Abstract
The transcription factor NF-κB in human intestinal epithelial cells plays a central role in regulating genes that govern the onset of mucosal inflammatory responses following intestinal microbial infection. Nod1 is a cytosolic pattern recognition receptor in mammalian cells that senses components of microbial peptidoglycans and signals the activation of NF-κB. The aim of these studies was to assess the functional importance of Nod1 in activating NF-κB and NF-κB proinflammatory target genes in human intestinal epithelium. Human colon epithelial cells that constitutively express Nod1 were used as a model intestinal epithelium. These cells do not signal through Toll-like receptor 4 (TLR4) or respond to bacterial lipopolysaccharide, but they express functional TLR5 and interleukin 1 (IL-1) receptors that signal the activation of NF-κB in response to bacterial flagellin or IL-1 stimulation. Stable expression of dominant negative (DN) Nod1 in colon epithelial cells prevented IκB kinase and NF-κB activation in response to infection with enteroinvasive Escherichia coli. In contrast, DN Nod1 did not eliminate IL-1 or flagellin-stimulated NF-κB activation. Inhibition of NF-κB was accompanied by inhibition of NF-κB target genes that provide signals for the mucosal influx of neutrophils during intestinal infection. We conclude that signaling through Nod1 is required for activating NF-κB in human intestinal epithelial cells infected with gram-negative enteric bacteria that can bypass TLR activation. Signaling through Nod1 provides the intestinal epithelium with a backup mechanism for rapidly activating innate immunity during infection with a group of highly invasive pathogenic gram-negative bacteria.
Enteric pathogens that infect the human gastrointestinal tract (e.g., Salmonella, Escherichia coli, Shigella, Yersinia) rapidly upregulate the expression of a program of intestinal epithelial cell genes, the products of which chemoattract and activate populations of leukocytes that are important for the onset of host protective mucosal inflammatory responses (4, 14, 37, 39, 40, 45, 56, 61). The transcription factor NF-κB is a key regulator of this proinflammatory gene program in intestinal epithelial cells (16, 19, 57).
NF-κB is a member of a family of dimeric transcription factors that regulate gene transcription by binding to κB elements in the promoter regions of specific target genes (54). Signaling cascades initiated by microbial products culminate in activation of the IκB kinase (IKK) complex, which phosphorylates inhibitory κB proteins, leading to their degradation (12, 41). This enables NF-κB to enter the nucleus and induce target gene transcription. Many of the NF-κB target genes are key elements in initiating and mediating host innate immunity.
The mammalian innate immune system recognizes pathogen-associated molecular patterns (PAMPs) through pattern recognition receptors (PRRs). PAMPs are conserved structures that are characteristic of bacterial pathogens but are not produced by mammalian cells. PRRs either are located in the cell membrane and respond to extracellular PAMPs or are located in the cytosol and respond to PAMPs that cross the plasma membrane (34, 46). Human Toll-like receptors (TLRs) mainly interact with extracellular PAMPs; for example, TLR5 interacts with bacterial flagellin, and TLR4 interacts with bacterial lipopolysaccharide (LPS) (10, 27, 50, 60). Signaling through TLRs leads to the activation of IKK, NF-κB, and NF-κB target genes (60).
Nod1 (also designated CARD4) is a cytosolic PRR that is a member of a family of nucleotide-binding oligomerization domain proteins which have been implicated in innate immune recognition (reviewed in reference 34). Nod1 structurally contains a caspase activation and recruitment domain (CARD), a centrally located nucleotide-binding oligomerization domain, and multiple C-terminal leucine-rich repeats. Nod1 engages in a homophilic CARD-CARD interaction with RICK (RIP2), a CARD-containing protein kinase (6, 32-34) that in turn activates IKK and results in NF-κB activation (6, 32, 33, 42). Both the CARD domain and the nucleotide-binding domain are required for NF-κB activation, whereas the leucine-rich repeats interact with PAMPs derived from gram-negative bacteria (21, 23, 35). Initially, Nod1 was thought to interact with intracellular LPS (23, 35). However, more recent studies revealed that this is not the case and resulted in identification of a diaminopimelate-containing N-acetylglucosamine-N-acetylmuramic acid tripeptide (21, 24) found in peptidoglycans from gram-negative bacteria and the dipeptide γ-d-glutamyl-meso-diaminopimelic acid (8) as the key structures recognized by Nod1. In contrast to Nod1, which is constitutively expressed by intestinal epithelial cells (28), a structurally and functionally related protein, Nod2, is expressed predominately in monocytes (49) and senses N-acetylmuramic acid-l-Ala-d-isoGln, also known as muramyl dipeptide, in peptidoglycans (22, 36).
Intestinal epithelium consists of a single layer of cells that, unlike other mucosal sites, normally are exposed to large numbers of commensal bacteria and their products. Intestinal epithelial cells also are the initial site of contact between the host and enteric bacterial pathogens. Nod1 signaling in response to bacterial PAMPs has been defined mainly in model systems in which Nod1 was transiently overexpressed in human embryonic kidney 293 cells (21, 23, 32, 35). However, little is known about the functional importance of Nod1 in activating NF-κB and NF-κB-dependent proinflammatory genes in bacterium-infected human intestinal epithelial cells.
In the present study we defined the functional importance of intestinal epithelial cell Nod1 in activating NF-κB and NF-κB target genes that provide signals essential for the onset of mucosal inflammation during enteric infections. We report here that signaling through Nod1 constitutes the major pathway by which NF-κB and NF-κB target genes are upregulated in human colon epithelial cells infected by bacterial pathogens that avoid activating host epithelial cell proinflammatory genes through TLRs.
MATERIALS AND METHODS
Cell lines and cell culture.
