Summary
Background
Previous studies have shown that administration of ghrelin exhibits protective and therapeutic effects in the gut. The aim of the present investigation was to examine the influence of ghrelin administration on the course of cysteamine-induced duodenal ulcers, as well as effects on mucosal production of oxygen free radicals and duodenal antioxidant defense.
Material/Methods
Duodenal ulcers were induced in male Wistar rats by cysteamine administered intragastrically at the dose of 200 mg/kg in 1 ml of saline, 3 times at 4-h intervals. Starting 24 h after the first dose of cysteamine, rats were treated intraperitoneally twice a day with saline or ghrelin given at the dose of 4, 8 or 16 nmol/kg/dose. Seven days after administration of the first dose of cysteamine, the study was terminated.
Results
Induction of ulcers by cysteamine was accompanied by a reduction in duodenal blood flow, mucosal DNA synthesis and mucosal activity of superoxide dismutase (SOD); whereas mucosal concentration of interleukin-1β and malonyldialdehyde (MDA – an index of lipid peroxidation) were increased. Treatment with ghrelin increased healing rate of duodenal ulcers and enhanced duodenal blood flow, mucosal DNA synthesis and mucosal activity of SOD, and reduced mucosal concentration of interleukin-1β and MDA.
Conclusions
Treatment with ghrelin increases the healing rate of duodenal ulcers and this effect is related, at least in part, to improvement of duodenal mucosal blood flow, mucosal cell proliferation and antioxidant defense, as well as being related to reduction in mucosal oxidative stress and inflammatory response.
Keywords: ghrelin, duodenal ulcer, mucosal blood flow, cell proliferation, oxidative stress
Background
Ghrelin is a circulating growth hormone-releasing peptide primarily isolated from human and rat stomachs [1,2]. The stomach is a main source of ghrelin, but this peptide has been also detected in other organs [1,3,4]. Ghrelin is a natural ligand for growth hormone secretagogue receptor (GHS-R) [1]. Ghrelin strongly and dose dependently stimulates release of growth hormone from the anterior pituitary and promotes release of adrenocorticotropic hormone, corticosterone, and prolactin [1,5,6].
Ghrelin stimulates the appetite and fat deposition in rats and humans and fasting plasma level of ghrelin is negatively correlated with body mass index [7–10].
Previous studies have shown that administration of ghrelin exhibits a protective effect in the gut. Pretreatment with ghrelin inhibits the development of cerulein- or ischemia/reperfusion-induced acute pancreatitis and accelerates pancreatic recovery in the course of cerulein-induced pancreatitis [11–14]. In the stomach, pretreatment with ghrelin reduces gastric mucosal damage induced by ethanol, stress or alendronate and accelerates healing of acetic acid-induced gastric and duodenal ulcers. Moreover, animal and clinical studies suggest that ghrelin reduces colonic inflammation [15–20].
The protective effect of ghrelin seems to be dependent on different mechanisms. Sibilia et al. reported that the gastroprotective effect of ghrelin is mediated by release of endogenous nitric oxide and requires activity of capsaicin-sensitive sensory nerves [15]. Other studies have shown that pretreatment with ghrelin dose-dependently reduces the area of the ethanol-induced gastric lesions, improves gastric blood flow and reduces mucosal expression of pro-inflammatory tumor necrosis factor-α (TNF-α) [16]. The anti-inflammatory effect of ghrelin was also found in our previous studies [11,12].
Recent research has also shown a relationship between plasma level of endogenous ghrelin and clinical gastric pathology. Presence of Helicobacter pylori in the stomach of patients with peptic ulcers increases plasma ghrelin level; whereas gastric cancer and atrophic gastritis are accompanied by a marked decrease in plasma concentration of ghrelin [21].
It is very interesting that obestatin, a 23-amino acid peptide derived from the same prohormone as ghrelin, exhibits similar protective and therapeutic effects as ghrelin. Recent studies have indicated that obestatin promotes survival of pancreatic islets, inhibits development of experimental acute pancreatitis and accelerates the healing of chronic gastric ulcers [21–24].
Cysteamine (β-mercaptoethylamine) is the chemical compound with the formula HSCH2CH2NH2. It is the simplest stable aminothiol and is a degradation product of the amino acid cysteine. Cysteamine is used in the body to form the essential biochemical coenzyme A (CoA) by combining with pantothenate (vitamin B 5) and adenosine triphosphate [25]. CoA may act as an acyl group carrier to form acetyl-CoA and other related compounds; this is a way to transport carbon atoms within the cell. CoA is critical in the metabolism and synthesis of carbohydrates, proteins and fats [26,27]. Clinically, cysteamine has been used in the treatment of cystinosis and cystinuria [28,29]. Cysteamine and its analog amifostine have been also used for treatment of radiation sickness [30]. Moreover, cysteamine, as a potent antioxidant, improves the maturation of oocytes and development of embryos in in vitro culture [31].
On the other hand, numerous experimental studies have shown that administration of cysteamine at high doses leads to induction of duodenal ulcers [32–34]. Pathogenesis of ulcerogenic activity of cysteamine is not fully understood. Previous studies have indicated that cysteamine increases gastric acid secretion and decreases neutralization of acid in the proximal duodenum. These effects seem to be related to somatostatin depletion in gastric mucosa and elevation of serum level of gastrin [35–37]. Some studies have suggested that transcription factors such as hypoxia-inducible factor-1α and early growth response factor-1 and their target genes are involved in pathogenesis of cysteamine-induced ulcers [38,39]. Moreover, a recent study performed by Khomenko et al. has produced strong evidence that cysteamine disrupts regulation of mucosal iron transport, leading to increased mucosal susceptibility to oxidative stress [40].
