Summary
Background
Matrix-Assisted Laser-Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS) has already proven to be a powerful tool for species identification in microbiological laboratories. As adequate and rapid screening methods for antibiotic resistance are crucially needed, the present study investigated the discrimination potential of MALDI-TOF MS among extended-spectrum-beta-lactamase (ESBL) or metallo-beta-lactamases- (MBL) producing and the nonproducing strains of Escherichia coli (n=19), Klebsiella pneumoniae (n=19), and Pseudomonas aeruginosa (n=38), respectively.
Material/Methods
We used a MALDI-TOF MS protocol, usually applied for species identification, in order to integrate a screening method for beta-lactamases into the routine species identification workflow. The acquired spectra were analyzed by visual inspection, statistical similarity analysis and support vector machine (SVM) classification algorithms.
Results
Neither visual inspection nor mathematical similarity analysis allowed discrimination between spectra of beta-lactamase-producing and the nonproducing strains, but classification within a species by SVM-based algorithms could achieve a correct classification rate of up to 70%.
Conclusions
This shows that MALDI-TOF MS has definite potential to discriminate antibiotic-resistant strains due to ESBL and MBL production from nonproducing strains, but this performance is not yet sufficiently reliable for routine microbiological diagnostics.
Keywords: MALDI-TOF MS, Enterobacteriaceae, Pseudomonas aeruginosa, ESBL, MBL, beta-lactamase
Background
Recent reports on new multidrug resistant bacteria from India (NDM-producing strains) show that adequate and rapid screening methods for antibiotic resistance due to those enzymes are crucially needed [1–3].
Beta-lactam antibiotics hydrolyzing enzymes, called beta-lactamases, have been known since the early 1950s and have been well-characterized since the 1980s [4, 5]. They are divided into 2 main groups based on description of their activity – Serine-beta-lactamases (Ambler class A, C and D) and metallo-beta-lactamases (Ambler class B). Enzymes of actual concern mostly derive from these 2 classes.
Ambler Class A extended-spectrum-beta-lactamases (ESBL) with a substrate specificity up to 3rd and 4th generation cephalosporins are a major cause of antibiotic resistance in Enterobacteriaceae and their presence is often linked to additional antibiotic resistance, such as fluoroquinolones and cotrimoxazole [5–9]. Escherichia coli and Klebsiella pneumoniae are the most common pathogens producing ESBLs and are ranking among the 3 most common pathogens causing nosocomial infections, predominantly urinary tract infections [7,9].
Ambler Class B metallo-Beta-Lactamases (MBLs) are a chief cause of antibiotic resistance in nonfermenting bacteria such as Pseudomonas aeruginosa, conferring resistance to all beta-lactams, including carbapenems [5,10]. MBL producing P. aeruginosa constitute a severe clinical threat since they often occur as multidrug-resistant pathogens in immunocompromised patients, as well as in those with cystic fibrosis [10,11].
E. coli, K. pneumoniae and P. aeruginosa are ranked among the 3 most common pathogens causing nosocomial infections, and their antibiotic resistance due to ESBLs and MBLs has become a worldwide problem [6,11,12]. Thus, diagnostic laboratories depend on systems suitable for rapid and exact identification of those resistance factors.
Currently, systems for the detection of ESBL- and MBL-producing bacteria are mostly phenotypical methods [13]. As a function of these methods, another incubation period and special growth media are necessary, leading to increased costs and time span until a specific antibiotic therapy can be initiated.
Matrix-Assisted Laser Desorption/Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) has already proven to be a powerful tool for species identification in microbiological laboratories [14–20]. Further studies attempted to evaluate the performance of MALDI-TOF MS in the detection of antibiotic-resistant strains, such as Methicillin-resistant Staphylococcus aureus (MRSA), but showed unsatisfactory results [21,22]. Subsequent publications showed the detection of ESBL-primer extensions by MALDI-TOF MS [23], revealed spectral distinctions among carbapenemase-gene harboring and non-harboring Bacteroides fragilis[24,25], and proved that MALDI-TOF MS can detect degradation products of antibiotics due to hydrolysis by beta-lactamases [26–28]. Most of the aforementioned protocols require further steps of incubation with antibiotics or primer extension prior to MALDI-TOF MS analysis, necessitating a longer time span compared to usual mass spectrometric species identification.