The human colon epithelial cell lines Caco-2, HT-29, HCA-7, LS174T, HCT-8, and I-407 were grown in Dulbecco's modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum. T84 human colon cells were grown in 50% Dulbecco's modified Eagle medium-50% Ham's F-12 medium supplemented with 5% newborn calf serum (39). Media were supplemented with 2 mM l-glutamine. Cells were maintained in 95% air-5% CO2 at 37°C.
Isolation of colon epithelial cells.
Epithelium from endoscopic colon biopsies obtained from normal human colon mucosa was isolated in sheets containing crypt and surface epithelium (31, 44). Biopsies were incubated in Ca2+- and Mg2+-free Hanks' balanced salt solution containing 30 mM EDTA at room temperature for 30 min, after which the colon epithelium was isolated mechanically by using needles and a dissecting microscope. These studies were approved by the University of California at San Diego Human Subjects Committee.
Bacteria, cytokines, and other reagents.
The following bacteria were used in these studies: enteroinvasive E. coli ATCC 43892 (serotype O29:NM), Shigella flexneri M90T (26, 56), enterohemorrhagic E. coli 86-24 (serotype O157:H7) (4), and Salmonella enterica serovar Dublin strain Lane (39). Recombinant human tumor necrosis factor alpha (TNF-α) and recombinant human interleukin 1α (IL-1α) were obtained from R&D Systems (Minneapolis, Minn.). Gamma interferon was obtained from BioSource International (Camarillo, Calif.). Bacterial LPS from E. coli O111:B4 and O55:B4 were obtained from Sigma Chemical Co. (St. Louis, Mo.). Flagellin isolated from enterohemorrhagic E. coli strain 86-24 by acid depolymerization was provided by Y. Miyamoto, University of California at San Diego (4). Anti-myc monoclonal antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.).
Plasmids, transfection, and luciferase assay.
A dominant-negative (DN) Nod1 expression vector (pcDNA3-Nod1ΔCARD-myc) with a deletion of the CARD domain and a control empty vector (pcDNA3) were provided by N. Inohara and G. Nunez (University of Michigan) (32, 35) and M. Karin (University of California at San Diego), respectively. Rous sarcoma virus β-galactosidase and 3XNF-κB-luciferase transcriptional reporters have been described previously (19). Cells in 24-well dishes were transfected with plasmid DNA by using Lipofectamine Plus (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. Luciferase activity was assayed and normalized relative to β-galactosidase activity (15, 52).
Transfection of Caco-2 cells and generation of stably transfected cell lines.
Caco-2 cells that express TLR5 and respond to bacterial flagellin (4, 58) and IL-1 (38, 61) but express little or no TLR4, lack MD-2, and do not respond to commercial preparations of bacterial LPS (1, 13, 39) were transfected with pcDNA3-Nod1ΔCARD-myc (DN Nod1) or with pcDNA3 by using Lipofectamine Plus (Invitrogen). G418 (0.5 mg/ml)-resistant colonies were isolated by using glass cloning cylinders (11). Production of DN Nod1 in cells stably transfected with pcDNA3-Nod1ΔCARD-myc was determined by immunoblotting with monoclonal anti-myc antibody. Six cell lines that stably expressed DN Nod1, as assessed by immunoblotting, were generated, and two of these lines were cloned further. One of the latter lines expressed a high level of DN Nod1 compared to the levels that other cell lines generated, and the other line expressed an intermediate level of DN Nod1 compared to the levels that other cell lines generated; these two lines were designated CDN10 and CDN1, respectively. The Caco-2 cell line that stably expressed empty vector was designated CEV1.
Infection protocols.
Epithelial cells grown to confluence in 24-well, 6-well, or 10-cm plates were infected with bacteria at a multiplicity of infection of 100 (19). Cells were incubated with bacteria for 1 h, after which extracellular bacteria were removed by washing. Cells were incubated for additional periods of time in the presence of 50 μg of gentamicin per ml to kill the remaining extracellular bacteria but not intracellular bacteria.
RT and real-time RT-PCR.
Total cellular RNA was extracted with an RNeasy mini kit (Qiagen, Valencia, Calif.) and treated with RNase-free DNase to remove any contaminating genomic DNA. For reverse transcription (RT)-PCR, 1 μg of total cellular RNA was reverse transcribed, and cDNA was amplified as described previously (39). Primers for Nod1 and Nod2 were designed from sequences available from GenBank (accession numbers AF113925 and AF178940, respectively). The Nod1 primers were sense primer 5′-TCCAAAGCCAAACAGAAACTC-3′ and antisense primer 5′-CAGCATCCAGATGAACGTG-3′, and the Nod2 primers were sense primer 5′-GAAGTACATCCGCACCGAG-3′ and antisense primer 5′-GACACCATCCATGAGAAGACAG-3′; these sets of primers yielded PCR products that were 180 and 174 bp long, respectively. After a hot start, the amplification profile was 45 s of denaturation at 95°C and 2.5 min of annealing and extension at 62°C for 30 cycles. Negative control reaction mixtures contained no added RNA in the RT reaction mixtures and no cDNA in the PCR amplification mixtures. Primers specific for IL-8 and ENA-78 have been described previously (39, 61). For real-time PCR, 1 μl of cDNA was amplified by using an ABI Prism 7700 sequence detection system (PE Applied Biosystems, Foster City, Calif.) with 2× SYBR Green master mixture (Applied Biosystems) as described previously (5, 26). Amplification of the expected single PCR product was confirmed on 1% agarose gels stained with ethidium bromide.
Nuclear extracts, electrophoretic mobility shift assay (EMSA), and IKK assays.