Fukuhara et al reported a relationship between cysteamine and endogenous ghrelin [41]. Induction of duodenal ulcer by cysteamine increases plasma level of ghrelin and reduces gastric level of this peptide [41]. However, the effect of ghrelin on the healing of ulcers induced by cysteamine is unknown. The aim of the present study was to examine the influence of ghrelin administration on the course of cysteamine-induced duodenal ulcers and on mucosal production of oxygen free radicals, duodenal antioxidant defense and release of pro-inflammatory interleukin-1β.
Material and Methods
Animals and treatment
Studies were performed on male Wistar rats weighing 200–220 g and were conducted following the experimental protocol approved by the Local Commission of Ethics for the Care and Use of Laboratory Animals. Experiments were performed in the Animal Laboratory of the Department of Physiology, Jagiellonian University Medical College. Animals were housed in cages with wire mesh bottoms, at normal room temperature (22±1°C) and a 12-h light-dark cycle. Rats had unlimited access to food and water.
Rats were divided into the following 6 experimental groups: (1) control rats without induction of ulcers and treated with saline; (2) rats without induction of ulcers and treated with ghrelin at the dose of 8 nmol/kg/dose; (3) rats with ulcers treated with saline; (4–6) rats with ulcers treated with ghrelin at the dose 4, 8 or 16 nmol/kg/dose, respectively. Experiments were repeated to obtain 10 observations in each experimental group.
Duodenal ulcers were induced by cysteamine (cysteamine-HCl, Sigma-Aldrich, St. Louis, MO, USA) administered intragastrically at the dose of 200 mg/kg 3 times at 4-h intervals. Each dose of cysteamine was dissolved in 1 ml of saline.
After intragastric administration of cysteamine (groups 3–6) or saline (groups 1–2), rats were treated with saline (groups 1 and 3) or ghrelin (groups 2 and 4–6) given intraperitoneally twice a day for 6 days at the doses shown above. The first dose of intraperitoneal saline or ghrelin was administered 24 h after the first dose of intragastric cysteamine or saline.
Active N-octanoyl rat ghrelin was synthesized at Yanaihara Institute by a solid phase methodology with Fmoc-strategy using automated peptide synthesizer (Applied Biosystem 9030 Pioneer, Foster, CA, USA) as described previously [12].
Determination of duodenal blood flow and mucosal lesions
Seven days after the first dose of cysteamine, rats were anesthetized with pentobarbital (30 mg/kg i.p., Vetbutal, Biowet, Puławy, Poland) and the abdomen was opened by a midline incision. The duodenum was exposed and the duodenal mucosal blood flow was measured using a laser Doppler flowmeter (PeriFlux 4001 Master monitor, Perimed AB, Järfälla, Sweden). Blood flow was measured in 5 areas of duodenal mucosa, and the mean value of 5 recordings was presented as percent of mucosal blood flow found in saline-treated control rats. After measurement of mucosal blood flow, the area of ulcerated mucosa was measured using a computerized planimeter (Morphomat, Carl Zeiss, Berlin, Germany) as described previously [42].
Biochemical analysis
After measurement of duodenal blood flow, biopsy samples from the duodenal mucosa were taken for determination of mucosal DNA synthesis (an index of mucosal cell proliferation), mucosal concentration of pro-inflammatory interleukin-1β, mucosal concentration of malonyldialdehyde (MDA) (an index of lipid peroxidation) and mucosal activity of superoxide dismutase (SOD).
DNA synthesis was determined by measurement of [3H]thymidine incorporation ([6-3H]-thymidine, 20–30 Ci/mmol (Institute for Research, Production and Application of Radioisotopes, Prague, Czech Republic) into mucosal DNA as described previously [43]. The incorporation of labeled thymidine into DNA was determined by counting 0.5 ml DNA-containing supernatant in a liquid scintillation system. DNA synthesis was expressed as tritium disintegrations per minute per ìg of DNA (dpm/μg DNA).
Lipid peroxidation was determined by measurement of malondialdehyde (MDA) using the commercial kit Bioxytech® LPO-586™ (OxisResearch™, OXIS Health Products, Inc., Portland, OR, USA), as described previously [44]. Prior to homogenization of tissue samples, 10 μl 0.5 M butylated hydroxytoluene in acetonitrate was added to prevent sample oxidation during homogenization. Mucosa was homogenized in ice-cold Tris buffer (20 mM, pH 7.4), centrifuged (3000 g at 4°C for 10 min) and supernatant was used for the assay. The Bioxytech® LPO-586™ is a colorimetric assay based on the reaction of a chromogenic reagent, N-methyl-2-phenylindole, with MDA and HAE at 45°C. One molecule of either MDA or 4-hydroxyalkens (HAE) may react with 2 molecules of N-methyl-2-phenylindole to yield a stable chromophore with maximal absorbance at 586 nm. We performed the assay using hydrochloric acid as the acid solvent. For this reason we only detected MDA content without detection of HAE. Results are expressed in nmol per g of duodenal mucosa.
To determine mucosal activity of SOD, tissue was homogenized in 20 mM HEPES buffer, pH 7.2, containing 1 mM EGTA, 210 mM mannitol, and sucrose. Homogenate was centrifuged at 1500 g for 5 min at 4°C. Activity of SOD in the supernatant was measured using the Superoxide Dismutase Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA). Results are expressed in units per g of duodenal mucosa.
Samples for determination of interleukin-1β in duodenal mucosa were homogenized in ice-cold phosphate-buffered saline (PBS, 20 mM, pH 7.4). Homogenate was centrifuged at 1500 g for 10 min at 4°C. Content of interleukin-1β in the supernatant was measured using the BioSource Cytoscreen rat IL-1β kit (BioSource International, Camarillo, California, USA) based on ELISA. Concentration of interleukin-1β in duodenal mucosa is expressed as ng per g of protein.
Statistical analysis
Results are expressed as mean ±SEM. Statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test using GraphPadPrism (GraphPad Software, San Diego, CA, USA). Differences were considered to be statistically significant when P was less than 0.05.
