Summary
Background
Dry cough is a common cause for the discontinuation of ramipril treatment. The aim of this pharmacoepidemiological study was to assess the incidence of ramipril-related cough among the Polish population and to characterize patients at risk of experiencing the adverse effect of cough during ramipril treatment.
Material/Methods
This was a prospective observational study involving 10,380 patients treated with ramipril for a period of no longer than 8 weeks, consisting of 3 visits: baseline, first follow-up (after 4–8 weeks) and second follow-up visit (after 4–8 weeks of cessation of ramipril, conducted only for evaluating coughing patients).
Results
The incidence of ramipril-related cough was 7.1%. Logistic regression analysis identified female sex (OR=1.35), cigarette smoking (OR=2.50), chronic obstructive pulmonary disease (OR=1.70), asthma (OR=1.60) and previous history of tuberculosis (OR=6.20) to be significantly and independently associated with the onset of ramipril-related cough.
Coughing subsided within a period of 2–20 days after ramipril was discontinued. In all patients reporting the appearance of cough within the first 5 days after therapy initiation, the adverse effect subsided after therapy discontinuation. If cough appeared within 6–10 days, it subsided after discontinuation in 81.6% of subjects. Cough persisted in 30.4% of those reporting later onset.
Conclusions
1. Female sex, cigarette smoking, COPD, asthma, and previous history of tuberculosis increase the risk of ramipril-related cough. 2. The later the cough occurs during treatment, the less often the drug is the causative agent and the cough and also less likely to disappear after discontinuation of ramipril.
Keywords: ramipril, angiotensin-converting enzyme inhibitor, cough
Background
Angiotensin-converting enzyme inhibitors (ACE-I) were first introduced in 1981. Initially only indicated for treatment of refractory hypertension, they are now widely used in hypertension, as well as to reduce morbidity or mortality in patients with congestive heart failure, myocardial infarction, diabetes mellitus, chronic kidney disease, and atherosclerotic cardiovascular disease [1]. ACE-I can also attenuate cardiac remodeling in different pathological models [2].
Ramipril is an ACE-I, primarily reducing the rate of conversion of angiotensin I to angiotensin II. Inhibition of angiotensin-converting enzyme is also associated with a decline of bradykinin degradation, which is likely to have beneficial effects on the circulation and kidneys [3,4]. Ramipril has been increasingly used after the publication of the HOPE trial, while the growth in rates of use of other ACE inhibitors remains constant or decline [5]. Due to its broad range of indications, especially for congestive heart failure treatment, ramipril is the second most widely prescribed ACE-I in Poland [6]. The number of patients on ramipril in Poland is estimated at 1.5 million. It is also the most prescribed pharmaceutical in Estonia and Lithuania in 2009 and is among the top 20 dispensed drugs in Canada in 2010 [7,8]. Annual sales of ramipril capsules in the United States were approximately $898 million USD for 12 months ending June 2008, according to IMS Health data [9].
During ACE-I treatment, different adverse effects have been reported, such as: hypotension, cough, hyperkalemia, renal impairment, headache, dizziness, fatigue, nausea, angioedema and allergic reactions [4].
Dry, persistent cough is a well-described adverse effect of the ACE-I class medications [10] and is the most frequently reported adverse drug reaction (ADR) [11,12]. In the general population, a significant and clinically relevant proportion of patients experiencing ACE-I-induced cough are treated with antitussive agents, which may subject them to extensive and unnecessary evaluations, diagnostic tests, and consultations [13,14]. The mechanism of ACE-I-induced cough remains unclear, but likely involves the protrusive mediators bradykinin and substance P, and is defined as extrathoracic airway hyper-responsiveness (EAHR) [15]. ACE-I-induced cough has not been demonstrated to be dose-dependent [10].
The use of ACE-Is can trigger the development of cough and also intensify stimulation of the cough reflex induced by other causes. ACE-I-related cough was reported more frequently in women treated for heart failure, in patients with respiratory diseases (bronchial asthma, chronic obstructive pulmonary disease), diabetes, concurrent use of other drugs, (indomethacin, amlodipine, nifedipine or theophylline) and in smokers [10,16–20]. It has been shown that a genetic predisposition, especially in women, may increase the risk of ACE-I-related cough [21].
The incidence of ACE-I-related cough has been reported to be in the range of 5% to 35% [10]. In large observational studies, the incidence of cough in patients treated with ramipril ranged from 3.0% to 24.3% [22,23]. The incidence and prevalence of ramipril-related cough in the Polish population is unknown at present. The onset of cough is a common reason for discontinuation of ACE-I therapy. In the ONTARGET study, 4.2% of ramipril-treated patients experienced cough, and 100% of these patients discontinued its use because of that [15].
