Summary
Background
Accumulating data support the atheroprotective role of the novel adipokines, apelin and ghrelin. The aim of the present randomized study was to investigate the effects of aerobic exercise training on these adipokines in patients with type 2 diabetes mellitus (T2DM).
Material/Methods
Fifty-four overweight (BMI >25 kg/m2) patients with T2DM, but without vascular complications, were randomized to either the aerobic exercise training group (EG, N=27), 4 times/week, 45–60 min/session; or to the control group (CG, N=27), orally instructed to increase physical activity. Clinical glycemic and lipid parameters, exercise capacity (VO2peak), insulin, HOMA-IR, and serum levels of apelin and ghrelin were assessed at baseline and after 12 weeks.
Results
Aerobic exercise significantly improved lipid and glycemic profile and insulin sensitivity compared to CG (p<0.05). Furthermore, between-groups comparison showed a considerable exercise-induced upregulation in apelin (p=0.007) and VO2peak (p<0.001) levels. Negligible changes in body-weight, waist-hip ratio and ghrelin concentrations were detected within and between groups after the completion of the study (p>0.05). However, subgroup analysis revealed a considerable increment in ghrelin levels only in the exercise-treated women compared to their control counterparts (p=0.038). LDL and HOMA-IR reduction were found to be independent predictors of apelin increment in multiple regression analysis (R2=0.391, p=0.011).
Conclusions
In patients with T2DM, systemic, long-term, aerobic exercise exerts positive effects on apelin and ghrelin (only in women), even in the absence of significant weight loss, suggesting its pleiotropic effects.
Keywords: exercise, apelin, ghrelin, adipokines, type 2 diabetes
Background
A growing body of evidence supports the predominant role of chronic, low-grade inflammation in mediating insulin resistance and all stages of atherosclerosis in type 2 diabetes mellitus (T2DM) [1]. Among pro-inflammatory mediators associated with cardiovascular diseases (CVD), adipose-tissue derived cytokines, known as adipokines, have emerged as potential key factors of atherosclerosis-related complications [2].
Apelin, an adipocyte-secreted factor upregulated by insulin, has been recently identified as a contributor to glucose homeostasis [3]. Moreover, previous trials have suggested apelin as a potent regulator of cardiovascular function [4]. We and other investigators have recently documented the inverse relationship of circulating apelin levels with coronary artery disease [5,6]. Until present, available data concerning pharmaceutical and non-pharmaceutical regulation of apelin have been extremely limited [7,8].
Ghrelin is a potent gastric, orexigenic factor with growth hormone-releasing effects [9]. The pathophysiological role of ghrelin derives from the association of low plasma ghrelin levels with elevated fasting insulin levels and insulin resistance [9]. In parallel, there are accumulating data on the protective role of ghrelin in atherosclerotic processes [10,11]. Numerous investigations suggest the anti-inflammatory properties of ghrelin in the presence of advanced lesions of atherosclerosis [12]. Taken together, ghrelin manipulation, at least theoretically, could be useful for the treatment of T2DM and its complications. Weight loss interventions have all been proved to promote the upregulation of ghrelin levels [9]. However, it is still unknown whether exercise training per se, without weight loss, can produce beneficial effects on ghrelin levels.
Physical exercise has been recommended world-wide as one of the mainstays of treatment of T2DM, along with diet and medications [13]. Reduced cardiovascular morbidity and mortality has been associated with increased regular physical activity in the diabetic population [14]. In addition to the favorable modulation of “classical” cardiovascular risk factors (eg, dyslipidemia and hypertension), numerous studies have indicated the anti-inflammatory effect of long-term exercise training [15]. However, the influence of chronic exercise on human circulating adipokines is not well-documented.
Taken together, the modulation of apelin and ghrelin seems quite promising, because they interact with numerous pathophysiological mechanisms in T2DM and cardiovascular diseases. The present study aimed to investigate the effect of long-term aerobic exercise on circulating levels of novel adipokines, including apelin and ghrelin, in type 2 diabetic patients without any history of CVD.