Results
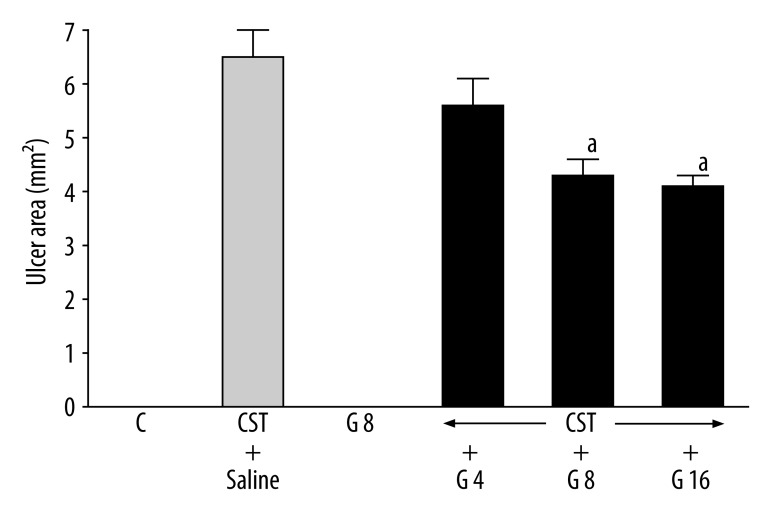
In control saline-treated rats, no lesions of duodenal mucosa were observed (Figure 1) and ghrelin given alone without cysteamine administration did not induce duodenal ulcers. Administration of cysteamine resulted in induction of duodenal ulcers; 7 days after the first dose of cysteamine, the mean ulcer area reached 6.5±0.5 mm2. Treatment with ghrelin reduced the ulcer area in the duodenum. Ghrelin given at the dose of 4 nmol/kg/dose tended to decrease the area of ulcers, but this effect was statistically insignificant. In contrast to that, higher doses of ghrelin (8 and 16 nmol/kg/dose) caused significant and similar reduction of the duodenal ulcer area by 34% and 37%, respectively (Figure 1).
Figure 1.
Influence of treatment with ghrelin given at the dose of 4, 8 or 16 nmol/kg (G 4, G 8 or G 16) on the area of cysteamine (CST)-induced duodenal ulcers. Mean ±SEM. N=10 in each group of animals. aP<0.05 compared to CST + saline.
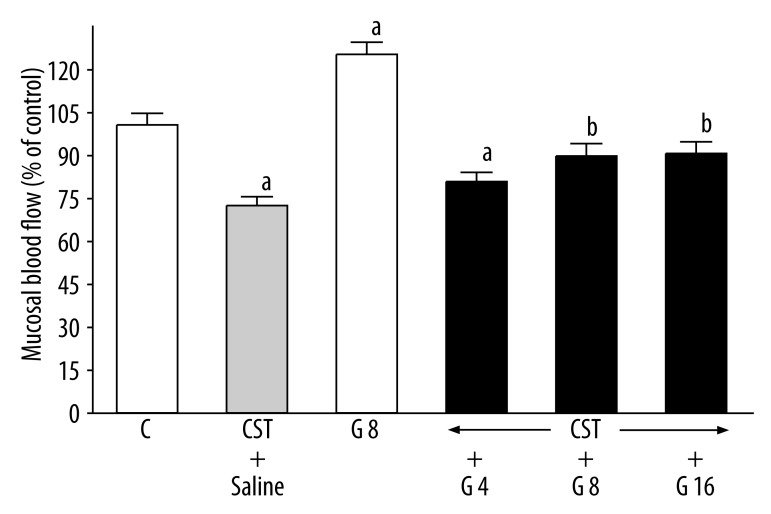
Seven days after administration of the first dose of cysteamine, duodenal blood flow was significantly reduced by 28% (Figure 2). Ghrelin given alone without induction of duodenal ulcers markedly increased duodenal blood flow by 25.5%. In rats with duodenal ulcers, treatment with ghrelin partly reversed the cysteamine-induced decrease in duodenal blood flow; this effect was statistically significant after administration of ghrelin at the dose of 8 or 16 nmol/kg/dose (Figure 2). Value of duodenal blood flow in rats with ulcers treated with ghrelin at the dose of 8 or 16 nmol/kg almost reached the control level and no significant differences were observed between these groups and control group (Figure 2).
Figure 2.
Influence of treatment with ghrelin given at the dose of 4, 8 or 16 nmol/kg (G 4, G 8 or G 16) on duodenal mucosal blood flow in rats with cysteamine (CST)-induced duodenal ulcers. Mean ±SEM. N=10 in each group of animals. aP<0.05 compared to control (C); bP<0.05 compared to CST + saline.
DNA synthesis, an index of cell proliferation, reached a value 54.2±2.1 dpm/μg DNA in duodenal mucosa of control saline-treated rats (Figure 3). In saline-treated rats with cysteamine-induced ulcers, DNA synthesis in duodenal mucosa was reduced by 26% as compared to control rats. In rats without induction of ulcers, administration of ghrelin increased mucosal DNA synthesis in the duodenum by 29%. In rats with duodenal ulcers, treatment with ghrelin partly reversed the cysteamine-induced decrease in DNA synthesis in duodenal mucosa. This effect reached statistical significance when ghrelin was given at the dose of 8 or 16 nmol/kg/dose; no statistical difference was observed between these groups and the control group (Figure 3).
Figure 3.
Influence of treatment with ghrelin given at the dose of 4, 8 or 16 nmol/kg (G 4, G 8 or G 16) on duodenal mucosal DNA synthesis in rats with cysteamine (CST)-induced duodenal ulcers. Mean ±SEM. N=10 in each group of animals. aP<0.05 compared to control (C); bP<0.05 compared to CST + saline.
