Summary
Background
Zoonotic Cutaneous Leishmaniasis (ZCL) is a polymorphic disease. It is generally accepted that bacterial superinfection may play a role in the clinical appearance of the lesions and may delay or prevent the healing process. However, the pattern of bacterial pathogens involved has rarely been investigated.
Material/Methods
The aim of this study was to identify the bacterial species contaminating the suspected ZCL and their susceptibility to commonly used antibiotics. Microscopic examination of stained smears and cultures were used to differentiate ZCL from non-ZCL lesions in a rural area north of Isfahan, Iran from July to December 2009. Bacteria were isolated from the lesions and identified and antibiotic susceptibility was determined by standard microbiological techniques.
Results
The results show that 602 (68%) of 855 patients were positive for ZCL, of which 83.4% with volcano-shape, 8.8% psoriasiform, 6.6% popular form and 1.2% with other atypical forms of ZCL. The bacteria were isolated from 66.8% of ZCL (70% of volcano-shape, 60% of psoriasiform and 25% of popular form) and 64.7% of non-ZCL lesions. The most common species were Staphylococcus aureus (41.7%) and S. epidermidis (28%) followed by Bacillus sp. Streptococcus pyogenes, Escherichia coli, Klebsiella sp., Proteus sp., Enterobacter sp. and Pseudomonas aeroginosa. Ciprofloxacin, Erythromycin, Cefazolin and Clindamycin were the most effective antibiotics.
Conclusions
Bacterial superinfection appears to be very common in ZCL, but its prevalence is not different from that of non-ZCL lesions and it has little effect on the clinical appearance of anthroponotic cutaneous Leishmaniasis (ACL). Local lesion care and management of bacterial superinfection must be considered in the treatment of ZCL.
Keywords: Cutaneous Leishmaniasis, bacterial superinfection, Leishmania major
Background
Zoonotic Cutaneous Leishmaniasis, a vector-borne protozoal infection, is among the most important causes of chronic ulcerating skin lesions in the tropics. It is a major public health problem in many parts of the world, including Iran [1,2]. The diseases are hyperendemic in rural areas, where it may cause considerable morbidity. ZCL shows various clinical manifestations, ranging from asymptomatic infection without apparent lesions, to extensive lesions that may cause severe disfiguring [3–5]. It is generally accepted that secondary bacterial infection of the ZCL lesions may a factors that can influence the size, shape and severity of these skin lesions and also may delay or prevent the healing process [6,7]. Furthermore, it has been suggested that Leishmania parasites that are present in the skin lesions may induce a local suppressive effect, providing a better condition for the growth and survival of contaminating bacteria [8]. However, the scant evidence regarding the relationship between the secondary bacterial infection and cutaneous leishmaniasis is currently restricted to the work of El-on et al. and Edrissian et al. [9,10]. The first report is based on an investigation in experimentally infected mice and the later is the only field study on the bacterial superinfection in ZCL [9]. Therefore, because of the lack of a systematic bacteriological investigation, in the present study the secondary bacterial infection associated with the 3 clinically different forms of ZCL lesions and their susceptibility to common antibiotics were studied in naturally infected human hosts and the results were compared to non-ZCL skin lesions in patients living under similar environmental conditions in the same geographical area.
Material and Methods
Study area and population
The subjects of the study were all patients aged between 1 to 50 years (average, 9 years) with clinically suspected ZCL lesions and a disease history of 2 to 4 weeks, living in the Borkhar area, a rural district of northern Isfahan, Iran, where ZCL is highly endemic [2,11,12]. They were referred to the research center for skin diseases, Isfahan University of Medical Sciences, Isfahan, Iran by primary health care centers and private clinics, for microscopic diagnosis during a 6-month period from July to December 2009, the season during which ZCL is most common in the area [5].
Sampling and culturing methods
The lesions and the surrounding skin areas were disinfected with a single piece of cotton wool moistened with 70% alcohol. For parasitological investigation, 3 samples were collected from the edge of the lesions using a flame-sterilized lancet. Two samples were used to prepare methanol-fixed and Giemsa-stained smears for direct microscopy and observation of amastigotes. The third sample was inoculated into sterile screw top tubes containing 10 ml of Novey-Nicol-Mac Neal (NNN) medium [13] and incubated at 20–25°C. They were examined microscopically for the development of promastigotes at 2-day intervals for 2 weeks [14].