Material and Methods
Patients
We enrolled 54 overweight (BMI >25 kg/m2) Caucasian men and women with T2DM, aged 50–70 years. All participants had poor glycemic control (HbA1c >6.5%) with oral anti-diabetic medications (sulfonylureas, metformin, for at least 4 months). Suitable patients, identified from review of case notes and/or computerized clinic registers, were contacted by the investigators in person or by telephone. Patients were excluded if they had any of the following conditions: a history of ketoacidosis within 6 months before study enrollment, excessive hyperglycemia (HbA1c >10%), diabetic micro-vascular complications, diabetic neuropathy, impaired hepatic function (plasma aminotransferase and/or γ-glutamyltransferase level higher than the upper limit of normal for age and sex), impaired renal function (serum creatinine level higher than the upper limit of normal for age and sex), severe anaemia, uncontrolled hypertension (blood pressure >170/100 mmHg), orthopaedic problems limiting exercise, malignancy, or autoimmune diseases. Patients with cardiovascular disease (New York Heart Association class I–IV congestive heart failure or a history of myocardial infarction), overt cardiac-related symptoms or history of cerebrovascular events also were excluded. Active patients reporting sport activities >1 per week and insulin-treated patients were considered ineligible.
Study design – Exercise intervention
Patients were randomly assigned to either a supervised, systemic, exercise training group (EG, N=27) or a control group (CG, N=27). Randomization process, based on computer software, created sex-matched groups.
The supervised exercise training program was similar to that performed in another exercise-study in our department [8]. Specifically, the EG group underwent a training program consisting of 4 sessions weekly. Each session included 10 min of warm-up, 30 min of aerobic activity (eg, walking/or running on treadmill, cycling, calisthenics of the upper and lower limbs) and 5 min of cool-down. The intensity and the duration of each session gradually increased across the first 4 weeks. After that, intensity achieved 60–75% of maximal heart rate and the duration of each session was constant at 60 min.
The control group was orally instructed, at baseline, to follow American Diabetes Association guidelines for cardiovascular diseases prevention [16]. In particular, the control patients were encouraged to perform a total of 150 minutes of self-controlled, moderate-intensity exercise, per week. Patients were asked to perform their preferred type of exercise (eg, walking, swimming) 3–5 times per week, with a minimum duration of 30 minutes per time. The recommended changes in physical activity were not assessed throughout the study. The latter modality of exercise intervention was selected as control group, because it is very common in clinical practice of a large general hospital. Finally, control patients were asked to record the weekly amount of physical activity at the end of the study.
Clinical evaluation
Before starting the study, all patients underwent an initial clinical evaluation including medical history, physical examination, measurements of body mass index (BMI), waist-hip ratio (WHR) and blood pressure (BP), as previously described [8]. The duration of the study was 12 weeks and clinical assessment was repeated at the end.
Cardiorespiratory fitness was estimated at the beginning and at the end of the study by ergospirometry, using an electronically-controlled ergocycle. Exercise testing protocol was supervised by an experienced physician as previously described [15]. During ergospirometry, gas exchanges were continuously measured by an analyzer (COSMED K4, Rome, Italy), using facemask and breath-by-breath technique. Patients were asked to maintain their eating patterns, anti-diabetic and anti-hypertensive medications throughout the study. Dietary habits of all patients were recorded at baseline by an expert dietician using a structured questionnaire. Additional weight control diet instructions were not provided throughout the study and patients were asked not to alter their dietary habits. During the study, monthly dietary records of each participant were assessed by the dietician at scheduled meetings.
The study design was approved by the ethics committee of Hippokratio General Hospital of Thessaloniki, Greece and the study was conducted in accordance with the Helsinki Declaration. All participants gave written informed consent before entering the study.