Nuclear extracts were prepared as described previously (4). Ten micrograms of nuclear extract was equilibrated for 20 min with 25,000 cpm of [γ-32P]ATP-end-labeled NF-κB oligonucleotide in binding buffer [10 mM Tris-Cl (pH 8.0), 75 mM KCl, 10% glycerol, 0.1 mM EDTA, 2.5 mM MgCl2, 1 μg of poly(dI/dC)]. The sequence of the NF-κB consensus oligonucleotide from the IL-8 promoter was TCGTGGAATTTCCTCTG (48). Bound and free DNA were resolved by electrophoresis through a 6% high-ionic-strength polyacrylamide gel containing 5% glycerol at 250 V in Tris-glycine-EDTA electrophoresis buffer (50 mM Tris, 375 mM glycine, 10 mM EDTA) (4, 18). NF-Y binding activity was used as a loading control.
For IKK assays, cells were lysed in kinase lysis buffer (43), and cell lysates were immunoprecipitated with anti-IKKγ antibody (PharMingen, San Diego, Calif.) as described previously (19, 30). Immune complexes were incubated in 25 μl of kinase reaction buffer containing 2 μg of glutathione S-transferase-IκBα (1-54) fusion protein as the substrate and 5.0 μCi of [γ-32P]ATP for 30 min at 30°C. Phosphorylation reactions were stopped by boiling in sample buffer, followed by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis. Phosphorylated substrates in the gel were transferred to nitrocellulose membranes, and kinase activity was quantitated by phosphorimaging. Recovery of IKK complexes was assessed by immunoblotting with antibody against IKKα (9).
Cell lysates and immunoblotting.
Cells were lysed in ice-cold lysis buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate, 0.1% sodium deoxycholate, 1 mM EDTA, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na+ vanadate, protease inhibitor cocktail set III [1:200; Calbiochem, La Jolla, Calif.]) for 30 min. Cell lysates were sonicated and centrifuged, and the protein contents of the lysates were assayed by using a Bio-Rad protein assay kit.
For immunoblots, cell lysates (7.5 μg of protein/lane) were electrophoresed on sodium dodecyl sulfate-10% polyacrylamide gels and transferred to nitrocellulose membranes (Hybond ECL; Amersham Pharmacia Biotech, Piscataway, N.J.). The membranes were blocked with Tris-buffered saline (TBS) (50 mM Tris base [pH 7.2], 150 mM NaCl, 2.6 mM KCl) containing 5% dry milk and 0.1% Tween 20 and incubated overnight at 4°C with mouse anti-myc monoclonal antibody in TBS containing 5% dry milk and 0.1% Tween 20. The blots were washed in TBS-Tween 20, incubated with horseradish peroxidase-conjugated sheep anti-mouse antibody (1:2,000; Amersham Pharmacia Biotech), and visualized by enzyme chemiluminescence. Equal protein loading was verified after the blot was stripped and reprobed with an antibody to β-actin.
IL-8 ELISA.
IL-8 in cell culture supernatants was measured by an enzyme-linked immunosorbent assay (ELISA) by using a monoclonal mouse anti-human IL-8 antibody for capture and a biotinylated polyclonal anti-human IL-8 antibody for detection (both obtained from R&D Systems). The ELISA was sensitive to 3 pg of IL-8 per ml.
RelA/p65 immunofluorescence.
Cells grown on chamber slides (Nunc, Naperville, Ill.) were fixed with 4% paraformaldehyde. Cells were permeabilized with 0.5% Triton X-100 and blocked with phosphate-buffered saline containing 1% bovine serum albumin and 5% goat serum, after which polyclonal anti-p65 immunoglobulin G (sc-372; Santa Cruz Biotechnology) in blocking buffer was added and the cells were incubated at 4°C overnight. Cy-3-conjugated goat anti-rabbit immunoglobulin (Jackson ImmunoResearch Labs, West Grove, Pa.) in blocking buffer was added as a secondary antibody. Cells in which the nuclear fluorescence intensity was the same as or greater than the cytoplasmic fluorescence intensity were considered positive for nuclear RelA/p65.
RESULTS
Nod1 mRNA expression by intestinal epithelial cells.
Human colon epithelial cells isolated from colon biopsies, as well as seven different human intestinal epithelial cell lines, constitutively expressed Nod1 mRNA (Fig. 1A and B). The expression levels of Nod1 were not significantly increased by stimulation of Caco-2 or T84 cells with gamma interferon or with other proinflammatory cytokines (IL-1α, TNF-α) alone or in combination, as shown by qualitative PCR (Fig. 1C) and confirmed by real-time PCR. This was also the case for three other cell lines tested (HCA7, HCT8, and HT-29). The Nod1 mRNA levels, as assessed by real-time PCR, were not increased in any of the cell lines tested (Caco-2, T84, and HT-29) by stimulation with titrated doses (0.1 to 100 ng/ml) of E. coli O111:B4 or E. coli O55:B5 LPS for up to 24 h or, as shown for Caco-2 cells in Fig. 1D, by infection of the cells with enteroinvasive E. coli O29:NM. This was also the case following infection with S. flexneri or Salmonella serovar Dublin. In further studies, qualitative PCR (35 cycles) revealed little, if any, constitutive Nod2 mRNA expression in Caco-2 cells (Fig. 1D) or in the other cell lines tested (T84, HT-29), which is consistent with a previous report (29). As assessed by real-time PCR, the Nod2 mRNA levels in these cell lines were at least 10- to 20-fold lower than the levels of Nod1 mRNA. The Nod2 mRNA levels in Caco-2 cells were not increased by stimulation with LPS but were upregulated 3 h after infection with enteroinvasive E. coli (Fig. 1D) (threefold increase as determined by real-time PCR) or Salmonella servovar Dublin (three- to sevenfold increase as determined by real-time PCR). Nonetheless, the Nod2 levels, even after infection, were consistently less than the levels of constitutively expressed Nod1. These data are consistent with a report that expression of Nod2 can be upregulated in some colon epithelial cells by proinflammatory mediators (e.g., TNF-α) (25, 53). In our additional studies we focused on the use of Caco-2 cells given their constitutive expression of Nod1, little, if any, constitutive Nod2 expression or no constitutive Nod2 expression, and lack of response to LPS stimulation, which is consistent with their expression of little, if any, TLR4, and a lack of MD-2, a key coreceptor for TLR4 signaling (1, 13, 39).
