Summary
Background
Considering the high prevalence of dementia, it would be of great value to develop effective tools to improve cognitive function. We examined the effects of a human-type communication robot on cognitive function in elderly women living alone.
Material/Methods
In this study, 34 healthy elderly female volunteers living alone were randomized to living with either a communication robot or a control robot at home for 8 weeks. The shape, voice, and motion features of the communication robot resemble those of a 3-year-old boy, while the control robot was not designed to talk or nod. Before living with the robot and 4 and 8 weeks after living with the robot, experiments were conducted to evaluate a variety of cognitive functions as well as saliva cortisol, sleep, and subjective fatigue, motivation, and healing.
Results
The Mini-Mental State Examination score, judgement, and verbal memory function were improved after living with the communication robot; those functions were not altered with the control robot. In addition, the saliva cortisol level was decreased, nocturnal sleeping hours tended to increase, and difficulty in maintaining sleep tended to decrease with the communication robot, although alterations were not shown with the control. The proportions of the participants in whom effects on attenuation of fatigue, enhancement of motivation, and healing could be recognized were higher in the communication robot group relative to the control group.
Conclusions
This study demonstrates that living with a human-type communication robot may be effective for improving cognitive functions in elderly women living alone.
Keywords: cognitive function, elderly, women, human-type communication robot, living alone
Background
The life expectancy of females is longer than that of males; therefore, many elderly females live alone, without a spouse [1]. Considering the high prevalence of dementia with advanced age, it is important in caring for elderly women living alone to evaluate how well they are functioning cognitively and to determine what assistance they require. In addition, it would be of great value to develop effective treatment methods for the cognitive decline of elderly women living alone.
Recently, a human-type communication robot was developed (Kabochan Nodding Communication ROBOT; PIP Co., Ltd., Osaka, Japan and WiZ Co., Ltd., Tokyo, Japan). Since the opportunity for communication with others often decreases with advanced age and a poor communication environment is associated with impaired cognitive function in the elderly [2], improvement of the communication environment may promote cognitive function in elderly women living alone. Therefore, it was hypothesized that living with the communication robot may improve cognitive outcomes in elderly women living alone.
The aim of our study was to determine whether the communication robot was effective for improving cognitive function in elderly women living alone. In this study, elderly female volunteers living alone were randomized to living with either the communication robot or the control robot at home for 8 weeks. Experiments were conducted to evaluate a variety of cognitive functions as well as variables such as physical, emotional, and lifestyle factors before interactions with the robot and 4 and 8 weeks after the start of living with the robot.
Material and Methods
Participants
Forty elderly [≥65 years of age (66–84 years of age)] women living alone were recruited. Subjects with dementia diagnosed during an examination by a medical doctor (M.T.) were excluded. In addition, we excluded current smokers, subjects with body weight less than 35 kg, those with blood hemoglobin levels less than 10.5 g/dl, and those with a Mini-Mental State Examination (MMSE) score less than 24. Good health was required for participation and was assessed by physical examination, blood chemistry panel (glucose, creatinine, uremic nitrogen, sodium, potassium, chloride, uric acid, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase, and creatine phosphokinase levels), lipid profile (total cholesterol and triacylglycerol levels), and complete blood count [3]. The study protocol was approved by the Ethics Committee of Osaka City University, and all the participants gave written informed consent to participate in this study.
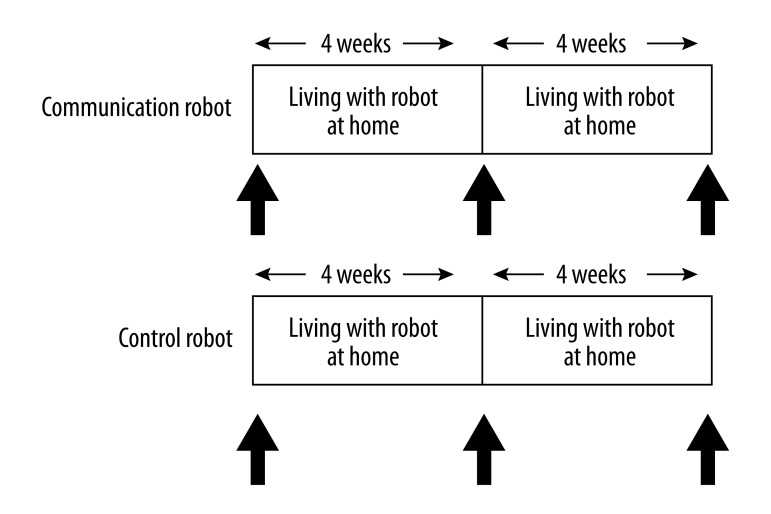
Experimental design (Figure 1)
Figure 1.
Experimental design. Participants were randomised to either the communication robot group or control robot group. Before and 4 and 8 weeks after the start of living with the robot at home, experiments (shown as arrows) were conducted.