Blood assays
Blood samples were collected in the morning after a 12 h overnight fast. Fasting plasma glucose (FPG) and lipid parameters were quantified using standard enzymatic methods (Roche/Hitachi 912 automatic analyzer; Roche Diagnostics, Basel, Switzerland). For low-density lipoprotein cholesterol (LDL-C) calculation, we used the Friedewald formula. Measurements of HbA1c were taken by high-performance liquid chromatography (Menarini Diagnostics, Florence, Italy). After centrifugation of blood samples (5000 g for 7 min), serum was collected and kept frozen at −80°C until analysis in the same assay. Using commercially available enzyme immunoassay (EIA) kits (Phoenix Pharmaceuticals, Belmont, CA, USA) we quantified serum levels of apelin (human apelin-12) and ghrelin. The intra-assay coefficients of variance (CVs) were 5% and <14% for apelin and ghrelin, respectively. The inter-assay CVs for the latter adipocytokines were 14% and 7.5%, respectively. We measured plasma insulin using ELISA method (DRG Diagnostics, Marburg, Germany). The inter- and intra-assay CVs were 3% and 3.4%, respectively. The homeostasis model assessment (HOMA-IR) index was determined as a surrogate index of insulin resistance and was calculated with the following formula: HOMA-IR = fasting insulin (mU/L) × fasting glucose (mg/dl)/405. Nephelometric procedure (Dade Behrin, BNII, Marburg, Germany) was used for serum high-sensitivity CRP (hsCRP) assay.
Statistical analysis
Data are expressed as mean ±SD. Normality of distribution was assessed by Kolmogorov-Smirnov test. Paired-samples T test and Student’s T test were used for comparisons of parametric variables within and between groups, respectively. Chi-square test was used for non-parametric data. The correlations of apelin and ghrelin with the other continuous variables at baseline, as well as with their changes post-treatment, were calculated by Pearson correlation coefficients. Standard multiple regression analysis, adjusted for sex and age, was performed to determine the independent correlations of either of the 2 novel adipokines with the other parameters. Statistical significance was set at 2-sided p<0.05. Statistical analyses were performed using the computer software package SPSS (version 16.0; SPSS Inc, Chicago, IL, USA).
Results
Anthropometric and metabolic data (means ±standard deviations) are presented in Tables 1 and 2 and Figure 1. Among initially recruited patients, only 1 stopped in the EG due to time constraint. Throughout the study, most pharmaceutical regimens remained constant; in 3 patients in the EG group the dosage of the anti-diabetic medications was reduced. However, noteworthy adverse events (eg, hypoglycemic episodes) were not reported. Exercise-treated patients showed high compliance, since they attended at least 85% of the scheduled sessions. On the other hand, only 22.22% of the control patients achieved the target of increased physical activity at the end of the study, suggesting a lack of motivation. No significant difference between groups was detected at baseline for any clinical characteristics, including sex, age, diabetes duration, smoking habits and current anti-diabetic, anti-hypertensive and lipid-lowering drug therapy. Fitness parameters, traditional CV risk factors and inflammatory biomarkers were also similar among groups.
Table 1.
Baseline characteristics of participants who completed the study.
| Exercise group (n=26) | Control group (n=27) | p | |
|---|---|---|---|
| Gender (Men/Women) | 9/17 | 10/17 | 0.912 |
| Age (y) | 58.4±5.7 | 62.9±4.2 | 0.420 |
| Diabetes duration (y) | 5.1±2.5 | 5.5±2.1 | 0.787 |
| Smokers | 5 (19.2%) | 4 (14.8%) | 0.291 |
| Anti-hypertensive medications (n) | 16 (61.5%) | 19 (70.4%) | 0.333 |
| Statins (n) | 15 (57.69%) | 18 (66.67%) | 0.456 |
| Anti-diabetic regimen (n) | |||
| Sulfonylurea | 3 | 2 | 0.123 |
| Metformin | 5 | 7 | 0.243 |
| Sulfonylurea + metformin | 12 | 14 | 0502 |
| DPP4 inhibitors + metformin | 6 | 4 | 0.091 |
| Apelin (ng/ml) | 0.209±0.102 | 0.213±0.114 | 0.824 |
| Ghrelin (ng/ml) | 2.51±1.81 | 2.76±1.86 | 0.729 |
| HOMA-IR | 5.91±3.69 | 4.97±2.16 | 0.286 |
| Insulin (mU/L) | 9.38±4.82 | 8.93±4.11 | 0.370 |
Data are mean ±SD. n – number of patients; y – years; DPP4 – dipeptidyl-peptidase 4; HOMA-IR – homeostasis model assessment-insulin resistance.