Camara et al were the first to demonstrate that MALDI-TOF MS can distinguish between wild-type and AmpC-plasmid-transformed strains of E. coli[29] in higher mass ranges – up to 30 kDalton (Da) – than actually recommended for species identification (ie, >20 kDa) [15,17]. The aim of the present study was to evaluate the performance of MALDI-TOF MS in the discrimination among ESBL-producing or MBL-producing and the nonproducing clinical isolates of E. coli, K. pneumoniae and P. aeruginosa, even in a mass range usual for species identification, in order to integrate the screening for resistance factors into routine species identification by MALDI-TOF MS.
Material and Methods
Chemicals
α-cyano-4-hydroxycinnamic acid was purchased from Bruker Daltonics, Bremen, Germany; trifluoroacetic acid (TFA) was from Merck, Darmstadt, Germany; and acetonitrile was from Sigma, Taufkirchen, Germany. Deionized water was used in all experiments. Columbia agar base and the other media used were purchased from Oxoid, Basingstoke, United Kingdom [20].
Bacterial strains, culture, phenotypic and resistance identification
A total of 76 clinical isolates of Enterobacteriaceae and P. aeruginosa were used in this study. Nineteen strains of E. coli, including 9 ESBL-producing strains, were recovered from urine (n=14), sputum (n=3), pharyngeal smear (n=1) and abscesses (n=1). Nineteen strains of K. pneumoniae, including 12 ESBL-producing strains, were recovered from urine (n=12), wound swabs (n=2), blood culture (n=1), bronchial lavage (n=1), and renal puncture (n=1). The 38 isolates of P. aeruginosa, of which 13 tested positive for MBL production, were recovered from tracheal secretions (n=13), wound swabs (n=10), sputum (n=6), bronchial lavages (n=4), vaginal swabs (n=2), urine (n=2), bile (n=2), and pleura puncture (n=1).
All isolates were subcultured at 37°C on 5% sheep blood agar. Biochemical identification was performed by ID 32 E and ID 32 GN systems (bioMérieux, Lyon, France), respectively. ESBL- and MBL-producing strains were identified by appropriate Etest (AB bioMérieux, Solna, Sweden). For further analysis, the strains were stored at −80°C in Cryobank preservation tubes (Mast Diagnostica GmbH, Reinfeld, Germany).
Standard samples
The following reference strains were used to validate appropriate species affiliation of the clinical samples: E. coli ATCC 25922, K. pneumoniae 911 (a strain from an interlaboratory test in Germany), and P. aeruginosa ATCC 27853.
Sample preparation for MALDI-TOF MS
The strains were cultivated on 5% sheep blood agar plates and incubated for 24 h at 37°C. Ten to 15 individual colonies were transferred into deionized water and washed twice. Afterwards, the sediment was dissolved in 50 μl 80% TFA and incubated for 10 min at ambient temperature. Then 150 μl of deionized water was added to reduce TFA-activity, followed by 200 μl acetonitrile. Thereafter, the samples were centrifuged at 13,000 rpm for 2 min; the supernatant was transferred into a new 1.5 ml Eppendorf tube, and then stored at −20°C. For further analysis, the thawed samples were dried in a vacuum centrifuge. The pellet was dissolved in 20 μl of 2.5% TFA/50% acetonitrile. One microliter of each sample was pipetted 5 times on a stainless steel MALDI target plate. After air drying, each sample was overlaid with 1 microliter of matrix (α-cyano-4-hydroxycinnamic acid as a saturated solution in 2.5% TFA/50% acetonitrile). The matrix/sample spots were crystallized by air drying [20].
MALDI-TOF MS parameters and data analysis
The mass spectra were acquired with an Autoflex II (Bruker Daltonics, Bremen, Germany) MALDI-TOF mass spectrometer with a nitrogen laser (λ=337nm) operated in the positive linear mode (delay 350 ns, voltage 20 kV; mass over charge ratio (m/z) ranging from 2,000 to 12,000) under control of FlexControl software (version 2.4; Bruker Daltonics). Each spectrum was obtained by averaging 500 laser shots acquired in automatic mode at the minimum laser power necessary for ionization of the samples. The calibration strain for each MALDI-TOF optical measurement was E. coli GSM 2163 from New England BioLabs. Data files were transferred to FlexAnalysis software (version 2.4; Bruker Daltonics) for automated peak extraction [20]. With this software, 50 peaks were automatically labeled in each spectrum according to their appearance above the background (threshold ratio 1.5). Correct labeling was controlled manually. Peak lists containing masses and intensities were exported as Excel files [20].