After the enrolment and initial assessment of cognitive function using the MMSE, the participants were randomly assigned to 2 groups, matched for age and MMSE score, to live with either a communication robot or a control robot at home for 8 weeks. Experiments were conducted before (baseline) and 4 and 8 weeks after the start of living with the robot. While living with the robot, subjects refrained from strenuous mental and physical activity and followed normal dietary behaviour, drinking patterns, and sleeping hours, and completed questionnaires dealing with diet, sleep, activity, physical and mental condition, life events, and communication with the robot every day at home.
After a visit at 10:00 a.m. on the experimental day, questionnaires were distributed to subjects and height and body weight were measured to assess body mass index (BMI) in a room at Osaka City University. BMI was calculated as body weight in kilograms divided by height in meters squared. Thereafter, subjects performed cognitive task trials and accelerated plethysmography (APG), and blood and saliva samples were collected. At the end of the all the experimental procedures, subjects answered questions about whether living with the robot for 8 weeks at home had effects on attenuation of fatigue (yes or no), enhancement of motivation (yes or no), healing (yes or no), pleasure (yes or no), and relaxation (yes or no). This study was conducted in a quiet temperature- and humidity-controlled environment and during the experiment subjects could only drink water.
Robots (Figure 2)
Figure 2.

Photograph of a communication or control robot. The robot is 28 cm in height and 680 g in weight. The features of shape, voice, and motion resemble those of a 3-year-old boy. The robot was programmed to behave as if communicating with customers and to release affective behavior that could develop a friendly relationship, in particular with elderly people. The control robot has the same shape as the communication robot, however, the control robot was not constructed to talk or nod to the participants.
The participants lived with the human-type communication robot (Kabochan Nodding Communication ROBOT) or a control robot at home for 8 weeks. The robots are 28 cm in height and 680 g in weight. The features of shape, voice, and motion resemble those of a 3-year-old boy. The robot was programmed to behave as if communicating with customers and to release affective behavior that could develop a friendly relationship, in particular with elderly people. Loaded with software developed by PIP Co., Ltd. (Osaka, Japan), this communication robot senses the situation and environmental surroundings using light, sound, and motion sensors, and is able to communicate by talking and nodding. The control robot has the same shape as that of the communication robot; however, it was not designed to talk or nod to the participants.
MMSE
The MMSE tests general cognitive function and the total score for the examination ranges from 0 to 30, with higher scores indicating a greater level of general cognitive function [4]. Normal cognitive function was determined as the MMSE score ≥24 [5].
Cognistat
Cognistat (formerly known as the Neurobehavioral Cognitive Status Examination) is widely used to evaluate a variety of cognitive functions and is useful in outlining recommendations for further evaluation [6]. Cognistat is a neuropsychological screen that takes 10 to 30 minutes to administer and was designed to give an independent assessment of 10 central cognitive domains. The following 10 corresponding subtests are scored: attention, naming, similarities/verbal abstraction, everyday/concrete judgement, understanding of simple commands, repetition of sentences, visuoconstruction, verbal memory, calculation, and orientation, each of which is scored separately. Therefore, it does not use a single summary score as the other screening tests do. Correct responses in each subtest are summed, and the test result was presented as a differentiated cognitive profile. If the participants pass the screening test, they are presumed to function normally in that domain, and the testers continue to the next domain. When a participant fails the screening test, it continues with a metric portion that explores a possible deficit further. This permits both streamlined administration and more in-depth evaluation of areas of deficit. It has been found to be more sensitive (less false-negatives) than the MMSE because it does not combine the results of performance in the different areas into a single score, but rather scores each domain separately [7]. Therefore, successful performance in one area will not obscure deficits in other areas. Furthermore, the use of a graded series of test items within each domain increases the likelihood of detecting mild deficits. It also independently assesses more areas of cognitive function, allowing for the detection of isolated deficits with greater frequency than the MMSE. Results of the Cognistat were analyzed using the Cognistat Composite Score [8]. The attention subset contains 8 questions (this subset does not contain any screening questions), each question was scored 0 (incorrect response) or 1 (correct response), and the total score of the subset (0 to 8) was transformed to 1 to 10, according to the Cognistat Composite Score. The naming subset contains 8 questions (this subset does not contain any screening questions), each question was scored 0 (incorrect response) or 1 (correct response), and the total score of the subset (0 to 8) was transformed to 0 to 10, according to the Cognistat Composite Score. The similarities/verbal abstraction subset contains 4 questions (this subset does not contain any screening questions), each question was scored 0 to 2, and the total score of the subset (0 to 8) was transformed to 6 to 11, according to the Cognistat Composite Score. The everyday/concrete judgement subset contains 3 questions (this subset does not contain any screening questions), each question was scored 0 to 2, and the total score of the subset (0 to 6) was transformed to 6 to 12, according to the Cognistat Composite Score. The understanding of simple commands subset contains 1 screening question and 6 metric questions, and if the