Table 2.
Baseline and end-values of variables in both groups.
| Exercise group (n=26) | Control group (n=27) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 weeks | P1 | Baseline | 12 weeks | P1 | P2 | |
| BMI (kg/m2) | 32.1±3.77 | 31.98±3.03 | 0.500 | 30.04±2.95 | 30.65±3.55 | 0.267 | 0.820 |
| WHR | 0.98±0.05 | 0.98±0.05 | 0.920 | 0.97±0.11 | 0.98±0.14 | 0.711 | 0.599 |
| Systolic BP (mmHg) | 137±15 | 128±16 | 0.016 | 135±15 | 136±19 | 0.714 | 0.017 |
| Diastolic BP (mmHg) | 82±11 | 80±10 | 0.085 | 81±11 | 81±10 | 0.879 | 0.163 |
| HbA1c (%) | 7.8±0.8 | 7.1±1 | <0.001 | 7.7±0.6 | 7.9±0.1.1 | 0.422 | 0.004 |
| FPG (mg/dl) | 191±40 | 162±41 | <0.001 | 188±36 | 194±33 | 0.656 | 0.008 |
| TChol (mg/dl) | 202±58 | 178±51 | 0.047 | 197±48 | 204±57 | 0.523 | 0.032 |
| HDL-C (mg/dl) | 42±11 | 47±10 | 0.007 | 44±11 | 43±10 | 0.417 | 0.018 |
| LDL-C (mg/dl) | 128±46 | 106±39 | 0.036 | 123±45 | 126±55 | 0.473 | 0.012 |
| TG (mg/dl) | 160±55 | 125±57 | 0.035 | 150±63 | 175±76 | 0.102 | 0.005 |
Data are mean ±SD. BMI – body mass index; WHR, – Waist-hip ratio; BP – blood pressure; FPG – Fasting plasma glucose; TChol – total cholesterol; TG – triglycerides. P1 – p values of changes of variables within groups; P2 – p values of changes of variables between groups.
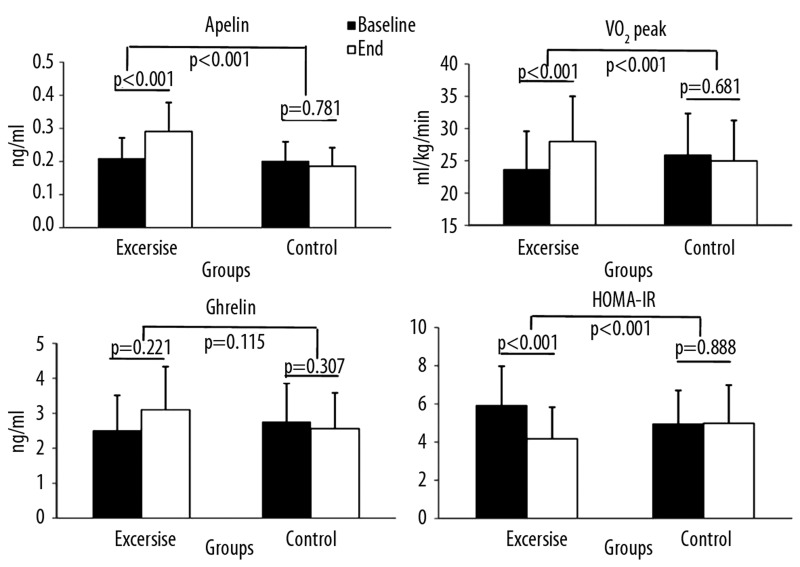
Figure 1.
