Summary
Gorlin-Goltz syndrome is a rare genetic condition showing a variable expressiveness. It is inherited in a dominant autosomal way. The strongest characteristic of the disease includes multiple basal cell carcinomas, jaw cysts, palmar and plantar pits, skeletal abnormalities and other developmental defects. Owing to the fact that the condition tends to be a multisystemic disorder, familiarity of various medical specialists with its manifestations may reduce the time necessary for providing a diagnosis. It will also enable them to apply adequate methods of treatment and secondary prevention. In this study, we present symptoms of the disease, its diagnostic methods and currently used treatments.
We searched 2 scientific databases: Medline (EBSCO) and Science Direct, for the years 1996 to 2011. In our search of abstracts, key words included nevoid basal cell carcinoma syndrome and Gorlin-Goltz syndrome.
We examined 287 studies from Medline and 80 from Science Direct, all published in English. Finally, we decided to use 60 papers, including clinical cases and literature reviews.
Patients with Gorlin-Goltz syndrome need particular multidisciplinary medical care. Knowledge of multiple and difficult to diagnose symptoms of the syndrome among professionals of various medical specialties is crucial. The consequences of the disease pose a threat to the health and life of patients. Therefore, an early diagnosis creates an opportunity for effective prevention and treatment of the disorder. Prevention is better than cure.
Keywords: Gorlin-Goltz syndrome (GGS), nevoid basal cell carcinoma syndrome (NBCCS), basal cell carcinoma (carcinoma basocellulare - BCC), keratocystic odontogenic tumour (KCOT)
Background
Gorlin-Goltz syndrome (GGS), also referred to as the nevoid basal cell carcinoma syndrome (NBCCS), is an infrequent inherited disease with a broad range of clinical symptoms, thus this multidisciplinary disorder constitutes a true challenge for medical specialists and, in particular, to dermatologists and dentists who often become primary care physicians for GGS patients.
The characteristic symptoms of the syndrome were first recorded by Jarish in 1894. In the 1960s, Gorlin and Goltz described them as a triad of disorders including multiple basal cell carcinoma, numerous keratocysts in the jaws and skeletal abnormalities, which gave rise to the Gorlin-Goltz syndrome designation [1–3]. Further research revealed a whole range of its clinical manifestations, consequences, and genetic background.
We searched 2 scientific databases – Medline (EBSCO) and Science Direct – for the years 1996 to 2011. In our search of titles and/abstracts, we used such key words as nevoid basal cell carcinoma syndrome and Gorlin-Goltz syndrome to select adequate scientific materials among clinical cases and literature reviews published in English.
Our search revealed 287 studies from Medline and 80 from Science Direct. We tried to choose the newest and, in our opinion, the most interesting papers, which presented the issue most extensively and precisely. Apart from earlier recalled articles, we decided to use slightly older sources, including articles by R. Gorlin (due to their educational value) and a few Polish-language articles. Moreover, we used information included on the website www.gorlingroup.co.uk and www.emedicine.com/PED/topic890.htm. Finally, we used 60 papers to prepare this article. The text below has been structured in a number of sections referring to: etiology and occurrence, symptoms and complications, treatment of a BCC, treatment of a KCOT, treatment of a medulloblastoma, discussion and conclusions.
Etiology and occurrence
Disregarding the most popular designation of the disease suggested by professor Gorlin (nevoid basal cell carcinoma syndrome), in 10% of patients no basal cell carcinoma develops in the skin [4,5].
In the scientific papers published in English there are many designations of the syndrome, which often stem from its symptoms (Table 1) [6].
Table 1.
Synonims of Gorlin-Goltz syndrome.
| Designations of the Gorlin-Goltz syndrome used in the scientific papers | |
|---|---|
| Basal cell naevus (carcinoma) syndrome | Multiple hereditary cutaneomandibular polyoncosis |
| Epithelioma naevique multiple | Multiple naevoid basal-cell carcinoma syndrome |
| Fifth phakomatosis | Naevous epitheliomatodes multiplex |
| Gorlin syndrome12 | Nevoid basal cell carcinoma syndrome |
| Hereditary cutaneo-mandibular polyoncosis | Nevoid basal cell carcinoma epithelioma – jaw cysts |
| Hermans-Grosfeld-Spaas-Valk syndrome | Multiple bifid rib syndrome |
| Multiple basal-cell carcinoma syndrome | Ward syndrome II |
| Multiple basal-cell naevi syndrome | |
NBCCS is a genetic disorder inherited in a dominant autosomal way [1,4,7,8]. Although its occurrence among family members an important diagnosing criteria, it has been found that between 20% and 40% of cases result from a de novo mutation of the PTCH1 [9q22.3] gene [4,9–13]. According to the current state of knowledge, mutations of other genes such as Patched2 [PTCH2], Smoothened [SMO] and Sonic Hedgehog [SHH], observed also in relation to basal cell carcinoma and medulloblastoma [7,13,14], may exert a certain influence on the occurrence of the syndrome.