participants passed the screening test, the testers continue to the next domain and the score of this domain was 6. If the participants did not pass, they performed the metric questions. Each metric question was scored 0 (incorrect response) or 1 (correct response), and the screening score (6) or the total score of the subset (0 to 6) was transformed to 1 to 10, according to the Cognistat Composite Score. The repetition of sentences subset contains 1 screening question and 6 metric questions, and if the participants passed the screening test, the testers continue to the next domain and the score of this domain was 12. If the participants did not pass, they performed the metric questions, each metric question was scored 0 to 2, and the screening score (12) or the total score of the subset (1 to 11) was transformed to 1 to 10, according to the Cognistat Composite Score. The visuoconstruction subset contains 1 screening question and 3 metric questions, each metric question was scored 0 equal to or (more than 60 sec), 1 (less than 60 sec), or 2 (less than 30 min) (screening question was not scored), and the total score of the subset (0 to 6) was transformed to 4 to 11, according to the Cognistat Composite Score. The verbal memory subset contains 4 questions (this subset does not contain any screening questions), each question was scored 0 to 3, and the total score of the subset (0 to 12) was transformed to 4 to 10, according to the Cognistat Composite Score. The calculation subset contains 1 screening question and 4 metric questions, and if the participants passed the screening test, the testers continue to the next domain and the score of this domain was 4. If the participants did not pass, they performed the metric questions, each metric question was scored 0 (incorrect response) or 1 (correct response), and the screening score (4) or the total score of the subset (0 to 4) was transformed to 2 to 10, according to the Cognistat Composite Score. Finally, the orientation subset contains 8 questions (this subset does not contain any screening questions), each question was scored 0 or 1 or 2, and the total score of the subset (0 to 12) was transformed to 0 to 10, according to the Cognistat Composite Score.
Questionnaires
Paper-and-pencil questionnaires were distributed to the participants. The questionnaires completed by each participant dealt with age, appetite, sleep, depressive symptoms, and activities of daily living (ADL). Subjects were asked to subjectively rate their levels of appetite on a visual analogue scale (VAS) from 0 (minimum) to 100 (maximum) [9]. Questions about sleep included items about nocturnal sleeping hours, difficulty in initiating sleep using a 4-level scale (no = 1, sometimes = 2, often = 3, and everyday = 4) scale, difficulty in maintaining sleep using a four-level (no = 1, sometimes = 2, often = 3, and everyday = 4) scale, and early morning awakening using a 4-level (no = 1, sometimes = 2, often = 3, and everyday = 4). The Geriatric Depression Scale-15 (GDS-15) was used to assess the number of depressive symptoms, as well as the depressive state. This questionnaire was specifically developed for older subjects and consists of 15 questions using a 2-level (0–1) scale that evaluates the functional and mood-associated symptoms of depression [10–12]. The total score for the 15-item depression scale ranges from 0 to 15, with higher scores indicating a greater number of depressive symptoms. The Tokyo Metropolitan Institute of Gerontology Index of Competence was used to assess the ADL level. This questionnaire consists of 13 questions using a 2-level (0–1) scale [13,14]. The total score of the 13-item scale ranges from 0 to 13, with a higher score indicating a greater level of ADL.
Blood and saliva sample analyses
Blood samples were collected from the brachial vein. The blood samples for the serum analysis of albumin were centrifuged at 1700 g for 10 minutes at 4°C. The blood samples for the analysis of blood cell counts were collected in an ethylenediamine-N,N,N′,N′-tetraacetic acid- and dipotassium salt-containing tube and kept on ice until analyzed. Saliva samples for the analyses of cortisol were collected in a tube (Salivette; Sarstedt, Rommelsdorf, Germany) and kept on ice until centrifuged at 1700 g for 5 minutes at 4°C. All supernatants were stored at −80°C until analysis. Assays were performed at Special Reference Laboratories (Tokyo, Japan).
Accelerated plethysmography (APG)
APG has been used for the evaluation of autonomic activities [15–18]. In the present study, APG was performed using a pulsimeter (Artett, U-Medica, Osaka, Japan) with the sensor positioned on the tip of the ventral side of the index finger. Photoplethysmography was used to measure changes in the absorption of light by hemoglobin, which is related to blood flow volume. The pulsimeter performed automatic analyses of the second derivative of the photoplethysmographic waveform, which is known as the APG waveform. The participants underwent APG sitting quietly with their eyes closed for 1 min. Sensor output of the pulsimeter was preprocessed by a second-order analogue low-pass filter with 23 Hz of cut-off frequency. Data were recorded (3.3 volts to 10 bits) using an analogue-to-digital converter and a real-time sampling rate of 1000 samples per second. These digital data were processed with the 67th order, finite impulse-response filter using the Hanning window. Detected peak times were interpolated to sub-millisecond order. Frequency analyses for pulse-interval variation were analyzed with fast Fourier transformation. The resolution ability for the power spectrum was 0.001 Hz. For the frequency analyses, the total power was calculated as the power within a frequency range of 0–0.4 Hz, the low-frequency component power (LF) was calculated as the power within a frequency range of 0.04–0.15 Hz, and the high-frequency component power (HF) was calculated as that within a frequency range of 0.15–0.4 Hz. The average power densities in these frequency bands were log-transformed (ln) for normalization. The HF is vagally mediated [19–21], whereas LF originates from a variety of sympathetic and vagal mechanisms [19–22]. The LF/HF ratio is considered to represent sympathetic activity [22].