FIG. 1.
Human colon epithelial cells constitutively express Nod1 mRNA. (A) RT-PCR analysis of Nod1 and β-actin mRNA transcripts in freshly isolated human colon epithelial cells (IEC), seven human intestinal epithelial cell lines, human embryonic kidney cells (HEK293T), HeLa cells, and a human macrophage cell line (U937). (B) Ratio of Nod1 to β actin transcripts constitutively expressed by the same cells, as assayed by real-time RT-PCR. (C) Nod1 mRNA expression by Caco-2 and T84 cells that were not stimulated or were stimulated for 3 h with proinflammatory cytokines, as indicated above the lanes. (D) Nod1 and Nod2 mRNA expression in Caco-2 cells that were not infected or were infected for up to 24 h with enteroinvasive E. coli 029:NM.
DN Nod1 inhibits IKK and NF-κB activation in E. coli O29:NM-infected cells.
To determine the functional role of Nod1 in bacterium-infected intestinal epithelial cells, we used enteroinvasive E. coli O29:NM, which activates IKK, NF-κB, and NF-κB proinflammatory target genes in Caco-2 cells, as shown previously (19, 39). Enteroinvasive E. coli O29:NM cells are highly invasive for intestinal epithelial cells. Nonetheless, they are nonmotile and lack flagella that can activate NF-κB by signaling through TLR5, which is expressed both by intestinal epithelial cell lines, including Caco-2 cells (4, 58), and by intestinal epithelial cells in vivo (7; Y. Miyamoto and M. Kagnoff, unpublished data). We hypothesized that IKK and NF-κB activation in enteroinvasive E. coli-infected Caco-2 cells depends on Nod1, which occupies a key position in a signal transduction pathway that leads to IKK and NF-κB activation (6, 32, 33, 42). Consistent with this, transient transfection of Caco-2 cells with a DN Nod1 expression vector inhibited activation of a cotransfected NF-κB reporter gene in response to E. coli O29:NM infection by ∼2.5-fold. Thus, following infection with E coli 029:NM, the increase in luciferase activity compared to the activity in uninfected controls in Caco-2 cells transiently transfected with a 3XNF-κB-luc reporter alone was 3.9- ± 0.2-fold, compared to the 1.6- ± 0.1-fold increase in Caco-2 cells transiently transfected with both DN Nod1 and the 3XNF-κB-luc reporter (P < 0.05; n = 3).
Based on these preliminary findings, we generated lines of Caco-2 cells that stably expressed various levels of DN Nod1. We then assessed IKK activity in E. coli O29:NM-infected Caco-2 cells that stably expressed DN Nod1 (i.e., CDN10 cells) or an empty vector (CEV1 cells) compared to the activity in infected wild-type Caco-2 cells. Consistent with our hypothesis, DN Nod1 significantly attenuated the activation of IKK in enteroinvasive E. coli-infected Caco-2 cells stably expressing DN Nod1 but not in CEV1 or wild-type Caco-2 cells (Fig. 2).
FIG. 2.
IKK activation in response to enteroinvasive E. coli infection in wild-type Caco-2 cells and Caco-2 cells expressing DN Nod1. Control Caco-2, Caco-2 cells that express an empty vector (CEV1), and Caco-2 cells that stably express DN Nod1 (CDN10) cells were infected with enteroinvasive E. coli O29:NM, after which IKK activity was determined by an in vitro kinase assay at different times after infection by using a glutathione S-transferase-IκBα fusion protein as the substrate. IKKα levels assessed by immunoblotting revealed equal loading (data not shown).
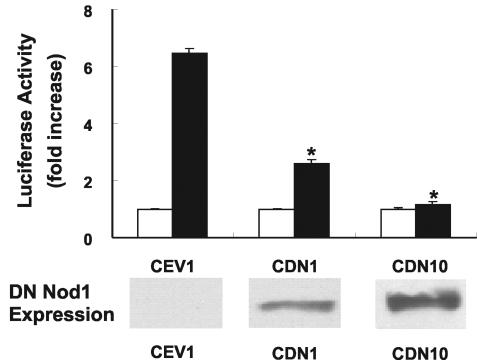
NF-κB activation was also significantly inhibited in Caco-2 cells expressing DN Nod1, and the greatest inhibition was found in cells that expressed the highest levels of DN Nod1 (Fig. 3). DN Nod1 expression did not alter enteroinvasive E. coli proliferation in Caco-2 cells, as the numbers of CFU recovered from infected wild-type and mutant Caco-2 cells were not significantly different at 5 and 24 h after infection. Consistent with the EMSA data, cytoplasmic-to-nuclear translocation of RelA/p65, as assessed by immunostaining, was almost completely eliminated in enteroinvasive E. coli-infected cells that expressed DN Nod 1 (Fig. 4).
FIG. 3.
Inhibition of E. coli-induced NF-κB activation in cells that express DN Nod1. (Top panel) Caco-2 cell lines that stably express DN Nod1 (CDN1 and CDN10) or control vector (CEV1) were transiently transfected with a 3XNF-κB-luc reporter construct and either infected with enteroinvasive E. coli O29:NM (solid bars) or not infected (open bars). The data indicate the fold increases in luciferase activity compared to the luciferase activity of uninfected cells. The data are from three independent experiments, and the error bars indicate standard errors of the means. An asterisk indicates that the P value is <0.05 for a comparison with CEV1-infected cells. (Bottom panel) Myc expression was assessed by immunoblotting as an indicator of DN Nod1 expression in cell lysates from uninfected CEV1, CDN1, and CDN10 cells.
FIG. 4.