The skin lesions were regarded as non-ZCL skin lesion when no amastigotes was found in the smears after careful examination and promastigotes were not observed in the culture media after 2 weeks. No attempt was made to determine the cause of non-ZCL skin lesions. Clinically different forms of ZCL were named according to [6] with respect to their size, shape and severity of the lesions. For bacterial examination, 3 sterile cotton swabs wetted with sterile saline were moved from the center to the edge of lesions immediately after collection of samples for parasitological studies. The samples were transferred to nutrient broth (NB; Merck, Germany), blood agar (BA; Merck, Germany) and MacConkey agar (MC; Merck, Germany) separately and incubated at 37°C for 24 to 72 hours under aerobic conditions. Identification of colonies was done by Gram staining and standard biochemical tests as described by [15]. The susceptibility of bacteria to 16 common antibiotics was assessed using the Kirby-Bauer method [15] and Muller Hinton agar medium (MHA; Merck, Germany).
Statistical analysis
The Fisher’s exact test was used to evaluate and analyze the data.
Result
Amastigotes were observed in Giemsa-stained smears taken from 602 (68%) of 885 patients with clinically suspected cases of ZCL who attended Isfahan research center for skin diseases during a 6 month-period from July to December 2009. The clinical feature of the ZCL-positive skin lesions is summarized in Table 1. The median age of 9 years of the patients reflects the age distribution of ZCL in the area. Table 2 shows the prevalence of 3 clinically distinguished different forms of ZCL and the distribution of secondary bacterial infection in these lesions.
Table 1.
The clinical feature of 3 different forms of ZCL present in Borkhar area, Isfahan, Iran.
| Type of ZCL* | Main characteristics | Size** | Duration*** | Site**** |
|---|---|---|---|---|
| Volcano-shape | Raised turgid margins. Central serous crust Resembles to a flattened Volcano. Painless | 2–3 | 2–4 | Face, Foot Leg, Hand |
| Psoriasiform | Superficial with raised patches of skin covered with silvery scales Resembles psoriasis, Painless Firm-levated erythematous | 6–8 | 2–4 | Face Hand Foot Face |
| Papular form | Papule with exudative Surface, Plainless | <0.5 | 3–4 | Upper arm |
The shape was named according to Griffithis (1987);
size of ulcerated area expressed in cm;
number of weeks after first appearance of the lesions according to the patients;
the most common site of the lesions.
Table 2.
Prevalence of clinically different forms of ZCL and the distribution of bacterial superinfection in Borkhar area Isfahan, Iran.
| Type of ZCL | Number and% of patients | ||
|---|---|---|---|
| Total | Infected | Non infected | |
| Volcano-shape | 502 (83.4)* | 355 (88.3) | 147 (73.5) |
| Psoriasiform | 53 (8.8) | 32 (8) | 21 (10.3) |
| Popular form | 40 (6.6) | 10 (2.5) | 30 (15) |
| Other atypical form | 7 (1.2) | 5 (1.2) | 2 (1) |
| Total | 602 (100) | 402 (100) | 200 (100) |
Figures in brackets are percentages.
Among 602 patients with ZCL and 283 patients with non-ZCL skin lesions, bacteria were isolated from the skin lesions of 402 (66.8%) and 183 (64.7%), respectively. The secondary bacterial infections were observed in about 70% of volcano-shape, 60% of psoriasiform and 25% of popular forms of ZCL. Table 3 shows the 9 bacterial species isolated from the lesions. Staphylococcus aureus and S. epidermidis were the most common bacteria isolated from both ZCL and non-ZCL lesions, followed by Bacillus sp. and Streptococcus pyogenes.
Table 3.