In saline-treated control rats, concentration of interleukin-1β in duodenal mucosa was 62.0±3.0 ng/g of protein (Figure 4). In saline-treated rats with cysteamine-induced ulcers, concentration of interleukin-1β in duodenal mucosa reached a value 176±8 ng/g of protein. Administration of ghrelin had no effect on interleukin-1β concentration in duodenal mucosa in saline-treated rats without induction of ulcers. In contrast, administration of ghrelin reduced the cysteamine-induced increase in mucosal interleukin-1β content in rats with duodenal ulcers; this effect was statistically significant after ghrelin given at the dose of 8 or 16 nmol/kg/dose (Figure 4).
Figure 4.
Influence of treatment with ghrelin given at the dose of 4, 8 or 16 nmol/kg (G 4, G 8 or G 16) on concentration of interleukin-1β in duodenal mucosa in rats with cysteamine (CST)-induced duodenal ulcers. Mean ±SEM. N=10 in each group of animals. aP<0.05 compared to control (C); bP<0.05 compared to CST + saline.
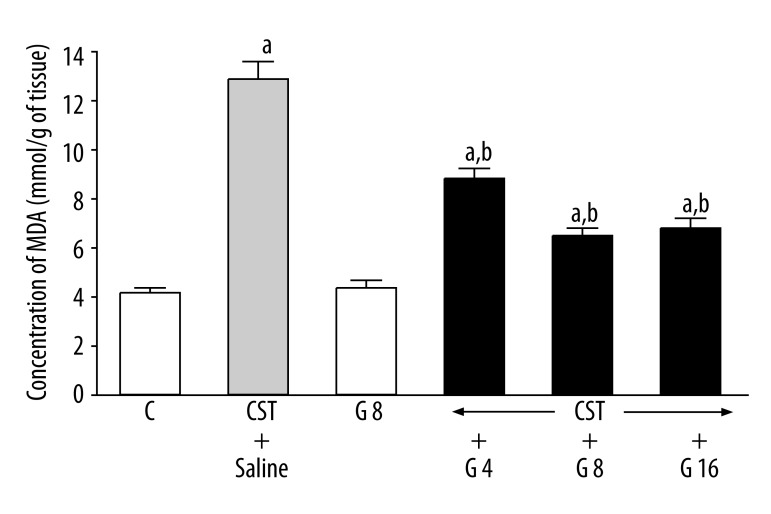
In duodenal mucosa of control saline-treated rats, concentration of MDA was 4.1±0.2 nmol/g of tissue (Figure 5). Induction of duodenal ulcers by administration of cysteamine caused a more than 3-fold increase in concentration of MDA in duodenal mucosa. Administration of ghrelin in rats without induction of ulcers failed to affect MDA concentration in the duodenum. In rats with ulcers, treatment with ghrelin significantly reduced the cysteamine-induced increase in mucosal concentration of MDA. This effect was the strongest and was similar to the effect of ghrelin at the dose of 8 or 16 nmol/kg/dose (Figure 5).
Figure 5.
Influence of treatment with ghrelin given at the dose of 4, 8 or 16 nmol/kg (G 4, G 8 or G 16) on concentration of MDA in duodenal mucosa in rats with cysteamine (CST)-induced duodenal ulcers. Mean ±SEM. N=10 in each group of animals. aP<0.05 compared to control (C); bP<0.05 compared to CST + saline.
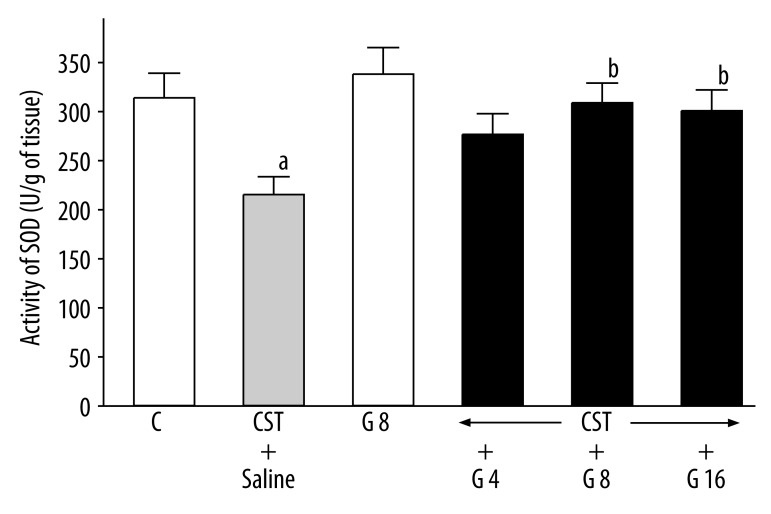
In rats with cysteamine-induced ulcers, mucosal activity of SOD was reduced by 32% (Figure 6). Administration of ghrelin failed to affect mucosal activity of SOD in rats treated with saline and without ulcers, but reversed the cysteamine-induced decrease in SOD activity in rats with ulcers. This last effect reached statistical significance after ghrelin given at the dose of 8 or 16 nmol/kg/dose (Figure 6).
Figure 6.
Influence of treatment with ghrelin given at the dose of 4, 8 or 16 nmol/kg (G 4, G 8 or G 16) on SOD activity in duodenal mucosa in rats with cysteamine (CST)-induced duodenal ulcers. Mean ±SEM. N=10 in each group of animals. aP<0.05 compared to control (C); bP<0.05 compared to CST + saline.
Discussion
Previous studies have shown that pretreatment with ghrelin inhibits the development of gastric ulcers [15–18] and accelerates healing of acetic acid-induced gastric and duodenal ulcers [19]. The present study confirms and extends these observations. Treatment with ghrelin increased the healing rate of the cysteamine-induced duodenal ulcers and this effect was accompanied with increased mucosal blood flow, DNA synthesis and activity of SOD. Mucosal concentrations of interleukin-1β and MDA were reduced.