RelA/p65 immunostaining of control and E. coli-infected cells. Control Caco-2 cells (top row), Caco-2 cells stably transfected with an empty vector (CEV1) (middle row), and Caco-2 cells stably transfected with DN Nod1 (CDN10) (bottom row) were not infected (left column) or were infected with enteroinvasive E. coli O29:NM (middle column) for 45 min and then immunostained with anti RelA/p65 antibody. Staining with an isotype control antibody is shown in the right column.
DN Nod1 does not inhibit NF-κB activation in cells stimulated with bacterial flagellin or IL-1α.
Stimulation with IL-1α activates IKK and NF-κB in Caco-2 cells (19, 38) by a pathway that uses many of the same signal transduction intermediates as TLR5 (3). To the extent that activation of NF-κB through Nod1 is independent of TLR signaling pathways, we predicted that NF-κB activation in response to IL-1α or flagellin stimulation would not be blocked in Caco-2 cells expressing DN Nod1. As shown in Fig. 5, this was the case. Consistent with this, bacteria expressing flagella (e.g., Salmonella serovar Dublin, enterohemorrhagic E. coli) activated NF-κB in these cells, as noted previously (5, 19).
FIG. 5.
DN Nod1 does not eliminate NF-κB activation in response to stimulation with IL-1 or bacterial flagellin. (A) Control Caco-2 and CDN10 cells were infected with enteroinvasive E. coli O29:NM as described in Materials and Methods or stimulated with IL-1α (20 ng/ml) or were not infected or stimulated (control lanes). NF-κB DNA binding was assessed by an EMSA 45 min after infection or stimulation. (B) Caco-2 and CDN10 cells were stimulated with H7 flagellin (100 ng/ml) for different times or were not stimulated for 60 min, after which NF-κB DNA binding was determined by an EMSA.
NF-κB DNA binding in E. coli O29:NM-infected Caco-2 cells.
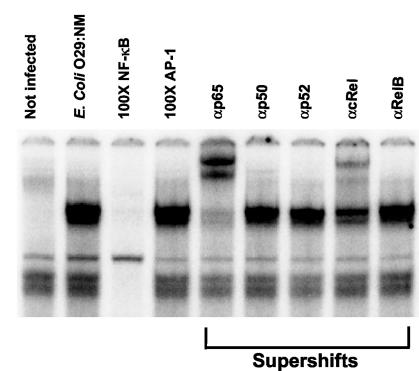
To determine if NF-κB DNA binding subunits other than RelA/p65 were activated in enteroinvasive E. coli-infected Caco-2 cells, EMSAs and supershift assays were performed by using nuclear extracts from E. coli O29:NM-infected cells and antibodies to RelB, cRel, p52, p50, and RelA/p65. NF-κB DNA binding after infection consisted mainly of RelA/p65 homodimers and RelA/p65-cRel heterodimers (Fig. 6).
FIG. 6.
NF-κB DNA binding in E. coli-infected Caco-2 cells. Caco-2 cells were not infected or were infected with E. coli O29:NM. The specificity of NF-κB DNA binding was assessed by using an excess of a specific oligonucleotide competitor (100× NF-κB) or a nonspecific oligonucleotide competitor (100× AP-1). Supershifts obtained with antibodies to RelB, cRel, p52, p50, and RelA/p65 are indicated on the right.
DN Nod1 inhibits enteroinvasive E. coli-induced expression of NF-κB target genes.
Several proinflammatory genes, including the potent neutrophil chemoattractant CXCL8, are upregulated in E. coli O29:NM-infected intestinal epithelial cells, as reported previously (19, 39). To determine if Nod1 governs the expression of neutrophil chemoattractants in enteroinvasive E. coli-infected Caco-2 cells, CEV1 cells and CDN10 cells were infected with E. coli O29:NM, after which CXCL8 and CXCL5 mRNA transcript levels were assayed. DN Nod 1 significantly inhibited the upregulated expression of CXCL8 and CXCL5 mRNA in response to E. coli infection (Fig. 7A). Furthermore, DN Nod1 almost completely inhibited epithelial cell production of the prototypic neutrophil chemoattractant CXCL8 by bacterium-infected cells (Fig. 7B).
FIG. 7.
DN Nod1 inhibits NF-κB target gene expression in E coli-infected Caco-2 cells. (A) CEV1, CDN1, and CDN10 cells were infected with enteroinvasive E. coli O29:NM. CXCL8 (open bars) and CXCL5 (solid bars) mRNA levels were assessed by real-time PCR 4 h after infection. The results are from three independent experiments, and the error bars indicate standard errors of the means. An asterisk indicates that the P value is <0.05 for a comparison with CEV1-infected cells. (B) CEV1 cells (open bars) and CDN10 cells (closed bars) were infected with E. coli O29:NM for 1 h, and CXCL8 secretion in culture supernatants was assessed at different times after infection. The results are from three or more independent experiments, and the error bars indicate standard errors of the means. An asterisk indicates that the P value is <0.05 for a comparison with CEV1-infected cells.
DISCUSSION
Nod1 is required for the activation of IKK, NF-κB, and NF-κB target genes in human colon epithelial cells infected with a gram-negative enteric pathogen that does not activate NF-κB through TLR signaling pathways. To demonstrate the functional importance of signaling through Nod1 in the absence of other extracellular or intracellular PRRs, we used a strategy in which cultured human colon epithelial cells that are not responsive to bacterial LPS (1, 13, 59) but are responsive to signaling through TLR5 and other TLRs (2, 4, 58) were infected with wild-type pathogenic invasive gram-negative bacteria that do not produce flagellin, which activates NF-κB by signaling through TLR5 (27). To the extent that signaling through Nod1 is the major pathway that leads to IKK and NF-κB activation in human colon epithelial cells during infection with bacteria that do not trigger TLRs or other PRRs, we hypothesized that IKK and NF-κB activation should be completely eliminated when DN Nod1 is expressed in Caco-2 cells and the cells are subsequently infected with enteroinvasive E. coli 029:NM. This was in fact the case. Nonetheless, signaling through the TLR pathway activated by bacterial flagellin, IL-1α, or bacteria that express flagella was intact in these cells. Taken together, these studies indicate that TLRs and PRRs other than Nod1 may have little, if any, role in activating NF-κB during infection with some highly invasive gram-negative epithelial cell pathogens that lack flagella.