Bacterial species isolated from ZCL and non-ZCL lesions in Borkhar area Isfahan, Iran.
| Bacterial | ZCL lesions %n=453 | Non-ZCL Lesions %n=415 |
|---|---|---|
| Staphylococcus aureus | 189 (41.7)* | 170 (41) |
| Staphylococcus epidermidis | 127 (28) | 110 (26.5) |
| Bacillus sp. | 36 (7.9) | 10 (2.4) |
| Streptococcus pyogenes | 30 (6.6) | 27 (6.5) |
| Escherichia coli | 24 (5.3) | 20 (4.8) |
| Klebsiella sp. | 19 (4.2) | 38 (9.2) |
| Proteus sp. | 11 (2.4) | 10 (2.4) |
| Enterobacter sp. | 10 (2.2) | 20 (4.8) |
| Pseudomonas aeroginosa | 7 (1.5) | 10 (2.4) |
The bacterial species isolated from clinically different forms of ZCL is summarized in Table 4. Staphylococcus aureus and S. epidermidis were isolated from all 3 types of ZCL and they were the only species isolated from the popular form. The bacterial species isolated from volcano-shape and psoriasiforms were similar, except for Bacillus sp. and Proteus sp., which were isolated from volcano shape and S. pyogenes, which was isolated from psoriasiform (Table 4).
Table 4.
Bacterial species isolated from 3 clinically different forms of ZCL.
| Volcano-shape | Psoriasiform | Popular form |
|---|---|---|
| S. aureus | S. aureus | S. aureus |
| S. epidermidis | S. epidermidis | S. epidermidis |
| Bacillus sp. | E. coli | |
| E. coli | Klebsiella sp. | |
| Klebsiella sp. | Enterobacter sp. | |
| Proteus sp. | P. aeroginosa | |
| Enterobacter sp. | S. pyogenes | |
| P. aeroginosa |
Only 1 bacterial species was isolated from the skin lesions of 337 (83.8%) patients with bacterial contaminated ZCL and 153 (83.6%) with non-ZCL lesions. Mixed infection with 2 or 3 bacterial species were observed in 59 (14.7%) and 6 (1.5%) of the ZCL lesions and 28 (15.3%) and 2 (1.1%) of the non-ZCL lesions, respectively. The most common combination was S. aureus and S. epidermidis.
The results of in vitro testing of bacterial resistance to 16 common antibiotics are summarized in Table 5.
Table 5.
Percentage resistance of bacteria isolated form ZCL lesions to common antibiotics.
| Antibiotics | Gram Positive Bacteria | Gram Negative Bacteria | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sa | Se | Sp | Bs | Ec | Ks | Es | Pa | Ps | |
| Amoxcillin | 87 | 89 | 17 | 41 | 99 | 60 | 94 | 91 | 94 |
| Ampicillin | 92 | 85 | 14 | 49 | 95 | 86 | 10 | 91 | 92 |
| Cefazolin | 12 | 20 | 10 | 27 | 20 | 18 | 16 | 25 | 11 |
| Ceftizoxime | 25 | 18 | 11 | 19 | 30 | 38 | 50 | 85 | 71 |
| Cephalothin | 13 | 11 | 20 | 28 | 65 | 62 | 51 | 75 | 38 |
| Ciprofloxacin | 8 | 10 | 12 | 14 | 20 | 17 | 13 | 13 | 15 |
| Clindomycin | 40 | 45 | 36 | 26 | 20 | 11 | 15 | 13 | 14 |
| Cloxacillin | 45 | 24 | 18 | 11 | 38 | 29 | 25 | 14 | 12 |
| Chloramphenicol | 60 | 50 | 35 | 43 | 50 | 70 | 81 | 93 | 85 |
| Cotrimaxozole | 83 | 90 | 35 | 35 | 80 | 78 | 70 | 91 | 93 |
| Erythromycin | 12 | 19 | 22 | 10 | 60 | 58 | 27 | 53 | 51 |
| Gentamycin | 36 | 30 | 20 | 12 | 55 | 60 | 29 | 71 | 59 |
| Kanamycin | 30 | 25 | 10 | 13 | 55 | 38 | 31 | 53 | 15 |
| Neomycin | 23 | 22 | 12 | 10 | 30 | 32 | 20 | 30 | 49 |
| Penicillin | 96 | 90 | 8 | 21 | 93 | 94 | 92 | 97 | 98 |
| Tetracycline | 71 | 63 | 35 | 43 | 89 | 60 | 81 | 62 | 69 |
Sa – Staphylococcus aureus; Se – Staphylococcus epidermidis; Sp – Streptococcus pyogenes; Bs – Bacillus sp.; Ec – Escherichia coli; Ks – Klebsiella sp.; Es – Entrobacter sp.; Pa – Pseudomonas aeroginosa; Ps – Proteus sp.