Cluster formation of the mass peaks
In order to refine spectra accuracy, peak lists were aligned for mass drift adjustment [20]. Briefly, a mass-dependent size of the mass window was used according to window size = sizeabs + (sizerel * peak mass) with sizeabs =0.8 m/z and sizerel =0.001. Thus, for each bacterial species we created a mean spectrum containing common m/z values. All spectra obtained for this species were aligned individually to the peaks of the mean spectrum by linear mass adjustment of the peaks [30]. Subsequently, peak clusters were formed which contained all peaks originating from different individual spectra but occurring in the same window. All peaks assigned to 1 cluster are represented by the respective mean cluster mass. This procedure represents the basis of the mass spectrometric approach.
Species identification and classification
For identification to the species level, similarity analysis between peak lists was carried out by using a hierarchical clustering procedure performed with MatLab software (Version 7.10.0.499; The Math-Works Inc., Natick, MA). Pair-wise comparison of the spectra determined the similarity between the latter by counting the number of clusters to which the 2 spectra contributed. By this procedure, a symmetric matrix of pair-wise similarities (peak mass-based similarity matrix) was formed. In addition, a similarity matrix (σi,j), which considers peak masses and differences in the peak intensities, was calculated according to the following equation:
The similarity of samples i and j was obtained over all clusters k contributing to either sample i or sample j.wki represents the intensity of peak i in spectrum k.
Distance matrices (δi,j) were calculated from normalized similarity matrices according to the following equation:
Dendrograms were calculated on the basis of the distance matrices by using a complete linkage function.
In order to discriminate between beta-lactamase producing and non-producing strains within a species, mass spectra were classified using the support vector machine (SVM) tool implemented in the Bioinformatic toolbox of MatLab. This software, which features an efficient 2-class classification, enables the user to define a number of parameters and to select from a choice of built-in kernel functions, including a radial basis function and a polynomial kernel (of a given degree). The SVM-algorithm was trained with a set of spectra of bacteria of known identity. An error estimate of the class prediction was carried out by calculation of a 10-fold cross-validation error for the training group. For this purpose, the training set was first divided into 10 subsets of equal size. Sequentially, 1 subset was tested by using the classifier trained on the remaining 9 subsets. Thus, each probe of the training set was predicted once. The cross-validation accuracy is the percentage of data which were correctly classified [20].
Results
In the mass spectra of the studied bacterial species the majority of the peaks were obtained below a mass over charge ratio (m/z) of 8,000; there were only few peaks above 10,000 and no peaks were observed above m/z 12,000 in a detection window up to m/z 20,000.
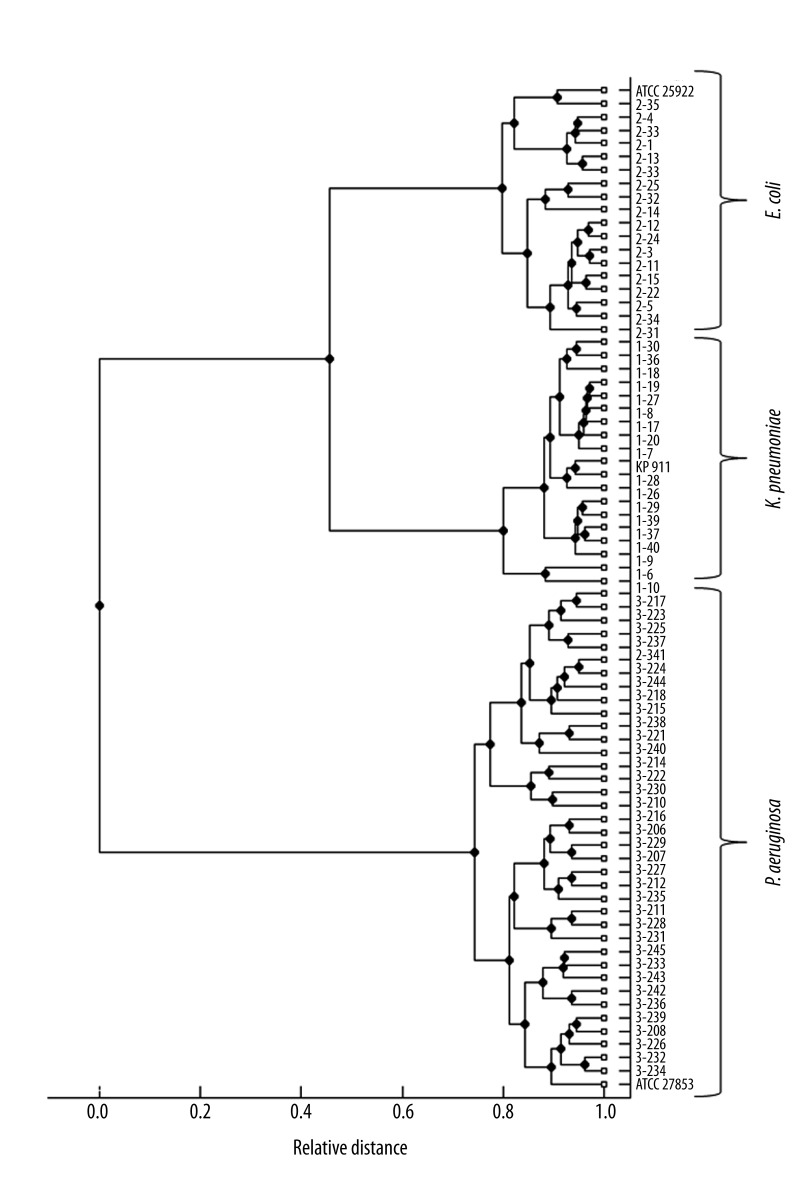
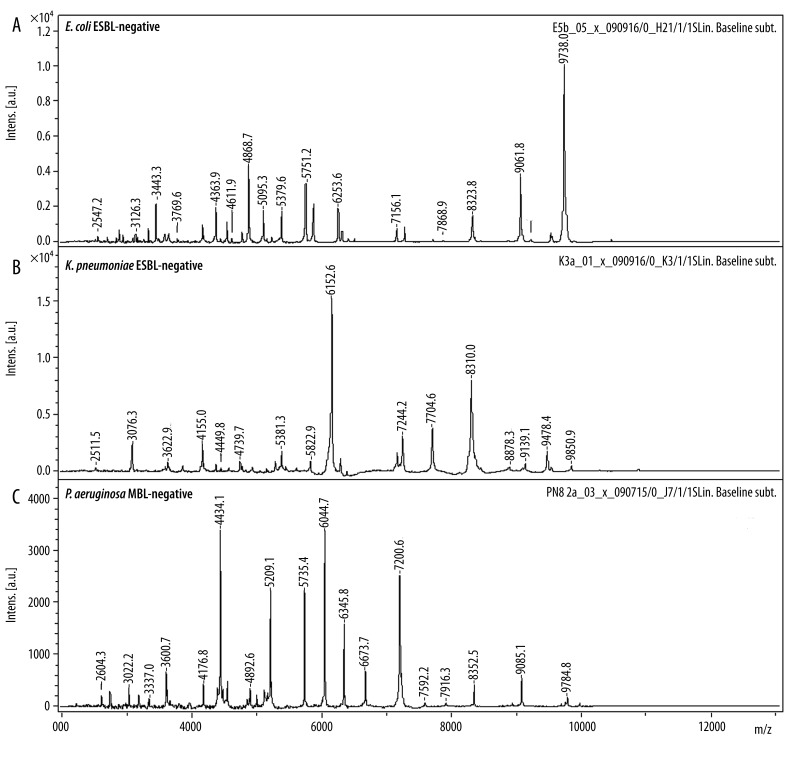
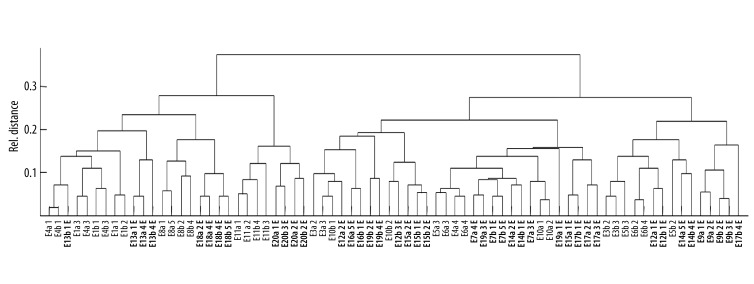
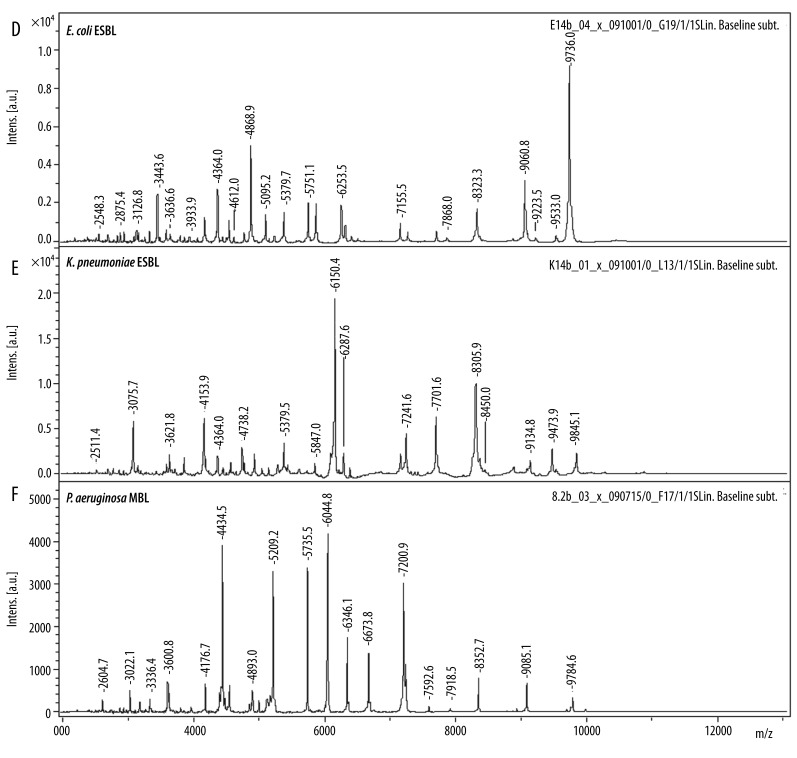
The reference and the collected strains of the 3 investigated species showed specific spectra which allowed a straightforward differentiation on the species level by similarity analysis (Figure 1). In a second step, the spectra of the strains that WERE previously tested positive for ESBL- and MBL-production