Mucosal blood flow plays an important role in the protection and healing of gastro-duodenal mucosa. [43,45–47]. Numerous experimental studies have shown that exposure of gastric mucosa to potentially noxious factors results in little or no damage as long as adequate blood flow is maintained, whereas reduction in mucosal blood flow leads to severe gastric injury [45]. Blood flow contributes to protection by supplying the mucosa with oxygen, bicarbonate and nutritious substances, and by removal of carbon dioxide, hydrogen ions and other toxic agents diffusing from the gastric lumen [45]. Hypoxia, resulting in accumulation of H+ within gastric mucosa leads to mucosal acidification and subsequently to the development of gastric ulcers [48]. On the other hand, improvement of mucosal blood flow reduces mucosal damage in the stomach [43,45–47]. The same influence of mucosal blood flow alterations on induction of mucosal damage and mucosal healing has been also shown in the duodenum [19,49]. These data are in agreement with our present observation indicating that improvement of mucosal blood flow plays an essential role in the healing effect of ghrelin on duodenal cysteamine-induced ulcers. Moreover, Ahluwalia et al. has shown that ghrelin stimulates sprouting of new capillary blood vessels and that deficiency of ghrelin is a major cause of the aging-related impairment of angiogenesis [50].
Peptic ulcer has been attributed to imbalance between the aggressive factors and mucosal resistance [51]. Mucosal resistance is related to, among other factors, mucosal blood flow, mucus and prostaglandin production, and rapid mucosal cell turnover. Inhibition of mucosal cell proliferation or excessive apoptosis results in the development of ulcers [52,53]. On the other hand, the increase in cell proliferation leads to reduction of mucosal damage and accelerates healing of mucosal ulcers [54–57]. Rate of DNA synthesis is an index of cell proliferation. Our present study has shown that administration of cysteamine leads to induction of ulcers and this effect is associated with inhibition of DNA synthesis in duodenal mucosa. Also, we have found that administration of ghrelin alone increases mucosal cell proliferation and treatment with ghrelin of rats with ulcers partly, but significantly, reverses the cysteamine-induced decrease in mucosal DNA synthesis. These findings indicate that ghrelin’s growth-promoting effect is involved in healing of duodenal ulcers induced by cysteamine.
Another finding of our present study is the observation that ghrelin reduces mucosal concentration of interleukin-1β in the duodenum of rats with cysteamine-induced ulcers. Interleukin-1β is a well known mediator of acute inflammation and plays a crucial role in the induction of systemic acute phase response and in the release of other members of the pro-inflammatory cytokine cascade [58]. This interleukin stimulates production and release of mediators of inflammation such as tumor necrosis factor, platelet activating factor, prostaglandins and pro-inflammatory interleukins [58]. These data taken together indicate that ghrelin reduces local inflammatory response in cysteamine-induced duodenal ulcers, but the mechanism of ghrelin’s anti-inflammatory effect is still not clear.
Numerous immune cells, including human leukemic B, T and myeloid cell lines, human peripheral lymphocytes and neutrophils, as well as mouse splenic T cells, exhibit the presence of receptors for ghrelin [59,60]. Biological action of ghrelin on the immune system includes attenuation of septic shock, promotion of thymopoiesis during aging in mice and inhibition of expression of pro-inflammatory cytokines by human monocytes and T lymphocytes [61–64]. Moreover, administration of ghrelin reduces phagocytic activity of peritoneal macrophages in rats exposed to cold-restraint stress [65].
Reactive oxygen species (ROS) are produced by inflammatory cells, particularly activated leukocytes, to attack and destroy pathogens. On the other hand, ROS can impair proteins, nucleic acid, lipids and other molecular species. ROS production and lipid peroxidation are well-established mechanisms of cellular and tissue injury, including the development of gastric and duodenal ulcers [66–68], as well as intestinal inflammation [69]. Moreover, oxidative stress and lipid peroxidation lead to fibroblast dysfunction and impairment of wound healing [70].
In normal conditions, cells and tissues are protected from the ROS-induced injury by antioxidant defense, involving oxygen scavenger enzymes such as superoxide dismutase (SOD), catalase and glutathione peroxidase, as well as vitamins E and C [69,71].
Conclusions
In agreement with previous data, our present study has shown that administration of cysteamine leads to the development of duodenal ulcers and that this effect is associated with an increase in mucosal concentration of MDA and reduction in mucosal activity of SOD [72]. Moreover, we have found that treatment with ghrelin partly reduces the cysteamine-induced mucosal oxidative stress. In rats with ulcers, treatment with ghrelin decreased mucosal concentration of MDA and increased mucosal activity of SOD. These findings indicate the therapeutic effect of ghrelin in the cysteamine-induced duodenal ulcers involves the reduction of cellular lipid peroxidation and the increase in mucosal antioxidant defense. This conclusion is in agreement with data obtained by Zhang et al. [73], who found that ghrelin reduces apoptosis and ROS level in rat aortic endothelial cells exposed to palmitate.
Therapeutic and anti-inflammatory effects of ghrelin in the treatment of cysteamine-induced duodenal ulcers are most likely based on 2 different mechanisms – direct influence on cells and tissues through ghrelin’s receptor (GHS-R), and indirect action by a release of growth hormone and IGF-1. For example, activation of GHS-R has been shown to attenuate the palmitate-induced apoptosis in pancreatic β cell line and this effect was related to a rapid stimulation of protein kinase B and inhibition of C-Jun N-terminal kinase, and mitochondrial pathway of apoptosis [74]. On the other hand, our previous studies have shown that protective and therapeutic effects of ghrelin in acute pancreatitis are mediated by a release of growth hormone and IGF-1 [12,14]. Hypophysectomy, performed prior to induction of acute pancreatitis, has eliminated serum growth hormone, reduced serum IGF-1 concentration by 90% and increased serum level of endogenous ghrelin, and effects were associated with aggravation of acute pancreatitis severity. Moreover, hypophysectomy has abolished the protective and therapeutic effect of ghrelin administration in the course of acute pancreatitis. In contrast to that, administration of IGF-1 reduced the severity of acute pancreatitis and accelerated healing in this disease to the same degree as ghrelin in pituitary-intact rats [12,14]. These observations indicate that the protective and therapeutic effects of ghrelin in the course of acute pancreatitis are based on an indirect mechanism related to release of endogenous growth hormone and IGF-1. A similar indirect therapeutic effect of ghrelin has been also found in the healing of acetic acid-induced gastric and duodenal ulcers [19].