The assumed prevalence of the disease is 1:60,000; however, in various studies its values range from 1:57,000 (in England) to 1:164,000, and even 1:256,000 (in Italy). The syndrome occurs with an equal frequency in men and women and in almost all ethnic groups except for the Caucasian race, which is most often affected by it [1,2,4–6,9]. NBCCS is sometimes diagnosed in very young patients, but in most cases it occurs in people aged between 17 and 35 years [1,15]. The condition is very difficult to diagnose in early childhood because its symptoms appear gradually as the child grows [3,16].
Symptoms and Complications
Skin anomalies
Basal cell carcinoma (BCC)
Multiple basal cell carcinoma of the skin constitutes the most characteristic feature of the syndrome. The highest incidence rate is observed in people between puberty and age 35, although it was also observed in children ages 3 to 4 years. It is diagnosed in 90% of Caucasians age 40 or older [4,17] and in 40% of the Negroid population [10,18,19]. The number of BCC lesions varies from several to thousands [10], their diameter ranges from 1 mm to 10 mm, and they may have various forms from skin-coloured nodules or papules to ulcerating plaques. They are usually located on the face, back and chest, but they may also be found on skin not exposed to the sun [10]. Aggressive forms of basal cell carcinomas, which infiltrate the facial bones, hardly ever occur [20]. The above-mentioned lesions are extremely challenging for therapists but, thanks to the combined efforts of various medical specialists such as maxillofacial surgeons, plastic surgeons, laryngologists, oncologists, radiation oncologists, restorative dental specialists and psychologists, the patients have a chance to recover and regain their regular social functions [8,15,21].
Milia
In 30% of patients, milia (small cysts filled with keratin) appear on the face, just below the eyes, and less frequently on the forehead [4,5,10].
Palmar and plantar pits
The presence of palmar (70%) and/or plantar (50%) pits is a very important diagnostic factor. They are small, with a diameter ranging from 2 to 3 mm and depth from 1 to 3 mm. They are red at the bottom in Caucasians and black in Negroids. From 30% to 65% of cases involve children under 10, but the prevalence in the age group above 20 years is 85%. The number of pits increases with age. They become more visible after the palms have been held in warm water for about 10 minutes [4,5,10].
Keratocystic odontogenic tumour (KCOT)
The most important manifestations of Gorlin-Goltz syndrome within the oral cavity are recurrent multiple jaw tumours called keratocysts. The lesions occur in as many as 90% of patients above age 40 [10,22]. They are most frequently located in the mandible − 44% are found in the mandibular angles and 18% in the zones adjacent to incisor and canine teeth [10,23]. In the maxilla, they accompany canines and incisors (15%), as well as molars (14%) (Figure 1). In spite of their less frequent occurrence as compared to in the mandible, they are more aggressive than those in the lower jaw area.
Figure 1.
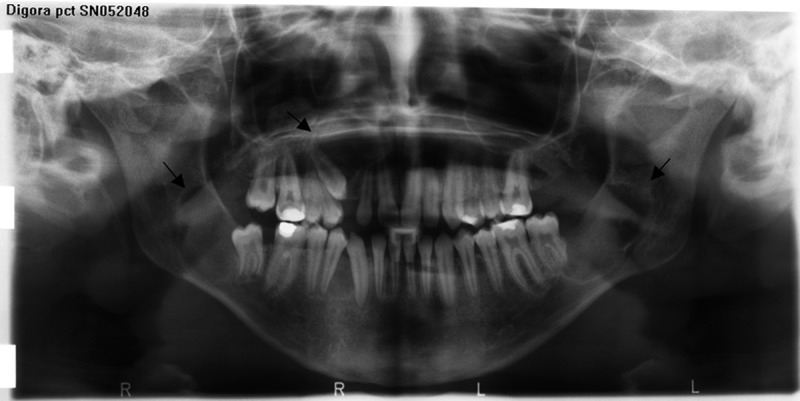
Panoramic view reveals multiple KCOT’s in patient with inherited GGS (daughter of T.S.)