Induction of the host epithelial cell proinflammatory gene program is essential for rapidly activating the host mucosal inflammatory response and is important for bacterial clearance and host survival following infection with enteric pathogens (56). Our studies indicate that human intestinal epithelial cells have at least two independent physiologically relevant signaling pathways for activating NF-κB and epithelial cell proinflammatory genes in response to bacterial infection. One pathway involves TLRs. For example, pathogenic bacteria that have flagella and adhere to or invade colon epithelium cells can activate IKK, NF-κB, and NF-κB target genes by signaling through the TLR5 receptor (4, 20, 58). Relevant to our study and Nod1 function, highly pathogenic gram-negative bacteria like enteroinvasive E. coli and perhaps closely related pathogens (51) that lack flagella have developed infection strategies to bypass TLR signaling in the intestinal epithelium. Signaling through Nod1 provides the host intestinal epithelium with an independent pathway for rapidly activating NF-κB and the subsequent mucosal inflammatory response, which can favor host survival following infection with these bacteria. Importantly, both pathways converge at IKK, making IKK a key molecular target for manipulating mucosal inflammatory responses that are initiated by host epithelium. An additional backup is provided later in infection, as bacteria that gain access to the subepithelial region activate the release of inflammatory cytokines (e.g., IL-1 and TNF-α) that act on the epithelium and upregulate the expression of NF-κB and epithelial cell NF-κB target genes that signal host inflammatory responses and alter epithelial cell function (17, 19, 39, 61).
Differences in NF-κB activation between wild-type and DN Nod1-expressing Caco-2 cells did not reflect differences in bacterial proliferation, as bacterial proliferation did not differ significantly in these cells. During bacterial invasion of intestinal epithelial cells, enteroinvasive bacteria activate signal transduction molecules (e.g., small GTPases of the Rho family), which cause rearrangements in the host cytoskeleton that facilitate bacterial entry (55). Our studies indicate that such cytoskeletal rearrangements do not trigger the activation of IKK and NF-κB in cells expressing DN Nod1, although these cells maintain functional TLR and IL-1R signaling pathways. This suggests either that cytoskeletal changes associated with cellular entry of enteroinvasive E. coli do not trigger NF-κB activation or alternatively that cell signals leading to NF-κB activation that are induced by cytoskeletal changes are transduced through Nod1. Currently, there is no evidence that supports or refutes the latter possibility.
Bacterial LPS produced by gram-negative bacteria has a major role in activating NF-κB in epithelial cells at most mucosal sites by signaling through the TLR4-MD-2 complex. In contrast, normal human colon epithelial cells are continually exposed to bacterial LPS. Like Caco-2 cells and several other long-term cell colon epithelial cell lines (1, 13, 59), normal human colon epithelial cells are poorly responsive to LPS, if they respond at all (39), and under normal conditions they express little, if any, TLR4 (7). Although human intestinal epithelial cells and cell lines like Caco-2 also express other potentially functional TLR receptors (e.g., TLR3 and TLR9) (2, 7, 47), those TLRs, like TLR5, did not play a role in the activation of NF-κB in the enteroinvasive E. coli-infected colon epithelial cells.
Our data also indicate that Nod2 is not likely to be important in signaling the onset of NF-κB activation in human intestinal epithelial cells infected with enteroinvasive E. coli. Thus, Nod1 was constitutively expressed at levels that were 10- to 20-fold greater than the levels of Nod2 in Caco-2 cells, and the Nod2 mRNA levels were not upregulated by infection with gram-negative pathogens in the early period after infection, when NF-κB was activated through constitutively expressed Nod1. Further supporting the importance of epithelial cell Nod1 compared to Nod2, supernatants from gram-negative bacteria activated NF-κB when they were microinjected into isolated murine intestinal epithelial cells from normal mice but not when they were microinjected into isolated murine intestinal epithelial cells from Nod1-deficient mice, although such cells also express detectable Nod2 (21). In summary, we conclude that Nod1 constitutively expressed by human intestinal epithelial cells is essential for the activation of NF-κB and the upregulated production of important epithelial cell chemoattractants in a physiologic system in which human intestinal epithelial cells are infected with pathogenic gram-negative enteropathogens that bypass TLR signaling.
Acknowledgments
This work was supported by NIH grants DK35108 and DK58960.