ACE-I-related cough may occur as early as after the first tablet or after many weeks or months. After discontinuation of ACE-I therapy, cough may persist for a few weeks, but generally no longer than 3 months [10]. The ACCP (The American College of Chest Physicians) Evidence-Based Clinical Practice Guidelines for ACE-I-related cough advocate that in patients in whom cough resolves after the cessation of ACE-I therapy, a repeat trial of such therapy may be attempted [10].
The only effective therapy for ACE-I-related cough is the cessation of therapy with the agent and substitution with another inhibitor of the renin-angiotensin system. The resolution of cough (usually within 1 to 4 weeks of the cessation of ACE-I use) confirms the diagnosis of ACE-I-related cough. [10]. Most guidelines recommend substitution with an angiotensin receptor blocker in case of troublesome and recurrent cough after ACE-I [24,25]. There are no specific Polish guidelines concerning this issue.
The aim of this pharmacoepidemiological study was to assess the incidence of ramipril-related cough among the Polish population, and characterize the patients particularly vulnerable to the adverse effect of dry cough and risk factors during ramipril therapy.
Material and Methods
This survey was conducted in 2010 and is comprised of responses from 517 general practitioners (out of 800 invited) working in primary care, private practice, and specialty clinics throughout Poland. The inclusion criteria for the study were the use of ramipril for no longer than 8 weeks, and 18 years of age or above. Patients treated with angiotensin II receptor type 1 antagonists (sartans) were excluded.
The patients were instructed (at the baseline visit) to report during the subsequent visit the onset of any adverse drug reactions (ADR) (hypotension, cough, headache, dizziness, fatigue, nausea, angioedema and other allergic reactions) that developed if and when they had stopped taking ramipril, and how such actions influenced the ADRs. Patients were not informed that incidence of cough was the main aim of the study, so there was no chance for Hawthorne effect [26]. At the baseline visit the incidence of cough, time of its appearance and disappearance after eventual ramipril discontinuation was analyzed retrospectively.
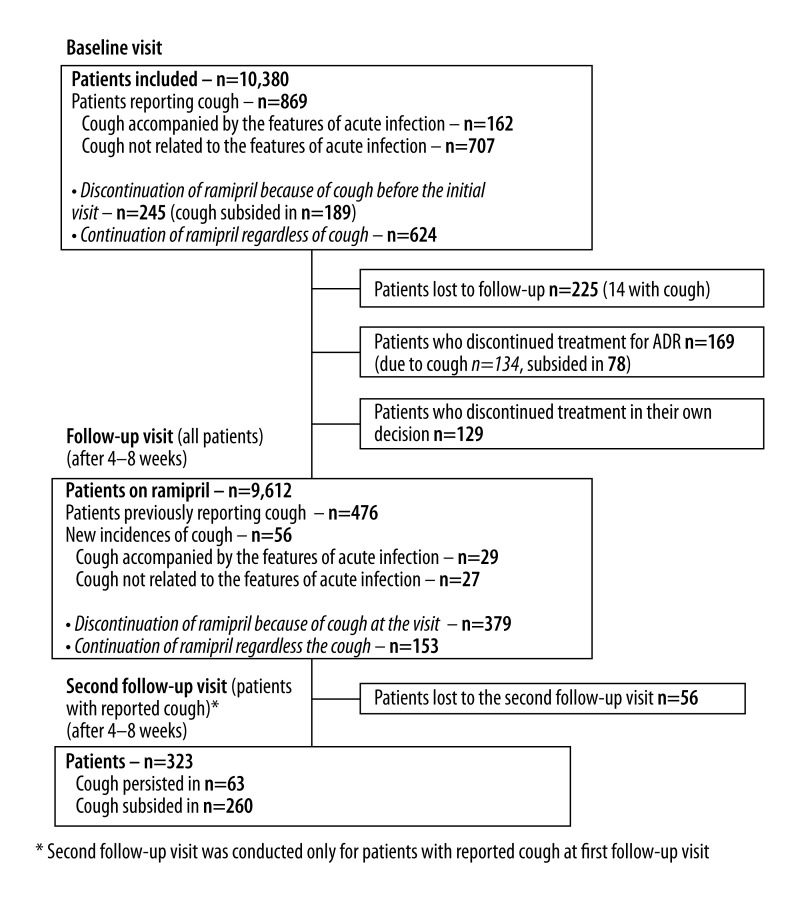
The basic study questionnaire consisted of 2 parts. An additional, third, part of the survey was conducted only for patients experiencing cough and discontinuing ramipril therapy (Figure 1). The patients did not actively participate in completing surveys. Surveys were completed by the physicians (as well as the monitoring of renal dysfunction and electrolyte imbalance).
Figure 1.