Finally, the present study demonstrated that treatment with ghrelin increases healing rate of cysteamine-induced duodenal ulcers. This effect is related, at least in part, to an improvement of duodenal mucosal blood flow and mucosal cell proliferation, as well as to a reduction in mucosal oxidative stress and inflammatory response.
Footnotes
Source of support: This study was supported by a grant from the Polish Ministry of Science and Higher Education (Project N N401 000635) and a grant from Jagiellonian University
References
- 1.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Ariyasu H, Takaya K, Tagami T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–58. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 3.Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–61. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 4.Gnanapavan S, Kola B, Bustin SA, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988–91. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 5.Takaya K, Ariyasu H, Kanamoto N, et al. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab. 2000;85:4908–11. doi: 10.1210/jcem.85.12.7167. [DOI] [PubMed] [Google Scholar]
- 6.Broglio F, Benso A, Castiglioni C, et al. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J Clin Endocrinol Metab. 2003;88:1537–42. doi: 10.1210/jc.2002-021504. [DOI] [PubMed] [Google Scholar]
- 7.Wren AM, Small CJ, Abbott CR, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–47. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 8.Nešić DM, Stevanović DM, Ille T, et al. Centrally applied ghrelin affects feeding dynamics in male rats. J Physiol Pharmacol. 2008;59:489–500. [PubMed] [Google Scholar]
- 9.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992–95. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 10.Shiiya T, Nakazato M, Mizuta M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87:240–44. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- 11.Dembiński A, Warzecha Z, Ceranowicz P, et al. Ghrelin attenuates the development of acute pancreatitis in rats. J Physiol Pharmacol. 2003;54:561–73. [PubMed] [Google Scholar]
- 12.Dembiński A, Warzecha Z, Ceranowicz P, et al. Role of growth hormone and insulin-like growth factor-1 in the protective effect of ghrelin in ischemia/reperfusion-induced acute pancreatitis. Growth Horm IGF Res. 2006;16:348–56. doi: 10.1016/j.ghir.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Warzecha Z, Ceranowicz P, Dembiński A, et al. Therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis in rats. J Physiol Pharmacol. 2010;61:419–27. [PubMed] [Google Scholar]
- 14.Ceranowicz D, Warzecha Z, Dembinski A, et al. Role of hormonal axis, growth hormone - IGF-1, in the therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis. J Physiol Pharmacol. 2010;61:599–606. [PubMed] [Google Scholar]
- 15.Sibilia V, Rindi G, Pagani F, et al. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology. 2003;144:353–59. doi: 10.1210/en.2002-220756. [DOI] [PubMed] [Google Scholar]
- 16.Konturek PC, Brzozowski T, Pajdo R, et al. Ghrelin-a new gastroprotective factor in gastric mucosa. J Physiol Pharmacol. 2004;55:325–36. [PubMed] [Google Scholar]
- 17.Brzozowski T, Konturek PC, Konturek SJ, et al. Exogenous and endogenous ghrelin in gastroprotection against stress-induced gastric damage. Regul Pept. 2004;120:39–51. doi: 10.1016/j.regpep.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Iseri SO, Sener G, Yuksel M, et al. Ghrelin against alendronate-induced gastric damage in rats. J Endocrinol. 2005;187:399–406. doi: 10.1677/joe.1.06432. [DOI] [PubMed] [Google Scholar]
- 19.Ceranowicz P, Warzecha Z, Dembinski A, et al. Treatment with ghrelin accelerates the healing of acetic acid-induced gastric and duodenal ulcers in rats. J Physiol Pharmacol. 2009;60:87–98. [PubMed] [Google Scholar]
- 20.Konturek PC, Brzozowski T, Engel M, et al. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J Physiol Pharmacol. 2009;60:41–47. [PubMed] [Google Scholar]
- 21.Zub-Pokrowiecka A, Rembiasz K, Konturek SJ, et al. Ghrelin in diseases of the gastric mucosa associated with Helicobacter pylori infection. Med Sci Monit. 2010;16(10):CR493–500. [PubMed] [Google Scholar]
- 22.Granata R, Settanni F, Gallo D, et al. Obestatin promotes survival of pancreatic beta-cells and human islets and induces expression of genes involved in the regulation of beta-cell mass and function. Diabetes. 2008;57:967–79. doi: 10.2337/db07-1104. [DOI] [PubMed] [Google Scholar]
- 23.Ceranowicz P, Warzecha Z, Dembinski A, et al. Pretreatment with obestatin inhibits the development of cerulein-induced pancreatitis. J Physiol Pharmacol. 2009;60(3):95–101. [PubMed] [Google Scholar]
- 24.Dembiński A, Warzecha Z, Ceranowicz P, et al. Administration of obestatin accelerates the healing of chronic gastric ulcers in rats. Med Sci Monit. 2011;17(8):BR196–200. doi: 10.12659/MSM.881897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller SL, Schlesinger G. Prebiotic syntheses of vitamin coenzymes: I. Cysteamine and 2-mercaptoethanesulfonic acid (coenzyme M) J Mol Evol. 1993;36:302–7. doi: 10.1007/BF00182177. [DOI] [PubMed] [Google Scholar]