Editor: F. C. Fang
REFERENCES
- 1.Abreu, M. T., P. Vora, E. Faure, L. S. Thomas, E. T. Arnold, and M. Arditi. 2001. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167:1609-1616. [DOI] [PubMed] [Google Scholar]
- 2.Akhtar, M., J. L. Watson, A. Nazli, and D. M. McKay. 2003. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-κB-independent pathway. FASEB J. 17:1319-1321. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 4.Berin, M. C., A. Darfeuille-Michaud, L. J. Egan, Y. Miyamoto, and M. F. Kagnoff. 2002. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cell NF-κB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell Microbiol. 4:635-648. [DOI] [PubMed] [Google Scholar]
- 5.Berin, M. C., M. B. Dwinell, L. Eckmann, and M. F. Kagnoff. 2001. Production of MDC/CCL22 by human intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G1217-G1226. [DOI] [PubMed] [Google Scholar]
- 6.Bertin, J., W. J. Nir, C. M. Fischer, O. V. Tayber, P. R. Errada, J. R. Grant, J. J. Keilty, M. L. Gosselin, K. E. Robison, G. H. Wong, M. A. Glucksmann, and P. S. DiStefano. 1999. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-κB. J. Biol. Chem. 274:12955-12958. [DOI] [PubMed] [Google Scholar]
- 7.Cario, E., and D. K. Podolsky. 2000. Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 68:7010-7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:702-707. [DOI] [PubMed] [Google Scholar]
- 9.Chen, L. W., L. Egan, Z. W. Li, F. R. Greten, M. F. Kagnoff, and M. Karin. 2003. The two faces of IKK and NF-κB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat. Med. 9:575-581. [DOI] [PubMed] [Google Scholar]
- 10.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 11.Davies, J., and A. Jimenez. 1980. A new selective agent for eukaryotic cloning vectors. Am. J. Trop. Med. Hyg. 29:1089-1092. [DOI] [PubMed] [Google Scholar]
- 12.DiDonato, J. A., F. Mercurio, and M. Karin. 1995. Phosphorylation of IκBα precedes but is not sufficient for its dissociation from NF-κB. Mol. Cell. Biol. 15:1302-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckmann, L., H. C. Jung, C. Schurer-Maly, A. Panja, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1993. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology 105:1689-1697. [DOI] [PubMed] [Google Scholar]
- 14.Eckmann, L., M. F. Kagnoff, and J. Fierer. 1993. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect. Immun. 61:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckmann, L., S. L. Reed, J. R. Smith, and M. F. Kagnoff. 1995. Entamoeba histolytica trophozoites induce an inflammatory cytokine response by cultured human cells through the paracrine action of cytolytically released interleukin-1α. J. Clin. Investig. 96:1269-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckmann, L., J. R. Smith, M. P. Housley, M. B. Dwinell, and M. F. Kagnoff. 2000. Analysis by high density cDNA arrays of altered gene expression in human intestinal epithelial cells in response to infection with the invasive enteric bacteria Salmonella. J. Biol. Chem. 275:14084-14094. [DOI] [PubMed] [Google Scholar]
- 17.Eckmann, L., W. F. Stenson, T. C. Savidge, D. C. Lowe, K. E. Barrett, J. Fierer, J. R. Smith, and M. F. Kagnoff. 1997. Role of intestinal epithelial cells in the host secretory response to infection by invasive bacteria. Bacterial entry induces epithelial prostaglandin h synthase-2 expression and prostaglandin E2 and F2α production. J. Clin. Investig. 100:296-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egan, L. J., D. C. Mays, C. J. Huntoon, M. P. Bell, M. G. Pike, W. J. Sandborn, J. J. Lipsky, and D. J. McKean. 1999. Inhibition of interleukin-1-stimulated NF-κB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J. Biol. Chem. 274:26448-26453. [DOI] [PubMed] [Google Scholar]
- 19.Elewaut, D., J. A. DiDonato, J. M. Kim, F. Truong, L. Eckmann, and M. F. Kagnoff. 1999. NF-κB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J. Immunol. 163:1457-1466. [PubMed] [Google Scholar]
- 20.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 21.Girardin, S. E., I. G. Boneca, L. A. Carneiro, A. Antignac, M. Jehanno, J. Viala, K. Tedin, M. K. Taha, A. Labigne, U. Zathringer, A. J. Coyle, P. S. DiStefano, J. Bertin, P. J. Sansonetti, and D. J. Philpott. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300:1584-1587. [DOI] [PubMed] [Google Scholar]
- 22.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869-8872. [DOI] [PubMed] [Google Scholar]
- 23.Girardin, S. E., R. Tournebize, M. Mavris, A. L. Page, X. Li, G. R. Stark, J. Bertin, P. S. DiStefano, M. Yaniv, P. J. Sansonetti, and D. J. Philpott. 2001. CARD4/Nod1 mediates NF-κB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2:736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girardin, S. E., L. H. Travassos, M. Herve, D. Blanot, I. G. Boneca, D. J. Philpott, P. J. Sansonetti, and D. Mengin-Lecreulx. 2003. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J. Biol. Chem. 278:41702-41708. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez, O., C. Pipaon, N. Inohara, A. Fontalba, Y. Ogura, F. Prosper, G. Nunez, and J. L. Fernandez-Luna. 2002. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J. Biol. Chem. 277:41701-41705. [DOI] [PubMed] [Google Scholar]
- 26.Hase, K., L. Eckmann, J. D. Leopard, N. Varki, and M. F. Kagnoff. 2002. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect. Immun. 70:953-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 28.Hisamatsu, T., M. Suzuki, and D. K. Podolsky. 2003. Interferon-gamma augments CARD4/NOD1 gene and protein expression through interferon regulatory factor-1 in intestinal epithelial cells. J. Biol. Chem. 278:32962-32968. [DOI] [PubMed] [Google Scholar]
- 29.Hisamatsu, T., M. Suzuki, H. C. Reinecker, W. J. Nadeau, B. A. McCormick, and D. K. Podolsky. 2003. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology 124:993-1000. [DOI] [PubMed] [Google Scholar]
- 30.Hu, Y., V. Baud, M. Delhase, P. Zhang, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science 284:316-320. [DOI] [PubMed] [Google Scholar]
