac magnetic resonance. Am J Cardiol. 2009;104:878–82. doi: 10.1016/j.amjcard.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 26.Carrabba N, Parodi G, Valenti R, et al. Comparison of effects of primary coronary angioplasty on left ventricular remodeling and heart failure in patients <70 versus > or =70 years with acute myocardial infarction. Am J Cardiol. 2009;104:926–31. doi: 10.1016/j.amjcard.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 27.Meta-Analysis Research Group in Echocardiography (MeRGE) AMI Collaborators. Independent prognostic importance of a restrictive left ventricular filling pattern after myocardial infarction: an individual patient meta-analysis: Meta-Analysis Research Group in Echocardiography acute myocardial infarction. Circulation. 2008;117:2591–98. [Google Scholar]
- 28.Pfeffer MA, Greaves SC, Arnold JMO, et al. Early versus delayed angiotensin converting enzyme inhibition therapy in acute myocardial infarction: In the Healing and Early Afterload Reducing Therapy Trial. Circulation. 1997;95:2643–51. doi: 10.1161/01.cir.95.12.2643. [DOI] [PubMed] [Google Scholar]