The KCOTs are divided into parakeratotic, orthokeratotic, and (rarely) mixed and solid lesions, and they are differentiated based on a histological image of the cells lining them. The tumour consists of a thin fibrous external pouch, whose interior is lined with a stratified squamous epithelium of a parakeratotic (96%) type [23,24]. The orthokeratotic form of tumour seldom (in 4% of cases) occurs, has a milder course and considerably fewer recurrences. That is why, according to the WHO regulations, the orthokeratotic form of the lesion is classified as an odontogenic cyst and the parakeratotic form is considered a benign neoplasm [23]. The cavity of the tumour is filled with thick keratinous material or a straw-coloured fluid [24]. The tumours are usually diagnosed accidentally during routine X-ray examinations performed in the course of a regular dental treatment [25]. Inflammatory symptoms occurring within the tumour sometimes force the patient to consult a medical practitioner, and this enables a faster diagnosis of KCOT [26].
An X-ray image of a KCOT in its early stage shows a spherical or oval unilocular lytic bone lesion often involving a wisdom tooth (Figure 2). It is well circumscribed and has a well-defined osteosclerotic rim, which may become less visible while the lesion grows and transforms into a multilocular form [25,27]. The latter form of the tumour needs to be differentiated from ameloblastoma [24,28]. In the course of its growth, the tumour causes bony expansion that may result in deformation or asymmetry of the facial structures. In spite of a considerable size of the tumours, pathological bone fractures hardly ever occur. Other rare anomalies in the oral cavity include occlusal problems related to the adjustment, shape and number of teeth, as well as mild mandibular prognathism manifested in soft tissues by protrusion of the lower lip [29]. In many cases, a high palatal arch or a close relationship between the canal and the lower border of the mandible [30] were observed. Less frequent symptoms included cleft lip, cleft palate [31] and alveolar process [27,29], as well as other deformities of alveolar processes caused by tumours. Tumours developing within the nasal sinuses may lead to a deteriorated patency of nasal passages [32]. It has been recently noted that patients with GGS have a bilateral hyperplasia of the coronoid process of the temporomandibular joint, which constitutes a useful diagnostic criterion, especially in the assessment of pediatric patients [10,33].
Figure 2.
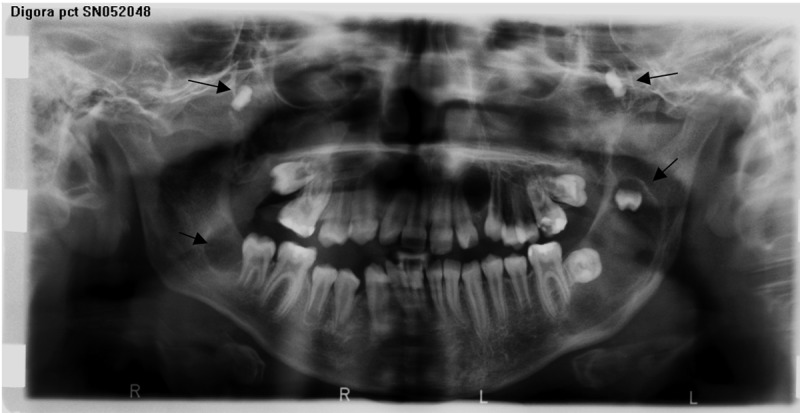
Panoramic view of the patient W.J. with GGS de novo, revealing multiple KCOT’s in both jaws involving and replacing germs of the molars.
Apart from the earlier mentioned symptoms of NBCCS, patients may have numerous disorders affecting various systems and organs (Table 2) [2,4,5,10,17,22,25,27,29,30,34–39].
Table 2.