Flow diagram of study desing.
The first part of the form (collected during baseline visit) included patient demographic data (sex, age, place of residence, education, and labor activity). Anthropometric data (weight, height, and waist circumference), 2 blood pressure measurements, date of initiation of treatment with ramipril, and the indications for the use of ramipril.
Current cigarette use and history of chronic diseases favoring the occurrence of cough (chronic obstructive pulmonary disease, asthma, allergic rhinitis, chronic rhinosinusitis, history of tuberculosis, mitral valve disease, and thoracic aorta aneurysm) were also recorded. We also collected data on eventual appearance of cough after ramipril treatment before the follow-up visit (whether it disappeared after ramipril discontinuation, and after how many days), nature of the cough, accompanying symptoms suggestive of acute respiratory tract infection (fever, rhinitis, muscle aches, bone and joint pain, shortness of breath), and data from an interview on the prevalence of cough during the previous ACE-I treatment.
The second part of the questionnaire was collected during the follow-up visit (after 4–8 weeks) and included the data on patient adherence to ramipril therapy, eventual reasons for discontinuation, and the occurrence of cough and associated symptoms (as at the baseline visit). For patients who stopped taking ramipril before the follow-up, there was the additional question of whether the cough resolved after discontinuation of the ramipril (after how many days).
The third part of the survey was conducted only for patients with cough who had discontinued ramipril therapy during their second follow-up visit. This survey was conducted between 4–8 weeks after the first follow-up visit (following the established method of diagnosing ACE-I-induced cough). It included only questions about the continuation or discontinuation of cough after cessation of treatment with ramipril.
This questionnaire-based survey did not fulfill the criteria of medical experimentation and thus did not require ethics committee approval.
Calculation of study size
Based on existing published studies, we postulated that ramipril-related cough would occur in approximately 10% of the population. Sample size with 0.5% error was calculated at 9,740. However, given the possible lack of response from about 20% of responders, the sample size was estimated at n=12,175 [27].
Data analysis
BMI was calculated on the basis of body weight and height. According to the widely accepted WHO criteria, overweight was defined as a BMI greater than 25 and lower than 30 kg/m2, obesity as a BMI equal to or more than 30 kg/m2, and morbid obesity as BMI ≤40 kg/m2. Visceral obesity was defined on the basis of the 2005 IDF criteria – waist circumference for Caucasians of ≥80 cm for adult women and ≥94 cm for adult men [28].
Ramipril-related cough was defined as cough not related to the signs of respiratory tract infections, and that disappeared up to 4–8 weeks after the cessation of the ramipril treatment. Patients experiencing cough who did not attend the second follow-up visit were included as ramipril-related cough.
The results of this analysis are presented as rates or as an average with standard deviation. Univariate and multivariate backward stepwise grouped logistic regression analysis were performed including factors potentially favoring the occurrence of chronic cough, including smoking. Age-adjusted odds ratios are presented with 95% confidence intervals. All variables were tested for the presence of multi co-linearity, which was assessed with the variance inflation factor (VIF) and the conditional index [29]. Based on the literature, to ensure that there is no co-linearity, the VIF value should not exceed 5, while the value of conditional factor should not exceed 30. The goodness of fit of the regression model was assessed with the Wald χ2 test. The frequency of categorical data was compared using the χ2 test, and 95% confidence intervals were calculated with continuity correction according to methods described by Newcomb [30]. Statistical analysis was performed with STATISTICA 8.0 PL software and R software. P<0.05 was considered as statistically significant. All tests were 2-sided.
Results
Characteristics of the study group
A total of 10,380 patients treated with ramipril, including 50.8% men and 49.2% women, participated in the study (Table 1); 25.8% of respondents were aged >65 years, and 28.9% of participates were currently smokers (17.3% women and 40.1% men, p<0.001).
Table 1.
The characteristics of the study participants treated with ramipril (n=10.380).