Statistical analyses
Differences between the baseline condition and the condition 4 or 8 weeks after living with the robot were compared using a paired t-test or Wilcoxon’s signed rank test where appropriate, with Bonferroni correction. Categorical variables were compared using Fisher’s exact test. In the analyses, the number of cases varied due to incidental missing values. All P values were 2-tailed, and P values less than .05 were considered statistically significant. Statistical analyses were performed using the SPSS 20.0 software package (SPSS, Chicago, IL).
Results
Among 40 subjects, 3 subjects decided not to participate in this study, 2 subjects were excluded because of misuse of the control robot, and 1 subject was excluded because of a technical error of the communication robot. The remaining 34 participants were enrolled (18 participants in the communication robot group and 16 in the control robot group).
The effects of living with the control or communication robot on various parameters are shown in Table 1. BMI and VAS score for appetite were not altered after living with the communication or control robot at home for 4 or 8 weeks. As for sleep, nocturnal sleeping hours tended to increase and difficulty in maintaining sleep tended to decrease after living with the communication robot for 8 weeks, while living with the control robot did not show this effect. GDS-15 score, ADL level, serum albumin level, and blood lymphocyte counts were not altered after living with the communication or control robot at home for 4 or 8 weeks. In addition, APG parameters, i.e., LF, HF, and LF/HF ratio, were not altered after living with the communication or control robot at home for 4 or 8 weeks. The saliva cortisol level was decreased after living with the communication robot for 8 weeks, while this level tended to increase after 4 weeks in subjects living with the control robot.
Table 1.
Effects of living with a control or communication robot.
| Control robot | Communication robot | |
|---|---|---|
| n | 16 | 18 |
| Age (years old) | 73.1±5.3 | 73.6±4.4 |
| BMI (kg/m2) | ||
| Baseline | 24.3±2.4 | 23.4±2.6 |
| After 4 weeks | 24.3±2.4 | 23.7±2.8 |
| After 8 weeks | 24.1±2.5 | 23.7±2.9 |
| VAS score for appetite | ||
| Baseline | 79.6±16.3 | 75.0±16.5 |
| After 4 weeks | 76.3±16.4 | 73.7±14.6 |
| After 8 weeks | 75.8±13.8 | 75.6±15.4 |
| Nocturnal sleeping hours | ||
| Baseline | 7.0±1.1 | 6.6±0.9 |
| After 4 weeks | 6.8±1.1 | 6.5±1.1 |
| After 8 weeks | 7.0±1.1 | 7.1±1.1# |
| Difficulty in initiating sleep | ||
| Baseline | 1.56±0.63 | 1.83±0.79 |
| After 4 weeks | 1.63±0.62 | 2.06±0.87 |
| After 8 weeks | 1.56±0.81 | 1.59±0.80 |
| Difficulty in maintaining sleep | ||
| Baseline | 1.63±0.81 | 1.61±0.85 |
| After 4 weeks | 1.56±0.81 | 1.56±0.78 |
| After 8 weeks | 1.44±0.63 | 1.28±0.57# |
| Early morning awakening | ||
| Baseline | 1.25±0.45 | 1.17±0.38 |
| After 4 weeks | 1.31±0.48 | 1.33±0.77 |
| After 8 weeks | 1.31±0.60 | 1.11±0.32 |
| GDS-15 score | ||
| Baseline | 3.3±4.4 | 2.6±2.9 |
| After 4 weeks | 3.1±4.2 | 2.1±2.7 |
| After 8 weeks | 2.3±4.1 | 2.1±2.1 |
| ADL score | ||
| Baseline | 12.6±0.9 | 12.5±0.9 |
| After 4 weeks | 12.4±1.0 | 12.3±1.0 |
| After 8 weeks | 12.5±1.0 | 12.4±1.0 |
| Serum albumin (g/L) | ||
| Baseline | 46.1±2.9 | 44.7±2.2 |
| After 4 weeks | 46.9±3.0 | 44.8±3.5 |
| After 8 weeks | 43.5±1.8 | 44.6±2.3 |
| Blood lymphocytes (109/L) | ||
| Baseline | 2.1±0.7 | 1.9±0.6 |
| After 4 weeks | 2.2±0.7 | 2.0±0.8 |
| After 8 weeks | 2.1±0.7 | 2.0±0.7 |
| Saliva cortisol (nmol/L) | ||
| Baseline | 2.51±0.92 | 3.12±1.61 |
| After 4 weeks | 3.48±1.35# | 2.79±1.69 |
| After 8 weeks | 2.21±0.87 | 2.16±0.89* |
| APG | ||
| LF (ms2) | ||
| Baseline | 5.9±2.0 | 5.3±1.3 |
| After 4 weeks | 5.5±1.1 | 5.3±1.3 |
| After 8 weeks | 6.1±1.3 | 5.6±1.5 |
| HF (ms2) | ||
| Baseline | 5.1±2.0 | 4.9±1.1 |
| After 4 weeks | 4.7±1.3 | 5.0±1.1 |
| After 8 weeks | 5.5±1.1 | 5.3±1.4 |
| LF/HF ratio | ||
| Baseline | 1.18±0.26 | 1.09±0.20 |
| After 4 weeks | 1.23±0.30 | 1.10±0.26 |
| After 8 weeks | 1.11±0.16 | 1.08±0.23 |
Data are shown as mean ±SD.