Other manifestations of NBCCS.
| Calcifications of the central nervous system and other lesions |
| Calcification of: |
| Cysts of the choroid plexus, third and lateral cerebral ventricles |
| Agenesis of corpus callosum |
| Meningioma |
| Medulloblastoma |
| Multiform glioblastoma |
| Astrocytoma |
| Foetal rhabdomyosarcoma |
| Grand mal |
| Congenital hydrocephalus |
| Mental retardation ~5% patients with NBCCS [10] |
|
|
| Ophtalmic and otologic anomalies |
| 20% patients with GGS |
| Hypertelorism 70% |
| Microcysts on eyelids |
| Congenital cataract, |
| Strabismus, |
| Nystagmus, |
| Orbital cysts |
| Congenital blindness. |
| Otosclerosis, |
| Conductive hearing loss |
| Posteriorly angulated ears |
|
|
| Skeletal anomalies |
| Significant height – average for females is 174 cm and for males 183 cm [30] |
| Increased pneumatisation of the paranasal sinuses (in particular frontal sinuses) |
| Increased head circumference [30,34] 50% |
| Strongly marked superciliary arches |
| Retracted and a wide base of the nose typical for pseudohypertelorism (in 5–40% cases true hypertelorism were reported) [22,25,35] |
| Wide eyes 70% |
Congenital skeletal anomalies:
|
| Spina bifida occulta 40–60% [5,10] |
| Sternal protrusion or depression 30–40% of patients [5] |
| Cysts within the phalanxes, long bones, pelvis and even calvaria – the symptoms may create an impression that the bone is occupied by medulloblastoma cells [10] |
|
|
| Urogenital anomalies |
25–50% of affected ♀ reveal ovarian cysts and fibromas:
|
|
|
| Gastro-enteric system |
| Limphomesenteric cysts Ø 2–14 cm, asymptomatic |
| Gastric polyps |
|
|
| Cardio-vascular system |
| Cardiac fibroma [22,36]: Absent internal carotid artery |
Treatment of a BCC
Owing to the possible occurrence of a varied number of neoplastic lesions, the patients must be provided with optimized treatment adjusted to the clinical conditions. The best method should result in a high percentage of successfully treated patients and a short period of healing. It should also save the biggest area of healthy skin possible, leave no scars and cause no adverse effects [40]. In spite of continually developing new and enhancing traditional treatment procedures (Table 3) [4,5,10,41–48], we should not forget prophylaxis. Prevention consists in following certain principles – patient self-control, avoiding sun, using UV sun block or wearing sun glasses and sun-protective clothing [4,5].
Table 3.
| Curettage and electrodessication |
|
| Cryosurgery |
|
| Laser ablation (CO2 laser vaporization) |
|
| Surgical excision |
|
| Mohs micrographic surgery |
|
| Photodynamic therapy (PDT) |
|
| Ionizing radiation |
|
| Chemotherapy of bcc: | |
| 5% imiquimod cream | |
| 0.1% tretinoin cream |
|
| 5-fluorouracil cream |
|
| SHH (sonic hedgehog) antagonist, | |
| Oral retinoid (isotretinoin) |
|
| Interferon |
|
Treatment of a KCOT
Keratocystic odontogenic tumour therapy depends on several important factors, including: the age of the patient; size, extent and location of the lesion; and possible perforation of the cortical bone lamellae or soft tissue infiltration. The methods may be divided into conservative, aggressive and radical. Conservative treatment consists of a regular enucleation of tumours from their bony beds in the course of a 1- or 2-stage procedure, which is used in the cases of ordinary intraosseous cysts [49]. Unfortunately, due to the presence of satellite micro-tumours in the surrounding bone, this method has the highest recurrence rate [7,49]. Much better results can be obtained if enucleation is followed by a chemical or mechanical curettage of the surrounding bone. For that purpose, either liquid nitrogen (−70°C) or Carnoy’s solution – a mixture of 6 ml of absolute alcohol, 3 ml of chloroform, 1 ml of glacial acetic acid and 1 g of ferric chloride [50] – may be applied to the bone cavity. In the case of Carnoy’s solution, the recurrence rate does not exceed 2%. Cryotherapy, however, has an 11% recurrence rate. Carnoy’s solution is most often used in the mandible, as in the maxilla the method involves the risk of necrosis of the mucosa lining the maxillary sinus or nasal cavity. Disregarding the above-mentioned facts and taking into account his clinical experience, Stoelinga considers the application of this method to be equally successful within the maxilla, as well as effective and relatively safe [51]. Radical treatment methods involve partial resection of the tumour-invaded bone together with a 5-millimetre margin of healthy bone tissue and are, undoubtedly, associated with the lowest recurrence rate. Nevertheless, in children whose tooth eruption or bone forming processes have not finished, the radical procedures should be replaced by conservative ones [7,52,53]. Whenever dental practitioners encounter cases of multiple or recurrent cysts (Figure 3), they are obliged to provide such patients with comprehensive dental care and carry out diagnostic tests or refer them for such tests, because the cysts might be the first noticeable symptoms of the nevoid basal cell carcinoma syndrome (GGS) [1,7,24].