| % | n | |
|---|---|---|
| Age [years] | 57.8±11.3 | |
| ≤65years [%] | 74.2 | 7707 |
| >65years [%] | 25.8 | 2689 |
| Gender m/f [%] | 50.8/49.2 | 5269/5111 |
| Smokers [%] | 28.9 | 3000 |
| Pack years | 20±14 | |
| BMI [kg/m2] | 28.6±4.2 | |
| Overweight [%] | 48.2 | 5005 |
| Obesity [%] | 33.2 | 3444 |
| Morbidly obese [%] | 1.3 | 133 |
| Waist circumference [cm] | 92±12 | |
| Visceral obesity [%] | 69.1 | 7174 |
| Left arm circumference [cm] | 33±8 | |
| Arm circumference >32 cm [%] | 49.9 | 5180 |
| Systolic blood pressure* [mmHg] | 149.8±15.6 | |
| Diastolic blood pressure* [mmHg] | 90.5±10.1 | |
| Place of residence [%] | ||
| Rural areas | 21.1 | 2194 |
| City with population of <50,000 residents | 27.9 | 2900 |
| City with population of 50,000–200,000 residents | 21.6 | 2240 |
| City with population of >200,000 residents | 29.4 | 3046 |
| Education [%] | ||
| Basic | 9.2 | 961 |
| Vocational | 28.8 | 2985 |
| Secondary | 41.7 | 4330 |
| Higher | 20.3 | 2104 |
| Co-morbidity [%] | ||
| Diabetes | 32.2 | 3338 |
| Hypertension | 96.4 | 10002 |
| Peptic ulcer disease | 13.8 | 1435 |
| Gastroesophageal reflux disease | 15.9 | 1654 |
| Chronic obstructive pulmonary disease | 8.9 | 920 |
| Asthma | 4.1 | 428 |
| Allergic rhinitis | 4.7 | 491 |
| Chronic rhinosinusitis | 3.1 | 326 |
| History of tuberculosis | 0.3 | 28 |
| Mitral valve disorder | 2.1 | 222 |
| Thoracic aorta aneurysm | 0.5 | 48 |
| Mental disease | 2.5 | 260 |
The average of 2 measurements.
Overweight or obesity was identified in 48.2% and 33.2% cases in the whole group, respectively; 1.3% of participants had severe obesity, and 69.1% of patients had abdominal obesity. Diabetes mellitus affected 32.2% of the respondents. Co-morbidity is summarized in Table 1.
A total of 21.4% of patients had pathologies conducive to the occurrence of chronic cough (chronic obstructive pulmonary disease, asthma, allergic rhinitis, chronic sinusitis, history of tuberculosis, mitral valve disorder, thoracic aortic aneurysm). Co-morbid mental illness was identified in 2.5% of participants. Arterial hypertension had been diagnosed in 96.4% of patients.
In 23.8% of respondents there was more than 1 registered indication for the use of ramipril (Table 2). The most common indication was hypertension (93.0%), followed by heart failure (21.4%). Diabetic nephropathy was the third most frequent indication (8.1%). Ramipril was obtained “off label” (outside of the registered indications) by 0.5% of patients
Table 2.
Indications for use of ramipril among study participants (n=10.380)*.
| [%] | |
|---|---|
| Hypertension | 93.0 |
| Heart failure after myocardial infarction | 12.3 |
| Heart failure without previous myocardial infarction | 9.1 |
| Diabetic nephropathy | 8.1 |
| Non-diabetic nephropathy | 2.4 |
| Patient of high cardiovascular risk not meeting the other criteria | 3.4 |
Some patients had more than one indication.
Occurrence of cough
A total of 869 patients (8.3%) (95% CI: 7.9–8.9%) complained of cough, mostly dry (91.8%), during the baseline visit. Cough occurred on average 13±9 days (from 1 to 60 days) after the initiation of ramipril therapy. After excluding patients with cough accompanied by the features of acute infection (fever, rhinitis, myalgia) from the analysis, the incidence of cough decreased to 6.8% (95%CI: 6.3–7.3%) (n=707). Of the study participants, 7.5% (n=695) had a previous history of cough related to etiology other than ramipril ACE-I. A total of 52.5% of responders who reported a cough that discontinued after cessation of ramipril, the adverse effect previously occurred during treatment with other ACE-Is. The cough did not appear after initiation of ramipril therapy until the baseline visit in 4.2% of all study participants with a positive history of cough during the use of another ACE-I.
A total of 28.2% (n=245) of respondents discontinued the use of ramipril because of cough. In 77.1% of patients who discontinued treatment, cough resolved after 2–15 days (median and interquartile range 5 [4–10] days), while in 23.9% of patients cough persisted despite the discontinuation of ramipril. At the baseline visit, 624 patients remained on ramipril therapy despite the occurrence of cough.
A total of 10,127 (97.6%) of participants attended the follow-up visit (after 4.1±0.5 weeks). A total of 9,612 (94.9%) were still on ramipril therapy, while 298 (3.1%) had discontinued the use of ramipril. Discontinuation of ramipril was reported secondary to ADRs in 56.8% (n=169) of patients, negative patient opinion concerning the necessity for the use of the medicine 41.2% (n=123), and economic reasons 2.0% (n=6). After therapy cessation, the cough resolved in 58.2% of affected participants who stopped the ramipril therapy. The median time of cough subsiding after discontinuation of ramipril treatment was 5 (interquartile range 4–11) days (range from 2 to 20 days).
ADRs, excluding cough, were reported in 171 (1.7%) patients on ramipril therapy, including hypotensive episodes, impairment of renal excretory function, and headaches.