Values of apelin ghrelin VO2peak and HOMA-IR at baseline and at the end of the study. * p<0.05, change of variables within groups. # p<0.05, change of variables between groups. Data are mean ±SD.
Anthropometric characteristics, lipid and glycemic profile, insulin resistance indexes
Although all patients were asked to maintain their dietary habits, the exercise-treated patients reported an almost 20% increase in water and energy intake compared to their untreated counterparts. Thus, the 12-week exercise intervention did not significantly affect body weight and anthropometric characteristics (p>0.05), but the intervention considerably reduced most lipid parameters, FPG and HbA1c compared to either baseline values or control group (p<0.05). Significant reductions (p<0.01) were noted in fasting insulin and HOMA-IR levels after the exercise training program. As expected, exercise training yielded a significant increase in the cardiorespiratory capacity, expressed by VO2peak (p<0.001) (Table 2).
Adipokines
As noted in Figure 1, after the exercise intervention there was a significant increment in apelin serum concentrations in the EG (from 0.209±0.102 ng/ml to 0.291±0.135 ng/ml; p<0.001), while there was a non-significant decrease in the CG (from 0.200±0.088 ng/ml to 0.186±0.109 ng/ml; p=0.781) (p<0.001). At the end of the study, the alterations in ghrelin concentrations did not reach statistically significant levels in either group (p>0.05). However, ghrelin was significantly reduced after exercise training in the women’s sub-group (from 2.14±0.71 ng/ml to 3.87±1.07 ng/ml, p=0.042) compared to control group women (from 2.20±1.36 ng/ml to 2.10±1.40 ng/ml, p=0.779) (p=0.038).
Pearson correlations were calculated for the novel adipokines at baseline as well as their changes post-exercise. At baseline, apelin correlated with age (r=0.311, p=0.024), HOMA-IR (r=−0.245, p=0.019), LDL-C (r=−0.521, p=0.022) and FPG (r=−0.431, p=0.041). Baseline values of ghrelin significantly correlated with BMI (r=0.469, p=0.038) and HOMA-IR (r=−0.399, p=0.010). The exercise-induced upregulation in apelin was inversely associated with changes in LDL (r=−0.363, p=0.002) and HOMA-IR (r=−0.411, p=0.003). The latter parameters retained their significant correlations with apelin in standard multiple regression analysis (R2=0.391, p=0.011). No significant correlations were found between the change of ghrelin and the rest of the variables within the exercise group.
Discussion
In the present study 12-week intensive aerobic exercise training significantly improved metabolic profile and cardiorespiratory capacity in overweight patients with T2DM. Furthermore, we conclusively demonstrated the positive effects of exercise training on serum concentrations of the novel adipokines, apelin and ghrelin. The latter results seemed to be mediated by the insulin-sensitizing effects of exercise, outlining a novel atheroprotective mechanism.
In our study body-weight and waist circumference remained essentially unaffected by intensive exercise training. This was not surprising, since exercise-treated patients increased food intake compensatory to increased energy expenditure. Consistent with previous studies, blood pressure, insulin sensitivity, glycemic and lipid control were substantially improved in exercise-treated patients, despite the absence of significant variations in anthropometric parameters [8,15].