P<0.05;
P<0.1, significantly different from the baseline condition (Paired t-test or Wilcoxon’s signed rank test where appropriate, with Bonferroni correction).
BMI – body mass index; VAS – visual analogue scale; GDS-15 – Geriatric Depression Scale-15; ADL – activity of daily living; APG – accelerated plethysmography; LF – low-frequency power; HF – high-frequency power.
As for cognitive functions, the MMSE score was increased after living with the communication robot for 8 weeks, although the score was not altered with the control robot (Table 2). Among the 10 Cognistat subtests, calculation and orientation were not included in the statistical analyses because most of the participants obtained high scores on all of the items. Although the attention, naming, similarities/verbal abstraction, understanding of simple commands, repetition of sentences and visuoconstruction scores were not altered after living with the communication or control robot for 4 or 8 weeks, the everyday/concrete judgement and verbal memory scores were increased after living with the communication robot for 8 weeks, while these scores were not altered with the control robot (Table 2).
Table 2.
Effects of living with control or communication robot on cognitive functions.
| Control robot | Communication robot | |
|---|---|---|
| MMSE score | ||
| Baseline | 28.3±2.2 24–30) |
28.2±1.5 25–30) |
| After 4 weeks | 29.0±1.5 26–30) |
29.2±1.4 26–30) |
| After 8 weeks | 29.2±1.6 25–30) |
29.7±0.7 28–30)** |
| Cognistat | ||
| Total score (25–105) | ||
| Baseline | 95.1±5.8 80–104) |
95.1±5.1 79–101) |
| After 4 weeks | 96.4±4.3 87–103) |
96.5±5.9 83–103) |
| After 8 weeks | 95.5±5.8 86–104) |
97.6±5.1 89–105) |
| Attention (1–10) | ||
| Baseline | 9.1±1.4 5–10) |
9.5±1.0 7–10) |
| After 4 weeks | 9.3±1.0 7–10) |
9.5±1.0 7–10) |
| After 8 weeks | 9.0±1.8 3–10) |
9.5±1.0 7–10) |
| Naming (0–10) | ||
| Baseline | 8.0±3.1 1–10) |
7.7±3.0 3–10) |
| After 4 weeks | 8.0±2.6 3–10) |
7.8±3.5 0–10) |
| After 8 weeks | 7.3±3.5 0–10) |
7.6±3.8 0–10) |
| Similarities/verbal abstraction (6–11) | ||
| Baseline | 9.7±0.9 8–11) |
9.9±0.6 9–11) |
| After 4 weeks | 9.6±1.0 7–11) |
10.2±0.6 9–11) |
| After 8 weeks | 9.8±0.7 8–11) |
10.2±0.6 9–11) |
| Every-day/concrete judgement (6–12) | ||
| Baseline | 9.9±0.9 9–10) |
9.9±1.1 8(11–) |
| After 4 weeks | 9.9±0.9 9–11) |
10.4±1.1 9–12) |
| After 8 weeks | 10.2±0.9 9–12) |
10.7±0.9 9–12)* |
| Understanding of simple commands (1–10) | ||
| Baseline | 9.8±0.8 7–10) |
10.0±0.0 10–10) |
| After 4 weeks | 10.0±0.0 10–10) |
9.8±0.7 7–10) |
| After 8 weeks | 9.6±1.0 7–10) |
10.0±0.0 10–10) |
| Repetition of sentences (1–11) | ||
| Baseline | 10.3±1.1 8–11) |
10.6±1.0 8–11) |
| After 4 weeks | 10.8±0.4 10–11) |
10.7±0.8 8–11) |
| After 8 weeks | 10.8±0.5 9–11) |
10.9±0.5 9–11) |
| Visuoconstruction (4–11) | ||
| Baseline | 8.6±1.6 6–11) |
8.2±1.3 5–11) |
| After 4 weeks | 8.8±1.2 7–11) |
8.2±1.1 6–11) |
| After 8 weeks | 8.8±1.4 7–11) |
8.8±1.3 6–11) |
| Verbal memory (4–10) | ||
| Baseline | 9.6±0.7 8–10) |
9.4±0.7 8–10) |
| After 4 weeks | 9.9±0.3 9–10) |
9.8±0.4 9–10) |
| After 8 weeks | 9.9±0.3 9–10) |
10.0±0.0 10–10)* |
Data are shown as mean ±SD (minimum-maximum).