Cumulative incidence of ramipril-related cough
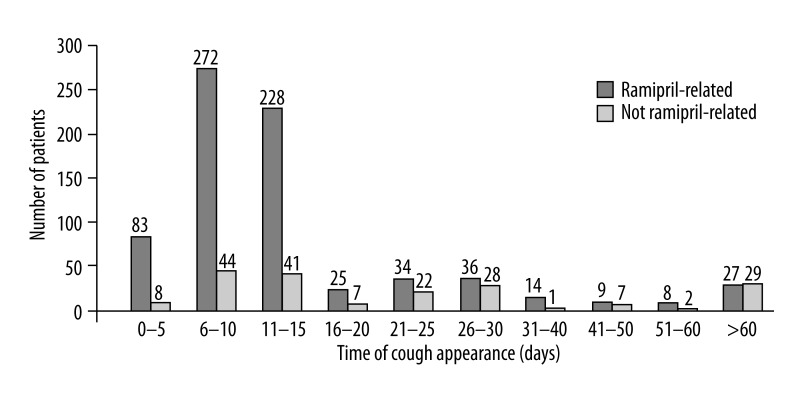
Ramipril-related cough not associated with infection, excluding cases with persistent cough 8 weeks after cessation of therapy, was observed in 736 participants (7.1% [95% CI: 6.6–7.6%]) of the (7.6% of women and 7.2% of men; ns) up to the end of the observation period, while episode of cough regardless of cause was observed in 925 of participants (8.9% [95% CI: 8.4–9.5%]) (Figure 2).
Figure 2.
Incidence of ramipril-related and non ramipril-related cough related to time to occurence.
Therapy with ramipril was discontinued in 702 of 925 patients with cough. The symptom subsided in 527 of them (75.1%). In all patients reporting the appearance of cough within the first 5 days after therapy initiation, the cough symptoms resolved after therapy was discontinued. If the cough appeared within 6–10 days, it subsided after discontinuation in 81.6%, and persisted in 30.4% of those reporting the appearance of cough later than 10 days after therapy initiation.
Ramipril-related cough occurred significantly more in patients with chronic diseases conducive to the occurrence of chronic cough – chronic obstructive pulmonary disease, asthma, allergic rhinitis, chronic sinusitis, history of tuberculosis, mitral valve disorder or thoracic aortic aneurysm (10.6% vs. 6.1%, p<0.001). In univariate age-adjusted logistic regression, ramipril-related cough occurred significantly more frequently in patients with hypertension, peptic ulcer disease, asthma, COPD, prior history of tuberculosis, and smokers. Ramipril-related cough occurred less frequently among those suffering from gastro-esophageal-reflux disease (GERD) and chronic rhinosinusitis (Table 3).
Table 3.
Factors influencing ramipril-related cough (age-adjusted univariate logistic regression).
| OR | (95% CI) | p | |
|---|---|---|---|
| Female gender | 1.02 | (0.89–1.12) | 0.76 |
| Cigarette smoking | 2.44 | (2.11–2.82) | <0.001 |
| Diabetes mellitus | 0.94 | (0.81–1.10) | 0.43 |
| Hypertension | 2.11 | (1.24–3.57) | <0.01 |
| Peptide ulcer disease | 1.32 | (1.09–1.59) | <0.01 |
| Asthma | 1.40 | (1.02–1.92) | <0.05 |
| Chronic obstructive pulmonary disease | 2.10 | (1.73–2.53) | <0.001 |
| Gastroesophageal reflux disease | 0.61 | (0.48–0.77) | <0.001 |
| Mitral valve disorder | 1.13 | (0.72–1.76) | 0.60 |
| Aorta aneurysm | 1.93 | (0.92–4.08) | 0.08 |
| History of tuberculosis | 5.91 | (3.47–10.04) | <0.001 |
| Allergic rhinitis | 0.86 | (0.59–1.26) | 0.45 |
| Chronic rhinosinusitis | 0.30 | (0.14–0.63) | <0.01 |
| Mental disease | 1.16 | (0.75–1.79) | 0.51 |
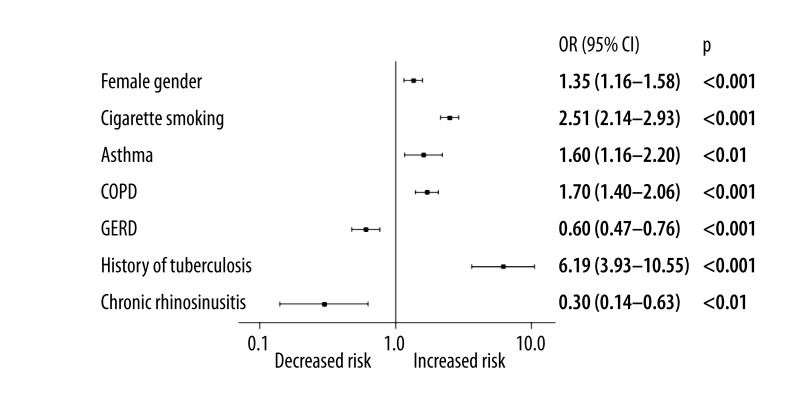
In this study of over 10,000 patients treated with ramipril, logistic regression adjusted for age analysis identified female sex (OR=1.35 [1.16–1.58]), cigarette smoking (OR=2.50 [2.14–2.93]), chronic obstructive pulmonary disease – COPD (OR=1.70 [1.40–2.06]), asthma (OR=1.60 [1.17–2.20]) and previous history of tuberculosis (OR=6.20 [3.63–10.55]) to be significantly and independently associated with the onset of cough not related to acute infection, as well as subsiding after ramipril therapy cessation (Figure 3). GERD and chronic rhinosinusitis were the only 2 factors demonstrating a decreased risk of cough in this model. For any variable included in the regression model, the VIF did not exceed the value of 1.11 (mean: 1.04±0.04). Maximal value of the conditional index was 3.48 (mean: 1.83±0.72). Based on these results, we may expect that our logistic regression model is free from multicollinearity.