It is well documented that strenuous exercise can ameliorate plasma levels of various adipokines, including IL-6, resistin and TNFa in type 2 diabetic individuals [17]. To our knowledge this is the first study demonstrating a significant upregulation of serum apelin levels after structured exercise treatment in the diabetic population. Apelin and its receptor (APJ) are localized in cardiomyocytes and vascular cells and seem to play a key role in the regulation of vascular tone and cardiovascular function [18]. Several lines of evidence support the association of low apelin concentrations with atherosclerotic complications [12]. Therefore, the observed apelin increase after exercise supports the hypothesis that an exercise training-induced increase in apelin might contribute to the beneficial effects of exercise training on cardiovascular disease risk.
Tasci et al. (2009) [7] reported an almost 4-fold increase in apelin levels after a 12-week therapeutic lifestyle program combining diet and self-controlled exercise. The study enrolled non-diabetic, statin-free, non-obese (BMI <30 kg/m2) individuals with dyslipidemia. Participants who achieved the LDL-lowering target after intervention showed increased apelin levels. Consistent with this, we observed LDL reduction was an independent predictor of the exercise-induced alterations in apelin levels. In addition to this, we found that insulin resistance attenuation was independently associated with apelin increase in our exercise-treated group. A growing body of evidence, derived from experimental and clinical trials, supports the interaction between apelin and insulin sensitivity [19]. Notably, a significant increment in serum apelin levels could be obtained in diabetic patients after treatment with insulin sensitizers, metformin and rosiglitazone [10]. It is still unclear whether apelin actively mediates insulin sensitivity modulation. However, the activation of AMP-activated protein kinase provides the underlying link between exercise-mediated insulin sensitization and apelin changes [20,21]. Future studies will be needed to verify the latter hypothesis in different stages of insulin resistance.
The novel adipokine ghrelin induces weight gain not only by stimulating appetite, but also by decreasing fat utilization [22]. Conversely, significant up-regulation of serum ghrelin levels has been recorded after weight loss interventions [23]. Furthermore, low plasma ghrelin levels have been associated with obesity, insulin resistance, inflammation and atherosclerosis [5,24,25]. Despite the emerging atheroprotective role of ghrelin, its regulation seems multi-factorial, depending predominantly on weight changes [26]. A limited number of studies have demonstrated increased total ghrelin levels after long-term exercise associated with weight loss [27]. In our study exercise training had negligible effects on body weight and concomitant serum levels of ghrelin in the whole study population. On the other hand, sub-group analysis revealed a considerable increase in ghrelin concentrations in exercise-treated women, despite the absence of weight loss. Our finding is consistent with results of a recent study showing increased ghrelin levels in non-diabetic, physically active women, but not in men, and that effect was independent of energy equilibrium [28].
The latter sexual dimorphic response of ghrelin to exercise intervention is of clinical importance. Looking for the underlying mechanisms, we initially hypothesized sex-related differences in appetite and energy homeostasis. After assessing the energy balance throughout the study, we observed an equivalent increase in energy expenditure and compensatory increased energy intake in both sexes. Therefore, the final energy balance did not change significantly. Another plausible explanation for the aforementioned ghrelin sex-based fluctuation may center on differences in sex hormones [29]. Peripheral adiposity and most adipokines are regulated by sex hormones [30]. Our findings indicate another sex-dependent cardioprotective mechanism and suggest that therapeutic interventions targeting ghrelin should optimally achieve weight loss. Further studies are needed to determine the magnitude of exercise-induced energy deficit resulting in positive effects on adipokines.
The principal limitation of the present study was the relative small sample size. However, we set numerous selection criteria in order to create homogeneous study groups. Another important shortcoming was the absence of dietary consultation throughout the study. Thus, the recorded diet changes in the exercise group might have moderated our findings, and the final results could have been even better. An additional consideration is that exercise-treated subjects had apparently more contact with study staff during supervised exercise training sessions than did the control group, raising concerns about the effect on other behaviors (eg, daily physical activities) during the study. Since unsupervised exercise was not monitored, no data are available from which to evaluate the potential role of such changes. Finally, we measured total ghrelin levels. The acylated form of ghrelin is thought to be more potent than non-acylated ghrelin in stimulating appetite. Our study might have had different results if we had assessed the latter form of ghrelin.