P<0.01;
P<0.05, significantly different from the baseline condition (Wilcoxon’s signed rank test with Bonferroni correction).
MMSE – Mini-Mental State Examination.
The proportions of the participants in whom effects on attenuation of fatigue, enhancement of motivation, and healing could be recognized were higher in the communication robot group relative to those in the control robot group. The proportion of the participants in whom effects on pleasure and relaxing could be recognized tended to be higher in the communication robot group relative to those in the control robot group (Table 3).
Table 3.
Comparisons between the control and communication robots.
| Control robot | Communication robot | |
|---|---|---|
| Attenuation of fatigue | 12 (75) | 17 (100)* |
| Enhancement of motivation | 10 (63) | 18 (100)** |
| Healing | 12 (75) | 18 (100)* |
| Pleasure | 13 (81) | 18 (100)# |
| Relaxation | 13 (81) | 18 (100)# |
Data are shown as number (%).
P<0.01;
P<0.05;
P<0.1, significantly different from the control robot (Fisher’s exact test).
Discussion
In this study, we demonstrated that the MMSE score and the judgement component of executive function and verbal memory function were improved after living with the communication robot for 8 weeks in the elderly women living alone, while the cognitive functions were not altered with the control robot. In addition, the saliva cortisol level was decreased, nocturnal sleeping hours tended to increase, and difficulty in maintaining sleep tended to decrease after living with the communication robot for 8 weeks, while alterations were not shown with the control robot. Finally, the proportions of the participants in whom effects on attenuation of fatigue, enhancement of motivation, and healing could be recognized were higher in the communication robot group relative to those in the control robot group.
Normal aging is associated with impairments in the executive and memory functions [23]. Impairments of these cognitive functions have been shown in patients with Alzheimer’s disease [24,25] and are also associated with the ADL decline and mortality in the elderly [26]. Interestingly, living with the communication robot improved these executive and memory functions in elderly women. This result emphasizes the important implications of living with the communication robot for the cognitive functions, as well as daily activities, morbidity, and mortality in the elderly. Neuroimaging studies using magnetic resonance imaging (MRI) suggest that normal aging is associated with the brain atrophy, primarily in frontal [27] and to a lesser extent in parietal [28,29] and temporal [29] cortices, and a positron emission tomography (PET) study showed that the frontal cortex is associated with executive and memory functions [30]. Therefore, living with the communication robot may have favorable effects on the frontal cortex in elderly women living alone.
One possible mechanism by which living with the communication robot is associated with the improved cognitive functions is that the increased opportunity for communication contributed to the favorable cognitive outcomes, since the improved communication environment promoted cognitive functions in the elderly [2]. Communication is essential to maintain motivation [31] and motivation improves cognitive functions [32,33]. Increased motivation caused by living with the communication robot thus may contribute to the cognitive benefits. Therefore, increased communication opportunities and enhanced motivation may lead to more favorable cognitive outcomes in the elderly women living alone.
We demonstrated that the saliva cortisol level was decreased after living with the communication robot and the proportions of the participants in whom effects on relaxing, healing, and pleasure could be recognized were higher in the communication robot group than in the control robot group in the study population. Stress impairs cognitive function by reducing the amount of available attentional resources [34] and by cognitive interference [35]. This may be another possible explanation for the improved cognitive function in elderly women living with the communication robot. Decreased levels of cortisol stress may have contributed to the favorable cognitive outcomes. Stress affects sleeping hours [35–38], sleep quality, the ability to maintain sleep [40], and fatigue [41,42]. Since poor sleep [43–46] and fatigue [47,48] are associated with cognitive impairment, improved sleep and decreased fatigue resulting from living with the communication robot may contribute to the cognitive benefits. Therefore, decreased stress accompanying the improved sleep and decreased fatigue caused favorable cognitive outcomes in the study population. Finally, the instruments used for the cognitive assessment were the cognitive function tests for screening and the clinical implication of the results are limited, and thus the use of more tests to evaluate specific cognitive functions such as executive function and memory would be beneficial.
The present study has 2 limitations. First, we performed this study with a limited number of participants. To generalize our results, studies involving a larger number of participants are essential. Second, the time span for living with the communication robot may be too short to sufficiently evaluate the effect of the robot on cognitive function. Future studies with longer observation periods are necessary to address this issue.