- 26.Halestrap AP, Denton RM. Hormonal regulation of adipose-tissue acetyl-Coenzyme A carboxylase by changes in the polymeric state of the enzyme. The role of long-chain fatty acyl-Coenzyme A thioesters and citrate. Biochem J. 1974;142:365–77. doi: 10.1042/bj1420365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxholm HJ, Pestana A, O’Connor L, et al. Protein acetylation. Mol Cell Biochem. 1982;46:129–53. doi: 10.1007/BF00239663. [DOI] [PubMed] [Google Scholar]
- 28.Belldina EB, Huang MY, Schneider JA, et al. Steady-state pharmacokinetics and pharmacodynamics of cysteamine bitartrate in paediatric nephropathic cystinosis patients. Br J Clin Pharmacol. 2003;56:520–25. doi: 10.1046/j.1365-2125.2003.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dohil R, Gangoiti JA, Cabrera BL, et al. Long-term treatment of cystinosis in children with twice-daily cysteamine. J Pediatr. 2010;156:823–27. doi: 10.1016/j.jpeds.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 30.Capizzi RL, Oster W. Chemoprotective and radioprotective effects of amifostine: an update of clinical trials. Int J Hematol. 2000;72:425–35. [PubMed] [Google Scholar]
- 31.Singhal S, Prasad S, Singh B, et al. Effect of including growth factors and antioxidants in maturation medium used for in vitro culture of buffalo oocytes recovered in vivo. Anim Reprod Sci. 2009;113:44–50. doi: 10.1016/j.anireprosci.2008.05.078. [DOI] [PubMed] [Google Scholar]
- 32.Selye H, Szabo S. Experimental model for production of perforating duodenal ulcers. Nature. 1973;244:458–59. doi: 10.1038/244458a0. [DOI] [PubMed] [Google Scholar]
- 33.Szabo S, Reynolds ES, Lictenberger LM, et al. Pathogenesis of duodenal ulcer. Gastric hyperacidity caused by propionitrile and cysteamine in rats. Res Commun Chem Pathol Pharmacol. 1977;16:311–23. [PubMed] [Google Scholar]
- 34.Szabo S. Duodenal ulcer disease. Animal model: cysteamine-induced acute and chronic duodenal ulcer in the rat. Am J Pathol. 1978;93:273–76. [PMC free article] [PubMed] [Google Scholar]
- 35.Adler RS, Gallagher GT, Szabo S. Duodenal ulcerogens cysteamine and propionitrile decrease duodenal neutralization of acid in the rat. Dig Dis Sci. 1983;28:716–23. doi: 10.1007/BF01312562. [DOI] [PubMed] [Google Scholar]
- 36.Szabo S, Reichlin S. Somatostatin depletion by cysteamine: mechanism and implication for duodenal ulceration. Fed Proc. 1985;44:2540–45. [PubMed] [Google Scholar]
- 37.Lichtenberger LM, Szabo S, Trier JS. Duodenal ulcerogens, cysteamine and propionitrile, stimulate serum gastrin levels in the rat. Gastroenterology. 1977;73:1305–8. [PubMed] [Google Scholar]
- 38.Khomenko T, Deng X, Sandor Z, et al. Cysteamine alters redox state, HIF-1alpha transcriptional interactions and reduces duodenal mucosal oxygenation: novel insight into the mechanisms of duodenal ulceration. Biochem Biophys Res Commun. 2004;317:121–27. doi: 10.1016/j.bbrc.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Khomenko T, Szabo S, Deng X, et al. Suppression of early growth response factor-1 with egr-1 antisense oligodeoxynucleotide aggravates experimental duodenal ulcers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1211–18. doi: 10.1152/ajpgi.00078.2005. [DOI] [PubMed] [Google Scholar]
- 40.Khomenko T, Szabo S, Deng X, et al. Role of iron in the pathogenesis of cysteamine-induced duodenal ulceration in rats. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1277–86. doi: 10.1152/ajpgi.90257.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuhara S, Suzuki H, Masaoka T, et al. Enhanced ghrelin secretion in rats with cysteamine-induced duodenal ulcers. Am J Physiol Gastrointest Liver Physiol. 2005;289:G138–45. doi: 10.1152/ajpgi.00298.2004. [DOI] [PubMed] [Google Scholar]
- 42.Dembiński A, Warzecha Z, Ceranowicz P, et al. Role of capsaicin-sensitive nerves and histamine H1, H2, and H3 receptors in the gastroprotective effect of histamine against stress ulcers in rats. Eur J Pharmacol. 2005;508:211–21. doi: 10.1016/j.ejphar.2004.11.059. [DOI] [PubMed] [Google Scholar]
- 43.Warzecha Z, Dembiński A, Brzozowski T, et al. Gastroprotective effect of histamine and acid secretion on ammonia-induced gastric lesions in rats. Scand J Gastroenterol. 2000;35:916–24. doi: 10.1080/003655200750022959. [DOI] [PubMed] [Google Scholar]
- 44.Dembiński A, Warzecha Z, Konturek SJ, et al. Extract of grapefruit-seed reduces acute pancreatitis induced by ischemia/reperfusion in rats: possible implication of tissue antioxidants. J Physiol Pharmacol. 2004;55:811–21. [PubMed] [Google Scholar]
- 45.Sorbye H, Svanes K. The role of blood flow in gastric mucosal defence, damage and healing. Dig Dis. 1994;12:305–17. doi: 10.1159/000171465. [DOI] [PubMed] [Google Scholar]
- 46.Warzecha W, Dembiński A, Brzozowski T, et al. Histamine in stress ulcer prophylaxis in rats. J Physiol Pharmacol. 2001;52:407–21. [PubMed] [Google Scholar]
- 47.West SD, Helmer KS, Chang LK, et al. Cholecystokinin secretagogue-induced gastroprotection: role of nitric oxide and blood flow. Am J Physiol Gastrointest Liver Physiol. 2003;284:G399–410. doi: 10.1152/ajpgi.00130.2002. [DOI] [PubMed] [Google Scholar]