- 31.Iimura, M., T. Nakamura, S. Shinozaki, B. Iizuka, Y. Inoue, S. Suzuki, and N. Hayashi. 2000. Bax is downregulated in inflamed colonic mucosa of ulcerative colitis. Gut 47:228-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inohara, N., T. Koseki, L. del Peso, Y. Hu, C. Yee, S. Chen, R. Carrio, J. Merino, D. Liu, J. Ni, and G. Nunez. 1999. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-κB. J. Biol. Chem. 274:14560-14567. [DOI] [PubMed] [Google Scholar]
- 33.Inohara, N., T. Koseki, J. Lin, L. del Peso, P. C. Lucas, F. F. Chen, Y. Ogura, and G. Nunez. 2000. An induced proximity model for NF-κB activation in the Nod1/RICK and RIP signaling pathways. J. Biol. Chem. 275:27823-27831. [DOI] [PubMed] [Google Scholar]
- 34.Inohara, N., and G. Nunez. 2003. NODs: intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3:371-382. [DOI] [PubMed] [Google Scholar]
- 35.Inohara, N., Y. Ogura, F. F. Chen, A. Muto, and G. Nunez. 2001. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J. Biol. Chem. 276:2551-2554. [DOI] [PubMed] [Google Scholar]
- 36.Inohara, N., Y. Ogura, A. Fontalba, O. Gutierrez, F. Pons, J. Crespo, K. Fukase, S. Inamura, S. Kusumoto, M. Hashimoto, S. J. Foster, A. P. Moran, J. L. Fernandez-Luna, and G. Nunez. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 278:5509-5512. [DOI] [PubMed] [Google Scholar]
- 37.Izadpanah, A., M. B. Dwinell, L. Eckmann, N. M. Varki, and M. F. Kagnoff. 2001. Regulated MIP-3α/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G710-G719. [DOI] [PubMed] [Google Scholar]
- 38.Jobin, C., S. Haskill, L. Mayer, A. Panja, and R. B. Sartor. 1997. Evidence for altered regulation of IκBα degradation in human colonic epithelial cells. J. Immunol. 158:226-234. [PubMed] [Google Scholar]
- 39.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kagnoff, M. F., and L. Eckmann. 1997. Epithelial cells as sensors for microbial infection. J. Clin. Investig. 100:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi, K., N. Inohara, L. D. Hernandez, J. E. Galan, G. Nunez, C. A. Janeway, R. Medzhitov, and R. A. Flavell. 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416:194-199. [DOI] [PubMed] [Google Scholar]
- 43.Li, Z. W., W. Chu, Y. Hu, M. Delhase, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. The IKKβ subunit of IkappaB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J. Exp. Med. 189:1839-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsubara, Y., M. Ichinose, N. Yahagi, S. Tsukada, M. Oka, K. Miki, S. Kimura, M. Omata, K. Shiokawa, N. Kitamura, Y. Kaneko, and H. Fukamachi. 1998. Hepatocyte growth factor activator: a possible regulator of morphogenesis during fetal development of the rat gastrointestinal tract. Biochem. Biophys. Res. Commun. 253:477-484. [DOI] [PubMed] [Google Scholar]
- 45.McCormick, B. A., S. P. Colgan, C. Delp-Archer, S. I. Miller, and J. L. Madara. 1993. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J. Cell Biol. 123:895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 47.Melmed, G., L. S. Thomas, N. Lee, S. Y. Tesfay, K. Lukasek, K. S. Michelsen, Y. Zhou, B. Hu, M. Arditi, and M. T. Abreu. 2003. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J. Immunol. 170:1406-1415. [DOI] [PubMed] [Google Scholar]
- 48.Mukaida, N., Y. Mahe, and K. Matsushima. 1990. Cooperative interaction of nuclear factor-κB- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J. Biol. Chem. 265:21128-21133. [PubMed] [Google Scholar]
- 49.Ogura, Y., N. Inohara, A. Benito, F. F. Chen, S. Yamaoka, and G. Nunez. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J. Biol. Chem. 276:4812-4818. [DOI] [PubMed] [Google Scholar]
- 50.Poltorak, A., P. Ricciardi-Castagnoli, S. Citterio, and B. Beutler. 2000. Physical contact between lipopolysaccharide and toll-like receptor 4 revealed by genetic complementation. Proc. Natl. Acad. Sci. 97:2163-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pupo, G. M., D. K. Karaolis, R. Lan, and P. R. Reeves. 1997. Evolutionary relationships among pathogenic and nonpathogenic Escherichia coli strains inferred from multilocus enzyme electrophoresis and mdh sequence studies. Infect. Immun. 65:2685-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roebuck, K. A., D. A. Brenner, and M. F. Kagnoff. 1993. Identification of c-fos-responsive elements downstream of TAR in the long terminal repeat of human immunodeficiency virus type-1. J. Clin. Investig. 92:1336-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenstiel, P., M. Fantini, K. Brautigam, T. Kuhbacher, G. H. Waetzig, D. Seegert, and S. Schreiber. 2003. TNF-α and IFN-γ regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology 124:1001-1009. [DOI] [PubMed] [Google Scholar]
- 54.Rothwarf, D. M., and M. Karin. 1999. The NF-κB activation pathway: a paradigm in information transfer from membrane to nucleus. Sci. STKE 1999:RE1. [DOI] [PubMed] [Google Scholar]
- 55.Sansonetti, P. J. 2001. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. III. Shigellosis: from symptoms to molecular pathogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G319-G323. [DOI] [PubMed] [Google Scholar]
- 56.Sansonetti, P. J., J. Arondel, M. Huerre, A. Harada, and K. Matsushima. 1999. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect. Immun. 67:1471-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 58.Sierro, F., B. Dubois, A. Coste, D. Kaiserlian, J. P. Kraehenbuhl, and J. C. Sirard. 2001. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc. Natl. Acad. Sci. 98:13722-13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki, M., T. Hisamatsu, and D. K. Podolsky. 2003. Gamma interferon augments the intracellular pathway for lipopolysaccharide (LPS) recognition in human intestinal epithelial cells through coordinated up-regulation of LPS uptake and expression of the intracellular Toll-like receptor 4-MD-2 complex. Infect. Immun. 71:3503-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 61.Yang, S. K., L. Eckmann, A. Panja, and M. F. Kagnoff. 1997. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology 113:1214-1223. [DOI] [PubMed] [Google Scholar]