No obvious differences were observed in the pattern of resistance to antibiotics between the bacteria isolated from ZCL and non-ZCL skin lesions.
The results of the present study show that secondary bacterial infections were not present in about 30%, 40% and 70% of volcano- shape, psoriasiform and popular forms of ZCL, respectively. Furthermore, the bacterial species isolated from volcano-shape and psoriasiform of ZCL were similar, and no obvious differences were observed between the clinical appearance of those with and without bacterial infection, except a more severe inflammatory reaction in the surrounding skin of those with bacterial infections. These observations suggest that the secondary bacterial infection does not have an important effect on the shape, size and severity of the skin lesions in ZCL (Figures 1–3).
Figure 1.
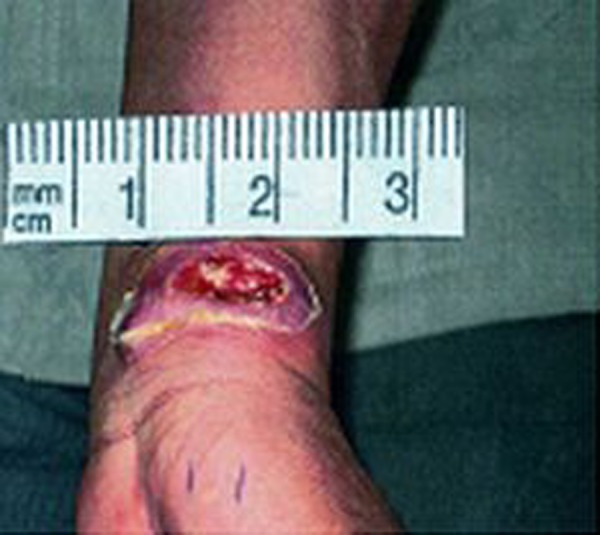
Volcano shape.
Figure 3.
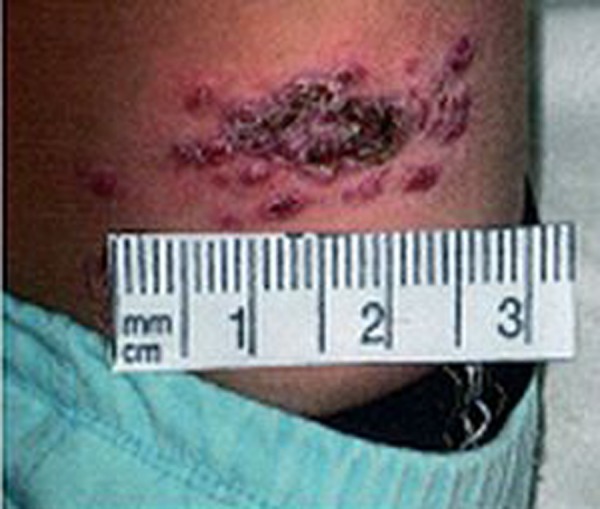
Popular form.
Discussion
Many authors believe that secondary bacterial infection of skin lesions in ZCL is common, particularly in areas where the disease is endemic. However, very little empirical evidence has been provided by systematic bacteriological studies. In fact, the only field study is that reported by Edrissian et al. [10], who reported secondary bacterial infection in 26.5% of ZCL skin lesions in military personnel deployed in Khuzestan province, an endemic area for ZCL in southwest Iran. Therefore, the present report is the first study in which native patients with clinically different forms of ZCL were examined bacteriologically and compared with non-ZCL skin lesions in the patients living in the same geographical area under similar condition.
In the present study, the results show that ZCL skin lesions are infected with bacterial pathogens in 66.8% of cases, and the differences in the prevalence of bacterial infection in ZCL lesions and non-ZCL lesions (64.7%) is not statistically significant (P<0.001). This finding confirms that in most cases the ZCL lesions are contaminated with potentially pathogenic bacteria, but does not support the idea that ZCL lesions are more susceptible to secondary bacterial infection as a result of a local immunosuppression induced by the parasite.