Conclusions
In conclusion, we demonstrated that living with the human-type communication robot was effective for the improvement of cognitive function, in particular executive and memory functions, in elderly women living alone. This is the first study to demonstrate favorable cognitive outcomes using a communication robot in the elderly and it is important to improve our understanding for the factors affecting cognitive decline in the elderly and to develop effective treatment strategies to prevent or minimize cognitive decline in elderly women living alone. Living with a human-type communication robot is a novel strategy to improve cognitive functions and prevent cognitive decline, and may provide beneficial outcomes for daily activities, morbidity, and mortality in the elderly.
Acknowledgments
We thank Mrs. Ayaka Fukuhata for helpful assistance. We also thank Forte Science Communication for editorial assistance with the manuscript. This work was supported in part by the Grant-in-Aid for Scientific Research B (KAKENHI: 23300241) from Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
Footnotes
Source of support: Departmental sources
References
- 1.Messinger-Rapport BJ, Thacker HL. Prevention for the older woman. A practical guide to assessing physical and cognitive function. Geriatrics. 2001;56:24–26. 29–31, 35. [PubMed] [Google Scholar]
- 2.Williams K, Kemper S, Hummert ML. Enhancing communication with older adults: overcoming elderspeak. J Psychosoc Nurs Ment Health Serv. 2005;43:12–16. doi: 10.3928/02793695-20050501-02. [DOI] [PubMed] [Google Scholar]
- 3.Nozaki S, Tanaka M, Mizuno K, et al. Mental and physical fatigue-related biochemical alterations. Nutrition. 2009;25:51–57. doi: 10.1016/j.nut.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Folstein MF, Folstein SE, McHugh PR. “Mini-mental stat”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 5.Allegri RF, Ollari JA, Mangone CA. El Mini Mental State Examinationen la Argentina: Instrucciones para su administración. Rev Neurol Arg. 1999;24:31–35. [Google Scholar]
- 6.Kiernan RJ, Mueller J, Langston JW, van Dyke C. The Neurobehavioral Cognitve Status Examination: A brief but differentiated approach to cognitive assessment. Ann Intern Med. 1987;107:481–85. doi: 10.7326/0003-4819-107-4-481. [DOI] [PubMed] [Google Scholar]
- 7.Perkins P, Annegers JF, Doody RS, et al. Incidence and prevalence of dementia in a multiethnic cohort of municipal retirees. Neurology. 1997;49:44–50. doi: 10.1212/wnl.49.1.44. [DOI] [PubMed] [Google Scholar]
- 8.Drane DL, Yuspeh RL, Huthwaite JS, et al. Healthy older adult performance on a modified version of the Cognistat (NCSE): Demographic issues and preliminary normative data. J Clin Exp Neuropsychol. 2003;25:133–44. doi: 10.1076/jcen.25.1.133.13628. [DOI] [PubMed] [Google Scholar]
- 9.Matsubayashi K, Okumiya K, Osaki Y, et al. Quality of life of old people living in the community. Lancet. 1997;350:1521–22. doi: 10.1016/S0140-6736(05)63944-X. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann N, Mittmann N, Silver IL, et al. A validation study of the geriatric depression scale short form. Int J Geriatr Psychiatry. 1996;11:457–60. [Google Scholar]
- 11.Pomeroy IM, Clark CR, Philp I. The effectiveness of very short scales for depression screening in elderly medical patients. Int J Geriatr Psychiatry. 2001;16:321–26. doi: 10.1002/gps.344. [DOI] [PubMed] [Google Scholar]
- 12.de Craen AJ, Heeren TJ, Gussekloo J. Accuracy of the 15-item geriatric depression scale (GDS-15) in a community sample of the oldest old. Int J Geriatr Psychiatry. 2003;18:63–66. doi: 10.1002/gps.773. [DOI] [PubMed] [Google Scholar]
- 13.Koyano W, Shibata H, Nakazato K, et al. Measurement of competence in the elderly living at home: development of an index of competence. Jpn J Pub Health. 1987;34:109–14. [in Japanese] [Google Scholar]
- 14.Koyano W, Shibata H, Nakazato K, et al. Measurement of competence: reliability and validity of the TMIG Index of Competence. Arch Gerontol Geriatr. 1991;13:103–16. doi: 10.1016/0167-4943(91)90053-s. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguti K. The evaluation of fatigue by using acceleration plethysmography. Nippon Rinsho. 2007;65:1034–42. [in Japanese] [PubMed] [Google Scholar]
- 16.Tajima K, Tanaka M, Mizuno K, et al. Effects of bathing in micro-bubbles on recovery from moderate mental fatigue. Ergonomia IJE&HF. 2008;30:135–45. [Google Scholar]
- 17.Mizuno K, Tanaka M, Tajima K, et al. Effects of mild-stream bathing on recovery from mental fatigue. Med Sci Monit. 2010;16:CR8–14. [PubMed] [Google Scholar]
- 18.Takada M, Ebara T, Kamijima M. Heart rate variability assessment in Japanese workers recovered from depressive disorders resulting from job stress: measurements in the workplace. Int Arch Occup Environ Health. 2010;83:521–29. doi: 10.1007/s00420-009-0499-1. [DOI] [PubMed] [Google Scholar]