were compared to those that tested negative. There was no significant difference in a first visual comparison of the spectra (Figure 2). In order to establish an objective differentiation method, similarity analysis was performed to see whether the ESBL- or MBL-positive strains form separable clusters in comparison to the nonproducing strains. As shown in Figure 3 for E. coli, the ESBL-positive strains did not form an individual cluster, but scattered among the non-producing strains. Similar results were found for K. pneumoniae and P. aeruginosa.
Figure 1.
Cluster analysis of the three investigated species’ mass spectra. Spectra of the reference strains are indicated. The cluster formation shows the relationship between E. coli and K. pneumoniae as members of the family Enterobacteriaceae and the clear separation to the genus P. aeruginosa.
Figure 2A–C.
MALDI-TOF mass spectra of nonproducing ESBL/MBL-negative strains. Mass spectra show no reproducible differences in the mass window we investigated.
Figure 3.
Inner species similarity analysis among wild type and ESBL-positive (printed in bold) strains of E. coli. ESBL-positive E. coli strains formed no individual cluster but are scattered among the ESBL-negative strains, allowing no dependable discrimination.
Moreover, we used SVM-based algorithms for classification as a more sensitive attempt to discover distinctions in the spectral patterns of the antibiotic resistant strains. For each species, 2 classes were defined to which the antibiotic susceptible and the antibiotic resistant strains were assigned (eg, class1: ESBL-negative, class 2: ESBL-positive). The spectra were classified by the algorithm according to their designated class. The classification performance was tested by 10-fold cross-validation, achieving rates for correct classification up to 70%.