Figure 3.
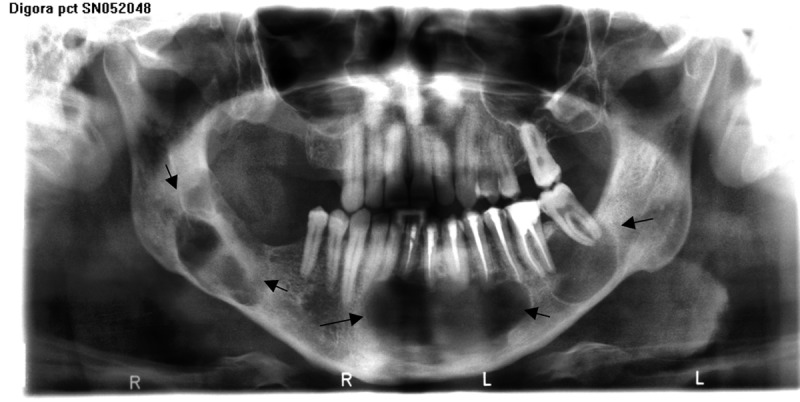
Panoramic view reveals KCOT’s in patient (T.S. father) with GGS.
Treatment of a Medulloblastoma
Medulloblastoma is a malignant tumour of the posterior cranial fossa, typically occurring in children between 7 to 8 years of age, whereas in people with Gorlin syndrome it occurs during the first 3 years of life [5,10]. Estimated prevalence of this disorder is 2%, and it is 3 times higher in boys than in girls [22]. It is assumed that about 10% of the patients in whom medulloblastoma was diagnosed at an early age have Gorlin syndrome. Early diagnosis of medulloblastoma should always lead to a suspicion of NBCCS. The best results of treatment are obtained when the treatment procedure combines aggressive tumour resection with both chemo- and radiotherapy [54]. The latter form is controversial because it induces invasive/multiple squamous cell carcinomas in the skin area submitted to radiation, as well as numerous neoplastic lesions in adjacent tissues [55–57]. Therefore, it should be avoided if possible or replaced with non-conformal radiation techniques conserving the skin, although their adverse effects include ototoxicity or radiation injury of the temporal lobes. Most of the GGS-associated medulloblastoma are desmoplastic lesions with a milder course and better prognoses [according to the clinical reports, the patient may even have a spontaneous recovery [54]], which is an important reason for why radiation therapy should not be used in such cases [4,5,10,56]. GGS rarely results in premature death (10%) but, if it does, the death is usually caused by medulloblastoma [4,5] or an X-ray therapy of invasive basal cell carcinomas that leads to secondary dissemination and re-initiates carcinogenesis of skin lesions, which in effect also causes death of the patient [4].
Discussion
Gorlin-Goltz syndrome (GGS) is a condition whose management requires the involvement of many different health professions. It may seem that the numerous symptoms of this disease make diagnosis a very simple task, but, in fact, it is quite difficult. Its variable expressiveness is proportional to the age of patients. Therefore, genetic tests are a matter of key importance for formulating adequate diagnosis, in particular during the first years of lives of the youngest patients. In most cases, however, GGS is detected using clinical criteria such as the presence of 2 symptoms of high importance or 1 of high and 2 or 3 symptoms of little importance (Table 4) [4,5,16,21,58,59].
Table 4.
Symptoms of GGS.
| The symptoms of high importance include: | The symptoms of little importance include: |
|---|---|
|
|
If there is a suspicion that the disease found in a child results from a de novo mutation, detailed radiological examinations of relatives need to be carried out. In the case where no abnormalities are detected, genetic tests should be the most helpful tool, and can provide definitive information [60].
In pregnant women at a high risk for GGS, ultrasonographic examination may reveal fetal anomalies such as increased head circumference or cardiac fibroma; however, they are extremely rare at that stage of development. In such cases, prenatal diagnosis may be formulated based on the genetic material collected in the course of amniocentesis performed between the 15th and 18th week of pregnancy or chorionic villus sampling (CVS) done between the 10th and 12th week of pregnancy [10]. Nevertheless, prenatal diagnosis is very rare and the necessary condition of its performance is a confirmed presence of a disease-causing allele in a patient’s relative [12].