Figure 3.
The results of age adjusted multivariate stepwise backward logistic regression of cough predisposing factors during ramipril treatment (χ2=232; p<0.001; log-pseudolikelihood =−5546).
Discussion
This study shows that ramipril-related cough occurred in 7.1% of Polish patients on ramipril therapy. Factors such as female sex, cigarette smoking, chronic obstructive pulmonary disease, asthma and previous history of tuberculosis seem to contribute to an increase in its occurrence. A baseline history of these factors may therefore be helpful in identification of patients particularly at risk of its occurrence. Careful attention to patients with these risk factors may prevent misdiagnosis and improper treatment of this well-known adverse-effect. Perhaps in these patients it would be reasonable to substitute ACE-I for an angiotensin II antagonist, bearing in mind that ACE-I-induced cough is a class-wide adverse effect and may occur with other agents in this class. There is also a 10-times increased risk for potentially fatal angioedema in patients with a history of ACE-I-related cough [31].
The CARE study evaluated the incidence of cough in a large population of Americans with hypertension (N=11,100) [22]. During an 8-week observational period, the incidence of dry cough was reported in 3.0% of patients. It is possible that not all incidences of cough were reported by the team of researchers. However, much higher rates were reported in a study conducted in India, where a rate of 24.39% was recorded [23]. The incidence of cough in patients treated with ramipril has also been reported in the ONTARGET and Pharao trials, in 4.2 and 4.8% of participants, respectively [15,32]. The most similar results to those reported in our study were reported by Lacourciere et al in n=405 Canadian patients and in n=1,048 patients in a study by Hathiala [33,34]. During a period of 14 weeks in the Canadian study and at the end of an 8-week period in the Hathiala study, the incidence of cough was reported as 10.1% and 10.0%, respectively [34]. The observed discrepancies may be partially explained by various racial differences, co-morbidities, and pharmacotherapies. The CARE study determined that cough appeared most frequently among Caucasian patients, constituting 77.8% of study participants [22]. This racial differentiation may not explain the markedly greater incidence of cough in the Polish population reported in this study, compared to the Americans in the CARE study.
In this study we found a higher incidence of ACE-I-related cough in women (OR=1.35). These results confirm results of another published study supporting the hypothesis that women are more susceptible to developing ACE-I-induced cough [21].
Co-morbid conditions are also factors that may influence variation in reported incidence of cough. In this study, we did not exclude patients with illnesses pre-disposing patients to chronic cough, which was 21.4% of the study participates. In these patients, the incidence of ramipril-related cough was 10.6%. One study identified a group of patients with asthma who exhibited cough during treatment with ACE-I, and found that the sensitivity of cough reflex increased during the treatment [16]. As shown, ACE-I sensitizes the cough reflex. It is therefore not surprising that cough is more common among patients with COPD (OR=1.7) or asthma (OR=1.6), or in smokers (OR=2.51).
Although according to some researchers ACE-I-related cough is less common in smokers [8], in our study cigarette smoking increased the risk of cough by more than 2-fold (OR=2.51). Such an association was not found by Singh et al. in their observation of a much smaller population of patients (n=250) [23].
We found that rhinosinusitis was not among the illnesses that independently increased the risk of ACE-1-related cough, probable as this etiology is frequently associated with asthma [35]. The absence of chronic rhinosinusitis among the independent factors demonstrating an increased risk of ramipril-related cough in our study may suggest that only a subset of patients with eosinophilic airway inflammation have an increased risk of ramipril-related cough.