Discussion
The correct classification rates achieved indicate that strains producing ESBLs and MBLs tend to form spectral patterns that are distinct from the nonproducing strains, but an accuracy of 70% does not allow a specific assignment of unknown samples to a single class. This indicates that neither statistical similarity analysis nor SVM-classification were able to reliably show significant distinctions in the spectral patterns of ESBL- or MBL-positive strains compared to the nonproducing strains of E. coli, K. pneumoniae and P. aeruginosa in terms of routine diagnostics.
Even randomly tested samples for peaks in higher mass-ranges up to 30,000 Da, as proposed by Camara et al. [29], showed no differences in the spectral patterns of the beta-lactamase-producing and the nonproducing strains (data not shown). The former success could be explained by a greatly increased protein synthesis level due to the in vitro plasmid-transformation, which cannot reflect the level of protein synthesis in clinical isolates already harboring those plasmids, and also partially by the utilization of a different matrix. Other studies with positive results [23,26–28] used protocols that require different steps of pre-analytical preparation or incubation and did not aim to integrate the screening method into the routine species identification workflow.
Conclusions
In conclusion, the MALDI-TOF MS protocol we used has potential to discriminate between beta-lactamase-negative strains of the species we investigated and strains producing ESBLs and MBLs, but it is not yet reliable enough for routine diagnostics. Thus, the integration of a dependable screening method for resistance factors into the routine species identification by MALDI-TOF MS could not satisfactorily be achieved with the protocol we used.
Figure 2D–F.
MALDI-TOF mass spectra of nonproducing corresponding ESBL/MBL-positive strains. Mass spectra show no reproducible differences in the mass window we investigated.
Footnotes
Source of support: Departmental sources
References
- 1.Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–54. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Göttig S, Pfeifer Y, Wichelhaus TA, et al. Global spread of New Delhi metallo-β-lactamase 1. Lancet Infect Dis. 2010;10:828–29. doi: 10.1016/S1473-3099(10)70275-9. [DOI] [PubMed] [Google Scholar]
- 3.Pfeifer Y, Witte W, Holfelder M, et al. NDM-1-producing Escherichia coli in Germany. Antimicrob Agents Chemother. 2011;55:1318–19. doi: 10.1128/AAC.01585-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambler RP. The Structure of Beta-Lactamases. Philosophical Transactions of the Royal Society B. Biological Sciences. 1980;289:321–31. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 5.Pfeifer Y, Cullik A, Witte W. Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens. Int J Med Microbiol. 2010;300:371–79. doi: 10.1016/j.ijmm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Winokur PL, Canton R, Casellas JM, Legakis N. Variations in the prevalence of strains expressing an extended-spectrum beta-lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clin Infect Dis. 2001;32:94–103. doi: 10.1086/320182. [DOI] [PubMed] [Google Scholar]
- 7.Stürenburg E, Mack D. Extended-spectrum beta-lactamases: Implications for the clinical microbiology laboratory, therapy, and infection control. J Infect. 2003;47:273–95. doi: 10.1016/s0163-4453(03)00096-3. [DOI] [PubMed] [Google Scholar]
- 8.DiPersio JR, Deshpande LM, Biedenbach DJ, et al. Evolution and dissemination of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae: Epidemiology and molecular report from the SENTRY Antimicrobial Surveillance Program (1997–2003) Diagn Microbiol Infect Dis. 2005;51:1–7. doi: 10.1016/j.diagmicrobio.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Pitout JDD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: An emerging public-health concern. Lancet Infect Dis. 2008;8:159–66. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 10.Mesaros N, Nordmann P, Plésiat P, et al. Pseudomonas aeruginosa: Resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect. 2007;13:560–78. doi: 10.1111/j.1469-0691.2007.01681.x. [DOI] [PubMed] [Google Scholar]