After birth, the child needs to have a careful clinical examination. If GGS is suspected, X-ray imaging is needed to show deformities of the rib or vertebrae. An echocardiogram is also recommended in order to exclude or confirm the presence of cardiac fibromas [25].
When the result of family medical history is positive, a newborn child should undergo a detailed assessment aimed at finding significant symptoms of GGS. If there is a suspicion of NBCCS in an adult patient, they too need to be carefully examined (Table 5) [1,4,5,10,16,21,35,59].
Table 5.
| a.i.1. A detailed medical and, in particular, dental history; |
a.i.2. A number of clinical examinations including:
|
| a.i.3. Genetic tests: PTCH (Patched) SMO (Smmothened), SHH (Sonic hedgehog); |
a.i.4. Radiological examinations:
|
| a.i.5. USG (ultrasonography) of the abdominal cavity and pelvis minor (focused on finding ovarian and mesentery fibromas and cysts); |
| a.i.6. USG (ultrasonography), ECG (electrocardiogram) of the heart (in search of fibromas) |
| a.i.7. Patients education: raising the awareness of the patient about one’s illness and the promotion pro-healthy behaviours and the self-control |
Conclusions
A patient with Gorlin-Goltz syndrome needs particular multidisciplinary medical care and what’s more he should understand nature of the problem. In the case of adults, self-control is crucial for maintaining good health, as it allows the noticing of even very subtle changes. Awareness of the risk related to radiation enables them to avoid its harmful influence by using UV filters and other available protective agents.
Medical specialists’ knowledge of the multiple and difficult to diagnose symptoms of the syndrome is a matter of key importance. The consequences of the disease pose a threat to both the health and life of a patient. Therefore, an early diagnosis creates an opportunity for effective prevention and treatment. Prevention is better than cure.
Footnotes
Source of support: Self financing
References
- 1.Baliga SD, Rao SS. Nevoid-basal cell carcinoma syndrome: a case report an overview on diagnosis and management. J Maxillofac Oral Surg. 2009;9:82–86. doi: 10.1007/s12663-010-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jawa DS, Sircar K, Somani R, et al. Gorlin-Goltz syndrome. J Oral and MaxilloFac Pathol. 2009;13:89–92. doi: 10.4103/0973-029X.57677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karthiga KS, Sivapatha Sundharam B, Manikandan R. Nevoid basal cell carcinoma syndrome. Indian J Dent Res. 2006;17:50–53. doi: 10.4103/0970-9290.29891. [DOI] [PubMed] [Google Scholar]
- 4.Walter AW. Gorlin Syndrome. www.emedicine.com/PED/topic890.htm.
- 5.www.gorlingroup.co.uk
- 6.Scully C, Langdon J, Evans J. Marathon of eponyms: 7 Gorlin-Goltz Syndrome (Naevoid basal-cell carcinoma syndorme) Oral diseases. 2010;16:117–18. doi: 10.1111/j.1601-0825.2009.01539.x. [DOI] [PubMed] [Google Scholar]
- 7.Casaroto AR, Loures DC, Moreschi E, et al. Early diagnosis of Gorlin-Goltz syndrome: case report. Head Face Med. 2011;25(7):1–5. doi: 10.1186/1746-160X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manfredi M, Vescovi P, Bonanini M, Porter S. Nevoid basal cell carcinoma syndrome: a review of the literature. Int J Oral and Maxillofac Surg. 2004;33:117–24. doi: 10.1054/ijom.2003.0435. [DOI] [PubMed] [Google Scholar]
- 9.Song YL, Zhang WF, Peng B, et al. Germline mutations of the PTCH gene in families with odontogenic keratocysts and nevoid basal cell carcinoma syndrome. Tumour Biol. 2006;27:175–80. doi: 10.1159/000093054. [DOI] [PubMed] [Google Scholar]