During ACE-I treatment, cough occurs most frequently in the early period of therapy. In our study, ramipril-related cough occurred on average of 13±9 days after initiation of treatment. The Hathial study reported an even earlier appearance of this adverse drug reaction. According to this research, during the first week of treatment coughing occurred in 7.1% of 1,048 patients with a high risk of cardiovascular disease, and at the end of the 8-week observation the prevalence of cough increased to 10.0% [34].
The causal relationship between ACE-I and cough also indicates a reduced resolution if the symptom onset appeared in the later period of observation. According to our findings, after discontinuation of ramipril treatment, cough resolved in 75.8% of patients if the symptom occurred before the baseline visit, and in 58.8% of those who stopped taking ramipril after the baseline visit.
The incidence of cough can also be affected by the use of other drugs. In this study, data on the concomitant treatment of cardiovascular and respiratory diseases were not collected. Thus the analysis of the impact of polypharmacotherapy on the incidence of cough cannot be performed and it is among the main limitations of this analysis.
In this study patients that experienced cough and did not attend the second follow-up visit were assumed to have ramipril-related cough. This may possibly have led to overestimation of the percentage of ramipril-related cough. On the other hand, the first follow-up visit was planned for after 4–8 weeks, while ACE-I-related cough sometimes starts after several weeks or months. This method therefore might lead to some underestimation of the percentage of ramipril-related cough. Both of these assumptions are limitations of this study.
Conclusions
Female sex, cigarette smoking, COPD, asthma and previous history of tuberculosis appear to increase the risk of ramipril-related cough.
The later the cough occurs during the treatment, the less often the drug is the cause and the less likely the cough will resolve after discontinuation of treatment.
Acknowledgments
This survey study did not require ethics approval.
Footnotes
Source of support: The study was carried out as a research project supported by a scientific grant of Sandoz Polska Sp. z o.o, organized by Europharma M. Rachtan Sp. z o.o. Participating general practitioners received payment for completing surveys
References
- 1.Bicket DP. Using ACE Inhibitors Appropriately. Am Fam Physician. 2002;66:461–69. [PubMed] [Google Scholar]
- 2.Duarte DR, Minicucci MF, Azevedo PS, et al. Influence of lisinopril on cardiac remodeling induced by tobacco smoke exposure. Med Sci Monit. 2010;16:255–59. [PubMed] [Google Scholar]
- 3.Sharpe N International HOPE TIPS Investigators. The HOPE TIPS: the HOPE study translated into practices. Cardiovasc Drugs Ther. 2005;19:197–201. doi: 10.1007/s10557-005-1375-1. [DOI] [PubMed] [Google Scholar]
- 4.Jafar TH, Stark PC, Schmid CH, et al. AIPRD Study Group. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–52. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 5.Hemels ME, Bennett HA, Bonari L, et al. HOPE Study Impact on ACE Inhibitors Use. Ann Pharmacother. 2003;37:640–45. doi: 10.1345/aph.1C339. [DOI] [PubMed] [Google Scholar]
- 6.Holecki M, Szewieczek J, Chudek J. Effects of angiotensin-converting enzyme inhibitors beyond lowering blood pressure – are they important for doctors? Pharmacological Reports. 2011;63:740–51. doi: 10.1016/s1734-1140(11)70586-2. [DOI] [PubMed] [Google Scholar]
- 7.Garuoliene K, Alonderis T, Marcinkevicius M. Pharmaceutical policy and the effects of the economic crisis: Lithuania. Eurohealth. 17(1) [Google Scholar]
- 8.IMS Brogan. Rx Database. Ottawa, ON: IMS Brogan; 2010. Canadian CompuScript Audit. Available from www.broganinc.com. [Google Scholar]
- 9.http://www.imshealth.com
- 10.Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough. Chest. 2006;129:169S–73S. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- 11.Sadanaga T, Yoshimura M, Sakamoto T, et al. Enalapril-induced cough is associated with non-severe heart failure. Int J Cardiol. 2009;26:275–76. doi: 10.1016/j.ijcard.2008.03.063. [DOI] [PubMed] [Google Scholar]