Nine different species of bacteria were isolated from both the ZCL and non-ZCL lesions and S. aureus and S. epidermidis were predominant, followed by Bacillus sp., S. pyogenes, E. coli, Klebsiella sp., Enterobacter sp. and P. aeroginosa, respectively, in both cases. The high frequency of S. aureus and S. epidermidis in both ZCL and non-ZCL lesions were not unexpected, as they are common on the skin and mucous surface of healthy people. They live harmlessly on the host, but when the skin is broken for any reason, they can enter the wound and cause an infection. These bacteria produce enzymes such as fibrinolysins, hyaluronidase and lipases, which break down components of tissue and facilitate invasion [16,17]. Most of these infections are minor, causing a mild to sever inflammation, as was observed in the present study; however, they can cause serious infections and under particular circumstances these bacteria should indeed be considered as true pathogens [18].
The rather high isolation rates of Enterobacteriaceae could be due to the use of night-soil as fertilizer on farms, resulting in an environment contaminated with fecal material. Furthermore, lack of hygiene, sewage disposal, walking barefoot and playing on the farms, particularly by children, provide favorable conditions for colonization of the skin, especially of the feet and hands, by these bacteria. This also may explain the total absence of these bacteria in popular form of ZCL, which are mostly located on the face and upper arms, with the consequence of less exposure to infection.
Although anaerobic cultures were not used in this study, microscopic examinations of Gram-stained smears did not suggest presence of any important anaerobic bacteria. This is not surprising in view of the erythema frequently surrounding the lesions, resulting in increased blood flow with a high oxygen concentration that inhibits the growth of anaerobic bacteria. Moreover, in the vast majority of cases the wound infections are caused by aerobic bacteria due to the lower number of anaerobic bacteria on the skin or in the environment [17].
The present study indicates that both Gram-positive and Gram-negative bacteria (except S. pyogenes) are highly resistant to penicillin, ampicillin and amoxicillin. Ciprofloxacin, cefazolin, and erythromycin are the most effective antibiotics against Gram-positive bacteria, and ciprofloxacin and clindamycin are the most effective antibiotics against Gram-negative bacteria.
Bacterial resistance to antibiotics has been widely investigated all over the world, but the present study is the first in this area and there is no previous information for comparison. However, in many cases, studies are difficult to compare due to the differences in patient demographics, etiologies and infections status of ulcers.
Conclusions
In conclusion, the present findings indicate that bacterial superinfection is very common in ZCL, but not more so than non-ZCL skin lesions. The bacterial superinfection has little if any effect on clinical appearance of ZCL which is a polymorphic disease. Local lesion care and management of secondary bacterial infection are essential and anti-Leishmanial therapy in ZCL may be more effective when combined with antibiotics.
Figure 2.
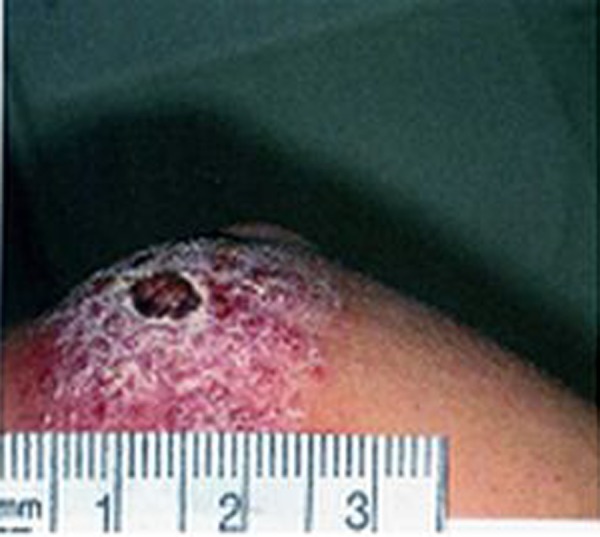
Psoriasiform.
Acknowledgments
We would thank from Dr. Mohammad Ali Nilforushzadeh, Head of the Skin and Leishmaniasis Center for providing the facilities and Ms. Leila Shirani-bidabadi for helping us in sampling. We also acknowledge Dr. Seyed Hossein Hejazi, School of Medicine, Isfahan University of Medical Sciences.