Conclusions
In conclusion, in patients with T2DM, exercise training led to significant increase of apelin and ghrelin (only in women) serum concentrations, despite the absence of body weight change. Increased insulin sensitivity and lowered LDL-C after training were most strongly related to changes in apelin, suggesting the multi-factorial, “pleiotropic” effects of exercise.
Acknowledgments
Nikolaos P.E. Kadoglou was awarded a grant by the Onassis Public Benefit Foundation.
Footnotes
Source of support: Departmental sources
References
- 1.Bonaterra GA, Zügel S, Kinscherf R. Novel systemic cardiovascular disease biomarkers. Curr Mol Med. 2010;10:180–205. doi: 10.2174/156652410790963330. [DOI] [PubMed] [Google Scholar]
- 2.Kadoglou NP, Avgerinos ED, Liapis CD. An update on markers of carotid atherosclerosis in patients with type 2 diabetes. Biomark Med. 2010;4:601–9. doi: 10.2217/bmm.10.79. [DOI] [PubMed] [Google Scholar]
- 3.Soriguer F, Garrido-Sanchez L, Garcia-Serrano S, et al. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes Surg. 2009;19:1574–80. doi: 10.1007/s11695-009-9955-y. [DOI] [PubMed] [Google Scholar]
- 4.Carpéné C, Dray C, Attané C, et al. Expanding role for the apelin/APJ system in physiopathology. J Physiol Biochem. 2007;63:359–73. [PubMed] [Google Scholar]
- 5.Kadoglou NP, Lampropoulos S, Kapelouzou A, et al. Serum levels of apelin and ghrelin in patients with acute coronary syndromes and established coronary artery disease – KOZANI STUDY. Transl Res. 2010;155:238–46. doi: 10.1016/j.trsl.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Kuklinska AM, Sobkowicz B, Sawicki R, et al. Apelin: a novel marker for the patients with first ST-elevation myocardial infarction. Heart Vessels. 2010;25:363–67. doi: 10.1007/s00380-009-1217-3. [DOI] [PubMed] [Google Scholar]
- 7.Tasci I, Erdem G, Ozgur G, et al. LDL-cholesterol lowering increases plasma apelin in isolated hypercholesterolemia. Atherosclerosis. 2009;204:222–28. doi: 10.1016/j.atherosclerosis.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Kadoglou NP, Tsanikidis H, Kapelouzou A, et al. Effects of rosiglitazone and metformin treatment on apelin, visfatin, and ghrelin levels in patients with type 2 diabetes mellitus. Metabolism. 2010;59:373–79. doi: 10.1016/j.metabol.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 9.García EA, Korbonits M. Ghrelin and cardiovascular health. Curr Opin Pharmacol. 2006;6:142–47. doi: 10.1016/j.coph.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Ukkola O, Poykko S, Paivansalo M, Kesaniemi YA. Interactions between ghrelin, leptin and IGF-I affect metabolic syndrome and early atherosclerosis. Ann Med. 2008:1–9. doi: 10.1080/07853890802084860. [DOI] [PubMed] [Google Scholar]
- 11.Yano Y, Toshinai K, Inokuchi T, et al. Plasma des-acyl ghrelin, but not plasma HMW adiponectin, is a useful cardiometabolic marker for predicting atherosclerosis in elderly hypertensive patients. Atherosclerosis. 2009;204:590–94. doi: 10.1016/j.atherosclerosis.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Kadoglou NP, Sailer N, Moumtzouoglou A, et al. Novel markers of carotid atherosclerosis in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2010;118:75–80. doi: 10.1055/s-0029-1237360. [DOI] [PubMed] [Google Scholar]
- 13.Sigal RJ, Kenny GP, Wasserman DH, et al. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433–38. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 14.Telford RD. Low physical activity and obesity: causes of chronic disease or simply predictors? Med Sci Sports Exerc. 2007;39(8):1233–40. doi: 10.1249/mss.0b013e31806215b7. [DOI] [PubMed] [Google Scholar]