- 12.Simpson CB, Amin MR. Chronic cough: state-of-the-art review. Otolaryngol Head Neck Surg. 2006;134:693–700. doi: 10.1016/j.otohns.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi TK, Burstin HR, Cook EF, et al. Drug complications in outpatients. J Gen Intern Med. 2000;15:149–54. doi: 10.1046/j.1525-1497.2000.04199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vegter S, de Jong-van den Berg LT. Misdiagnosis and mistreatment of a common side-effect – angiotensin-converting enzyme inhibitor-induced cough. Br J Clin Pharmacol. 2010;69:200–3. doi: 10.1111/j.1365-2125.2009.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–59. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 16.McEwan JR, Choudry N, Street R, Fuller RW. Change in cough reflex after treatment with enalapril and ramipril. BMJ. 1989;299:13–16. doi: 10.1136/bmj.299.6690.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morimoto T, Gandhi TK, Fiskio JM, et al. Development and validation of a clinical prediction rule for angiotensin-converting enzyme inhibitor-induced cough. J Gen Intern Med. 2004;19:684–91. doi: 10.1111/j.1525-1497.2004.30016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YJ, Chiang YF, Tsai JC. Severe nonproductive cough and cough-induced stress urinary incontinence in diabetic postmenopausal women treated with ACE inhibitor. Diabetes Care. 2000;23:427–28. doi: 10.2337/diacare.23.3.427. [DOI] [PubMed] [Google Scholar]
- 19.Fogari R, Zoppi A, Tettamanti F, et al. Effects of nifedipine and indomethacin on cough induced by angiotensin-converting enzyme inhibitors: a double-blind, randomized, cross-over study. J Cardiovasc Pharmacol. 1992;19:670–73. [PubMed] [Google Scholar]
- 20.Visser LE, Stricker BH, van der Velden J, et al. Angiotensin converting enzyme inhibitor associated cough: a population-based case-control study. J Clin Epidemiol. 1995;48:851–57. doi: 10.1016/0895-4356(94)00231-e. [DOI] [PubMed] [Google Scholar]
- 21.Grilo A, Sáez-Rosas MP, Santos-Morano J, et al. Identification of genetic factors associated with susceptibility to angiotensin-converting enzyme inhibitors-induced cough. Pharmacogenet Genomics. 2011;21:10–17. doi: 10.1097/FPC.0b013e328341041c. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan NM. The CARE Study: a postmarketing evaluation of ramipril in 11,100 patients. The Clinical Altace Real-World Efficacy (CARE) Investigators. Clin Ther. 1996;18:658–70. doi: 10.1016/s0149-2918(96)80216-5. [DOI] [PubMed] [Google Scholar]
- 23.Singh NP, Uppal M, Anuradha S, et al. Angiotensin converting enzyme inhibitors and cough - a north Indian study. J Assoc Physicians India. 1998;46:448–51. [PubMed] [Google Scholar]
- 24.Mancia G, De Backer G, Dominiczak A, et al. The 2007 ESH/ESC guidelines for the management of arterial hypertension. J Hyperten. 2007;25:1751–62. doi: 10.1097/HJH.0b013e3282f0580f. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Sendon J, Swedberg K, McMurray J. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. Eur Heart J. 2004;25:1454–70. doi: 10.1016/j.ehj.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 26.McCarney R, Warner J, Iliffe S, et al. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrie A, Sabin C. Medical Statistics at a Glance. 3rd ed. John Wiley & Sons; United Kingdom: 2009. [Google Scholar]
- 28.www.idf.org/metabolic-syndrome, website of the International Diabetes Federation
- 29.O’Brien RM. A Caution Regarding Rules of Thumb for Variance Inflation Factors. Quality and Quantity. 2007;41:673–90. [Google Scholar]
- 30.Newcombe RG. Two-Sided Confidence Intervals for the Single Proportion: Comparison of Seven Methods. Stats in Med. 1998;17:857–72. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 31.Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract. 2004;10:499–509. doi: 10.1111/j.1365-2753.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- 32.Lüders S, Schrader J, Berger J, et al. PHARAO Study Group. The PHARAO study: prevention of hypertension with the angiotensin-converting enzyme inhibitor ramipril in patients with high-normal blood pressure: a prospective, randomized, controlled prevention trial of the German Hypertension League. J Hypertens. 2008;26:1487–96. doi: 10.1097/HJH.0b013e3282ff8864. [DOI] [PubMed] [Google Scholar]
- 33.Lacourcière Y, Neutel JM, Davidai G, Koval S. A multicenter, 14-week study of telmisartan and ramipril in patients with mild-to-moderate hypertension using ambulatory blood pressure monitoring. Am J Hypertens. 2006;19:104–12. doi: 10.1016/j.amjhyper.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Hathial M. Safety and tolerability of ramipril 10 mg in patients at high risk of cardiovascular events: an observational study. Indian Heart J. 2008;60:200–4. [PubMed] [Google Scholar]
- 35.Joe SA, Thakkar K. Chronic rhinosinusitis and asthma. Otolaryngol Clin North Am. 2008;41:297–309. doi: 10.1016/j.otc.2007.11.001. [DOI] [PubMed] [Google Scholar]