Footnotes
Source of support: Departmental sources
References
- 1.Desjeux P. The increase in risk factors for Leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001;95:239–43. doi: 10.1016/s0035-9203(01)90223-8. [DOI] [PubMed] [Google Scholar]
- 2.Yaghoobi-Ershadi MR, Javadian E. Zoonotic Cutaneous Leishmaniassis to the north of Isfahan. Bulletin. Societe. Patholigie. Exotique. 1995;88:42–43. [PubMed] [Google Scholar]
- 3.Kebaier C, Louzir H, Chenik M, et al. Heterogeneity of Wild Leishmania major Isolates in Experimental Murine Pathogenicity and Specific Immune Response. Infecti Immun. 2001;69:4906–15. doi: 10.1128/IAI.69.8.4906-4915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alrajhi AA. Cutaneous Leishmaniasis of the Old World. Skin Therapy Lett. 2003;8:1–4. [PubMed] [Google Scholar]
- 5.Baghaei M. Intraspecific Variation in Leishmania major Isolated from Different Forms of Zoonotic Cutaneous Leishmaniasis. Iran J Med Sci. 2005;30:51–54. [Google Scholar]
- 6.Griffiths WAD. Old World Cutaneous Leishmaniasis. In: Peters W, Kellick-Kendrick R, editors. The Leishmaniasis in Biology and Medicine. Vol. 2. London: Academic; 1987. pp. 617–36. [Google Scholar]
- 7.Bailey MS, Lockwood DN. Cutaneous leishmaniasis. Clin Dermatol. 2007;25:203–11. doi: 10.1016/j.clindermatol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Shahian M, Alborzi A. Effect of meglumine antimoniant on the pancreas during treatment of visceral leishmaniasis in children. Med Sci Monit. 2009;15(6):CR290–93. [PubMed] [Google Scholar]
- 9.El-on J, Sneier R, Elias E. Leishmania major: bacterial contamination of Cutaneous Lesions in experimental animals. Isr J Med Sci. 1992;28:847–51. [PubMed] [Google Scholar]
- 10.Edrissian GH, Mohammadi M, Kanani A, et al. Bacterial infections in suspected Cutaneous Leshmaniasis lesions. Bull World Health Organ. 1990;68:473–77. [PMC free article] [PubMed] [Google Scholar]
- 11.Tashakori M, Ajdary S, Kariminia A, et al. Characterization of Leishmania Species and L. major Strains in different endemic areas of Cutaneous Leishmaniasis in Iran. Iran Biomed J. 2003;7:43–50. [Google Scholar]
- 12.Paravizi P, Mauricoi I, Aransay AM, et al. First detection of Leishmania major in peridomestic Phlebotomus papatasi from Isfahan province, Iran: comparison of nested PCR of nuclear ITS ribosomal DNA and semi-nested PCR of minicircle kinetoplast DNA. Acta Trop. 2005;93:75–83. doi: 10.1016/j.actatropica.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Minodier P. Cutanous Leishmaniasis treatment. Travel Med Infect Dis. 2007;5:150–58. doi: 10.1016/j.tmaid.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Ash LR, Orihel TC. Parasites, a guide to laboratory procedures and identification. American Society of Clinical Pathologists; Chicago: 1987. [Google Scholar]
- 15.Baron EJ, Finegold SM. Diagnostic Microbiology. 8th ed. 1997. pp. 171–94.pp. 323–408. [Google Scholar]
- 16.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 17.Doudi M, Hejazi SH, Razavi MD, et al. Comparetive molecular epidemiology of Leishmania major and Leishmania tropica by PCR-RFLP technique in hyper endemic cities of Isfahan and Bam. Iran Med Sci Monit. 2010;16(11):CR530–35. [PubMed] [Google Scholar]
- 18.Lapins J, Jarstrand C, Emestam L. Coagulase-negative staphylococci are the most common bacteria found in cultures from the deep portions of hidradenitis suppurativa lesions, as obtained by carbon dioxide laser surgery. Br J Dermatol. 1999;140:90–95. doi: 10.1046/j.1365-2133.1999.02613.x. [DOI] [PubMed] [Google Scholar]