- 15.Kadoglou NP, Iliadis F, Angelopoulou N, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. 2007;14:837–43. doi: 10.1097/HJR.0b013e3282efaf50. [DOI] [PubMed] [Google Scholar]
- 16.Buse JB, Ginsberg HN, Bakris GL, et al. American Heart Association; American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30:162–72. doi: 10.2337/dc07-9917. [DOI] [PubMed] [Google Scholar]
- 17.Kadoglou NP, Iliadis F, Liapis CD, et al. Beneficial effects of combined treatment with rosiglitazone and exercise on cardiovascular risk factors in patients with type 2 diabetes. Diabetes Care. 2007;30:2242–44. doi: 10.2337/dc07-0341. [DOI] [PubMed] [Google Scholar]
- 18.Quazi R, Palaniswamy C, Frishman WH. The emerging role of apelin in cardiovascular disease and health. Cardiol Rev. 2009;17:283–86. doi: 10.1097/CRD.0b013e3181b3fe0d. [DOI] [PubMed] [Google Scholar]
- 19.Dray C, Debard C, Jager J, et al. Apelin and APJ regulation in adipose tissue and skeletal muscle of type 2 diabetic mice and humans. Am J Physiol Endocrinol Metab. 2010;298:E1161–69. doi: 10.1152/ajpendo.00598.2009. [DOI] [PubMed] [Google Scholar]
- 20.Kelly M, Gauthier MS, Saha AK, Ruderman NB. Activation of AMP-activated protein kinase by interleukin-6 in rat skeletal muscle: association with changes in cAMP, energy state, and endogenous fuel mobilization. Diabetes. 2009;58:1953–60. doi: 10.2337/db08-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdem G, Dogru T, Tasci I, et al. Low plasma apelin levels in newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008;116:289–92. doi: 10.1055/s-2007-1004564. [DOI] [PubMed] [Google Scholar]
- 22.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 23.Adams CE, Greenway FL, Brantley PJ. Lifestyle factors and ghrelin: critical review and implications for weight loss maintenance. Obes Rev. 2011;12(5):e211–18. doi: 10.1111/j.1467-789X.2010.00776.x. [DOI] [PubMed] [Google Scholar]
- 24.Pöykkö SM, Kellokoski E, Horkko S, et al. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes. 2003;52:2546–53. doi: 10.2337/diabetes.52.10.2546. [DOI] [PubMed] [Google Scholar]
- 25.Dezaki K, Sone H, Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol Ther. 2008;118:239–49. doi: 10.1016/j.pharmthera.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Ueno H, Shiiya T, Mizuta M, et al. Plasma ghrelin concentrations in different clinical stages of diabetic complications and glycemic control in Japanese diabetics. Endocr J. 2007;54:895–902. doi: 10.1507/endocrj.k07-007. [DOI] [PubMed] [Google Scholar]
- 27.Littman AJ, Vitiello MV, Foster-Schubert K, et al. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int J Obes. 2007;31:466–75. doi: 10.1038/sj.ijo.0803438. [DOI] [PubMed] [Google Scholar]
- 28.Hagobian TA, Sharoff CG, Stephens BR, et al. Effects of exercise on energy-regulating hormones and appetite in men and women. Am J Physiol Regul Integr Comp Physiol. 2009;296:R233–42. doi: 10.1152/ajpregu.90671.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gayle DA, Desai M, Casillas E, et al. Gender-specific orexigenic and anorexigenic mechanisms in rats. Life Sci. 2006;79:1531–36. doi: 10.1016/j.lfs.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Nedungadi TP, Clegg DJ. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J Cardiovasc Transl Res. 2009;2:321–27. doi: 10.1007/s12265-009-9101-1. [DOI] [PubMed] [Google Scholar]