- 19.Akselrod S, Gordon D, Ubel FA, et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–22. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 20.Pomeranz B, Macaulay RJ, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:151–53. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 21.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–92. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 22.Appel ML, Berger RD, Saul JP, et al. Beat to beat variability in cardiovascular variables: noise or music? J Am Coll Cardiol. 1989;14:1139–48. doi: 10.1016/0735-1097(89)90408-7. [DOI] [PubMed] [Google Scholar]
- 23.Light LL. Memory and aging: four hypotheses in search of data. Annu Rev Psychol. 1991;42:333–76. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- 24.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Rabin LA, Borgos MJ, Saykin AJ, et al. Judgment in older adults: development and psychometric evaluation of the Test of Practical Judgment (TOP-J) J Clin Exp Neuropsychol. 2007;29:752–67. doi: 10.1080/13825580601025908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62:1134–41. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raz N, Gunning-Dixon FM, Head D, et al. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- 28.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 29.Van Petten C, Plante E, Davidson PS, et al. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42:1313–35. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Reed LJ, Lasserson D, Marsden P, et al. Correlations of regional cerebral metabolism with memory performance and executive function in patients with herpes encephalitis or frontal lobe lesions. Neuropsychology. 2005;19:555–65. doi: 10.1037/0894-4105.19.5.555. [DOI] [PubMed] [Google Scholar]
- 31.Levin RP. Office communication is necessary for staff to maintain motivation. Dent Off. 1989;1(10):13. [PubMed] [Google Scholar]
- 32.Velligan DI, Kern RS, Gold JM. Cognitive rehabilitation for schizophrenia and the putative role of motivation and expectancies. Schizophr Bull. 2006;32:474–85. doi: 10.1093/schbul/sbj071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuno K, Tanaka M, Fukuda S, et al. Relationship between cognitive function and prevalence of decrease in intrinsic academic motivation in adolescents. Behav Brain Funct. 2011;7:4. doi: 10.1186/1744-9081-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasher L, Zacks RT. Automatic and effortful processes in memory. J Exp Psychol: General. 1979;108:356–88. [Google Scholar]
- 35.Stawski RS, Sliwinski MJ, Smyth JM. Stress-related cognitive interference predicts cognitive function in old age. Psychol Aging. 2006;21:535–44. doi: 10.1037/0882-7974.21.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakata A, Haratani T, Takahashi M, et al. Job stress, social support at work, and insomnia in Japanese shift workers. J Hum Ergol (Tokyo) 2001;30:203–9. [PubMed] [Google Scholar]
- 37.Nakata A, Haratani T, Takahashi M, et al. Job stress, social support, and prevalence of insomnia in a population of Japanese daytime workers. Soc Sci Med. 2004;59:1719–30. doi: 10.1016/j.socscimed.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Murata C, Yatsuya H, Tamakoshi K, et al. Psychological factors and insomnia among male civil servants in Japan. Sleep Med. 2007;8:209–14. doi: 10.1016/j.sleep.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Nomura K, Nakao M, Takeuchi T, Yano E. Associations of insomnia with job strain, control, and support among male Japanese workers. Sleep Med. 2009;10:626–29. doi: 10.1016/j.sleep.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Knudsen HK, Ducharme LJ, Roman PM. Job stress and poor sleep quality: data from an American sample of full-time workers. Soc Sci Med. 2007;64:1997–2007. doi: 10.1016/j.socscimed.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlson LE, Garland SN. Impact of mindfulness-based stress reduction (MBSR) on sleep, mood, stress and fatigue symptoms in cancer outpatients. Int J Behav Med. 2005;12:278–85. doi: 10.1207/s15327558ijbm1204_9. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka M, Fukuda S, Mizuno K, et al. Stress and coping styles are associated with severe fatigue in medical students. Behav Med. 2009;35:87–92. doi: 10.1080/08964280903231979. [DOI] [PubMed] [Google Scholar]
- 43.Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28:981–89. [PubMed] [Google Scholar]
- 44.Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20:41–48. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 45.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Study of Osteoporotic Fractures Group. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 46.Nebes RD, Buysse DJ, Halligan EM, et al. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:180–87. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka M, Mizuno K, Tajima S, et al. Central nervous system fatigue alters autonomic nerve activity. Life Sci. 2009;84:235–39. doi: 10.1016/j.lfs.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Mizuno K, Tanaka M, Fukuda S, et al. Relationship between cognitive functions and prevalence of fatigue in elementary and junior high school students. Brain Dev. 2011;33:470–79. doi: 10.1016/j.braindev.2010.08.012. [DOI] [PubMed] [Google Scholar]