- 11.Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: Our worst nightmare? Clin Infect Dis. 2002;34:634–40. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 12.Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-beta-lactamases: The quiet before the storm? Clin Microbiol Rev. 2005;18:306–25. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradford PA. Extended-spectrum beta-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14:933–51. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holland RD, Wilkes JG, Rafii F, et al. Rapid identification of intact whole bacteria based on spectral patterns using matrix-assisted laser desorption/ionization with time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 1996;10:1227–32. doi: 10.1002/(SICI)1097-0231(19960731)10:10<1227::AID-RCM659>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Krishnamurthy T, Ross PL, Rajamani U. Detection of pathogenic and non-pathogenic bacteria by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 1996;10:883–88. doi: 10.1002/(SICI)1097-0231(19960610)10:8<883::AID-RCM594>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 16.Claydon MA, Davey SN, Edwards-Jones V, Gordon DB. The rapid identification of intact microorganisms using mass spectrometry. Nat Biotechnol. 1996;14:1584–86. doi: 10.1038/nbt1196-1584. [DOI] [PubMed] [Google Scholar]
- 17.Demirev PA, Ho YP, Ryzhov V, Fenselau C. Microorganism identification by mass spectrometry and protein database searches. Anal Chem. 1999;71:2732–38. doi: 10.1021/ac990165u. [DOI] [PubMed] [Google Scholar]
- 18.Fenselau C, Demirev PA. Characterization of intact microorganisms by MALDI mass spectrometry. Mass Spectrom Rev. 2001;20:157–71. doi: 10.1002/mas.10004. [DOI] [PubMed] [Google Scholar]
- 19.Rupf S, Breitung K, Schellenberger W, et al. Differentiation of mutans streptococci by intact cell matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Oral Microbiol Immunol. 2005;20:267–73. doi: 10.1111/j.1399-302X.2005.00223.x. [DOI] [PubMed] [Google Scholar]
- 20.Friedrichs C, Rodloff AC, Chhatwal GS, et al. Rapid identification of viridans streptococci by mass spectrometric discrimination. J Clin Microbiol. 2007;45:2392–97. doi: 10.1128/JCM.00556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards-Jones V, Claydon MA, Evason DJ, et al. Rapid discrimination between methicillin-sensitive and methicillin-resistant Staphylococcus aureus by intact cell mass spectrometry. J Med Microbiol. 2000;49:295–300. doi: 10.1099/0022-1317-49-3-295. [DOI] [PubMed] [Google Scholar]
- 22.Du Z, Yang R, Guo Z, et al. Identification of Staphylococcus aureus and determination of its methicillin resistance by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Chem. 2002;74:5487–91. doi: 10.1021/ac020109k. [DOI] [PubMed] [Google Scholar]
- 23.Ikryannikova LN, Shitikov EA, Zhivankova DG, et al. A MALDI TOF MS-based minisequencing method for rapid detection of TEM-type extended-spectrum beta-lactamases in clinical strains of Enterobacteriaceae. J Microbiol Methods. 2008;75:385–91. doi: 10.1016/j.mimet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Wybo I, Bel A, Soetens O, et al. Differentiation of cfiA-negative and cfiA-positive Bacteroides fragilis isolates by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49:1961–64. doi: 10.1128/JCM.02321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagy E, Becker S, Sóki J, et al. Differentiation of division I (cfiA-negative) and division II (cfiA-positive) Bacteroides fragilis strains by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Med Microbiol. 2011;60:1584–90. doi: 10.1099/jmm.0.031336-0. [DOI] [PubMed] [Google Scholar]
- 26.Burckhardt I, Zimmermann S. Using matrix-assisted laser desorption ionization-time of flight mass spectrometry to detect carbapenem resistance within 1 to 2.5 hours. J Clin Microbiol. 2011;49:3321–24. doi: 10.1128/JCM.00287-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hrabák J, Walková R, Studentová V, et al. Carbapenemase activity detection by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49:3222–27. doi: 10.1128/JCM.00984-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sparbier K, Schubert S, Weller U, et al. MALDI-TOF MS based functional assay for the rapid detection of resistance against β-lactam antibiotics. J Clin Microbiol. 2012;50:927–37. doi: 10.1128/JCM.05737-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camara JE, Hays FA. Discrimination between wild-type and ampicillin-resistant Escherichia coli by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Bioanal Chem. 2007;389:1633–38. doi: 10.1007/s00216-007-1558-7. [DOI] [PubMed] [Google Scholar]
- 30.Morris JS, Coombes KR, Koomen J, et al. Feature extraction and quantification for mass spectrometry in biomedical applications using the mean spectrum. Bioinformatics. 2005;21:1764–75. doi: 10.1093/bioinformatics/bti254. [DOI] [PubMed] [Google Scholar]