- 48.Allen A, Flemstrom G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev. 1993;73:823–57. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- 49.Leung FW, Reedy TJ, Van Deventer GM, Guth PH. Reduction in index of oxygen saturation at margin of active duodenal ulcers may lead to slow healing. Dig Dis Sci. 1989;34:417–23. doi: 10.1007/BF01536265. [DOI] [PubMed] [Google Scholar]
- 50.Ahluwalia A, Li A, Cheng G, et al. Reduced ghrelin in endothelial cells plays important mechanistic role in aging-related impairment of angiogenesis. J Physiol Pharmacol. 2009;60:29–34. [PubMed] [Google Scholar]
- 51.Tulassay Z, Herszényi L. Gastric mucosal defense and cytoprotection. Best Pract Res Clin Gastroenterol. 2010;24:99–108. doi: 10.1016/j.bpg.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Greant P, Delvaux G, Willems G. Influence of stress on epithelial cell proliferation in the gut mucosa of rats. Digestion. 1988;40:212–18. doi: 10.1159/000199657. [DOI] [PubMed] [Google Scholar]
- 53.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: The importance of apoptosis. J Cell Sci. 1994;107:3569–77. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 54.Beckert S, Class N, Farrahi F, Coerper S. Growth hormone enhances gastric ulcer healing in rats. Med Sci Monit. 2004;10(8):BR255–58. [PubMed] [Google Scholar]
- 55.Konturek SJ, Dembinski A, Warzecha Z, et al. Role of epidermal growth factor in healing of chronic gastroduodenal ulcers in rats. Gastroenterology. 1988;94:1300–7. doi: 10.1016/0016-5085(88)90667-1. [DOI] [PubMed] [Google Scholar]
- 56.Perez Aisa A, Szopena Biarge F, Arceiz Gonzalo E, et al. Effect of exogenous administration of platelet-derived growth factor and epidermal growth factor on duodenal ulcer healing in rats treated with indomethacin. Gastroenterol Hepatol. 2002;25:299–305. doi: 10.1016/s0210-5705(02)79023-7. [DOI] [PubMed] [Google Scholar]
- 57.Kusstatscher S, Sandor Z, Szabo S, et al. Different molecular forms of basic fibroblast growth factor (bFGF) accelerate duodenal ulcer healing in rats. J Pharmacol Exp Ther. 1995;275:456–61. [PubMed] [Google Scholar]
- 58.Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–17. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 59.Hattori N, Saito T, Yagyu T, et al. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab. 2001;86:4284–91. doi: 10.1210/jcem.86.9.7866. [DOI] [PubMed] [Google Scholar]
- 60.Xia Q, Pang W, Pan H, et al. Effects of ghrelin on the proliferation and secretion of splenic T lymphocytes in mice. Regul Pept. 2004;122:173–78. doi: 10.1016/j.regpep.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 61.Dixit VD, Yang H, Sun Y, et al. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117:2778–90. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang L, Du JB, Gao LR, et al. Effect of ghrelin on septic shock in rats. Acta Pharmacol Sin. 2003;24:45–49. [PubMed] [Google Scholar]
- 63.Wu R, Dong W, Zhou M, et al. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am J Respir Crit Care Med. 2007;176:805–13. doi: 10.1164/rccm.200604-511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dixit VD, Schaffer EM, Pyle RS, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tümer C, Bilgin HM, Obay BD, et al. Effect of ghrelin administration on phagocytic activity in acute cold-restraint stress exposed rats. Regul Pept. 2007;138:113–17. doi: 10.1016/j.regpep.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 66.Bagchi D, Bhattacharya G, Stohs SJ. Production of reactive oxygen species by gastric cells in association with Helicobacter pylori. Free Radic Res. 1996;24:439–50. doi: 10.3109/10715769609088043. [DOI] [PubMed] [Google Scholar]
- 67.Pohle T, Brzozowski T, Becker JC, et al. Role of reactive oxygen metabolites in aspirin-induced gastric damage in humans: gastroprotection by vitamin C. Aliment Pharmacol Ther. 2001;15:677–87. doi: 10.1046/j.1365-2036.2001.00975.x. [DOI] [PubMed] [Google Scholar]
- 68.Kwiecień S, Brzozowski T, Konturek PC, et al. The role of reactive oxygen species and capsaicin-sensitive sensory nerves in the pathomechanisms of gastric ulcers induced by stress. J Physiol Pharmacol. 2003;54:423–37. [PubMed] [Google Scholar]
- 69.Yamada T, Grisham MB. Role of neutrophil-derived oxidants in the pathogenesis of intestinal inflammation. Klin Wochenschr. 1991;69:988–94. doi: 10.1007/BF01645144. [DOI] [PubMed] [Google Scholar]
- 70.Clark RA. Oxidative stress and “senescent” fibroblast in non-healing wounds as potential therapeutic targets. J Invest Dermatol. 2008;128:2361–64. doi: 10.1038/jid.2008.257. [DOI] [PubMed] [Google Scholar]
- 71.Zadák Z, Hyspler R, Tichá A, et al. Antioxidants and vitamins in clinical conditions. Physiol Res. 2009;58(Suppl 1):S13–17. doi: 10.33549/physiolres.931861. [DOI] [PubMed] [Google Scholar]
- 72.Chen SH, Pan S, Okita K, Takemoto T. Role of oxygen-derived free radicals in the mechanism of cysteamine-induced duodenal ulcer in rats. J Formos Med Assoc. 1994;93:11–14. [PubMed] [Google Scholar]
- 73.Zhang D, Wang W, Zhou D, et al. Ghrelin inhibits apoptosis induced by palmitate in rat aortic endothelial cells. Med Sci Monit. 2010;16(12):BR396–403. [PubMed] [Google Scholar]
- 74.Wang W, Zhang D, Zhao H, et al. Ghrelin inhibits cell apoptosis induced lipotoxicity in pancreatic beta cells line. Regul Pept. 2010;161:43–50. doi: 10.1016/j.regpep.2009.12.017. [DOI] [PubMed] [Google Scholar]