- 10.Muzio L. Nevoid Basal Cell Carcinoma Syndrome(Gorlin Syndrome) Orphanet J Rare Dis. 2008;3:32–48. doi: 10.1186/1750-1172-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acocella A, Sacco R, Bertolai R, Sacco N. Genetic and clinicopathologic aspects of Gorlin-Goltz syndrome (NBCCS): presentation of two case reports and literature review. Minerva Stomatologica. 2009;58:43–53. [PubMed] [Google Scholar]
- 12.Le Brun Keris Y, Jouk PS, Saada-Sebag G, et al. Prenatal manifestation in a family affected by nevoid basal cell carcinoma syndrome. Eur J Med Genet. 2008;51:472–78. doi: 10.1016/j.ejmg.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Huang YF, Chen YJ, Yang HW. Nevoid basal cell carcinoma syndrome – case report and genetic study. J Dent Sciences. 2010;5:166–70. [Google Scholar]
- 14.Cohen MM., Jr Nevoid basal cell carcinoma syndrome: molecular biology and new hypotheses. Int J Oral and Maxillofac Surg. 1999;28:216–23. doi: 10.1034/j.1399-0020.1999.283280314.x. [DOI] [PubMed] [Google Scholar]
- 15.Honavar SG, Shields JA, Shields CL, et al. Basal cell carcinoma of the eyelid associated with Gorlin-Goltz syndrome. Ophthalmolog. 2001;108:1115–23. doi: 10.1016/s0161-6420(01)00560-7. [DOI] [PubMed] [Google Scholar]
- 16.Veenstra-Knol HE, Scheewe JH, van der Vlist GJ, et al. Early recognition of basal cell naevus syndrome. Eur J Pediatr. 2005;164:126–30. doi: 10.1007/s00431-004-1597-4. [DOI] [PubMed] [Google Scholar]
- 17.Evans DG, Ladusans EJ, Rimmer S, et al. Complications of the naevoid basal cell carcinoma syndrome: results of a population based study. J Med Genet. 1993;30:460–64. doi: 10.1136/jmg.30.6.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulkarni P, Brashear R, Chuang T. Nevoid basal cell carcinoma syndrome in a person with dark skin. J Am Acad Dermatol. 2003;49:332–35. doi: 10.1067/s0190-9622(03)00415-8. [DOI] [PubMed] [Google Scholar]
- 19.Hall J, Johnston KA, McPhillips JP, et al. Nevoid basal cell carcinoma syndrome in a black child. J Am Acad Dermatol. 1998;38:363–65. doi: 10.1016/s0190-9622(98)70585-7. [DOI] [PubMed] [Google Scholar]
- 20.Ortega García de Amezaga A, García Arregui O, Zepeda Nuño S, et al. Gorlin-Goltz syndrome: clinicopathologic aspects. Med Oral Patol Oral Cir Bucal. 2008;13:338–43. [PubMed] [Google Scholar]
- 21.Nagy K, Kiss E, Erdei C, et al. Complex care by multiple medical and dental specialists of a patient with aggressive Gorlin-Goltz syndrome. Postgrad Med J. 2008;84:330–32. doi: 10.1136/pgmj.2007.066282. [DOI] [PubMed] [Google Scholar]
- 22.Gorlin RJ. Nevoid basal cell carcinoma (Gorlin) syndrome. Genet Med. 2004;6:530–39. doi: 10.1097/01.gim.0000144188.15902.c4. [DOI] [PubMed] [Google Scholar]
- 23.Brzozowski F, Wanyura H, Stopa Z, Kowalska K. Odontogenic keratocysts in the material of the Department of Craniomaxillofacila Surgery, Medical University of Warsaw. Czas Stomatol. 2010;2:69–78. [Google Scholar]
- 24.Kaczmarzyk T, Stypułkowska J, Tomaszewska R, Zapała J. Nowotwory zębopochodne i guzy nowotworopodobne kości szczękowych. Wyd Kwintesencja sp. z.o.o; Warszawa: pp. 81–96. [in Polish] [Google Scholar]
- 25.Lazaridou MN, Dimitrakopoulos I, Tilavreridis I, et al. Basal cell carcinoma arising with a maxillary keratocyst in a patient with Gorlin-Goltz syndrome. Report of a case. Oral Maxilliofac Surg. 2012;16(1):127–31. doi: 10.1007/s10006-011-0270-0. [DOI] [PubMed] [Google Scholar]
- 26.Ahn SG, Lim YS, Kim DK, et al. Nevoid basal cell carcinoma syndrome: a retrospective analysis of 33 affected Korean individuals. Int J Oral Maxillofac Surg. 2004;33:458–62. doi: 10.1016/j.ijom.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Jędrusik-Pawłowska M, Adamczyk W, Łangowska-Adamczyk H, Borgile-Marek H. Rodzinne występowanie zespołu Gorlina-Goltza. Czas Stomat. 2002;4:229–36. [Google Scholar]
- 28.Eslami B, Lorente C, Kieff D, et al. Ameloblastoma associated with the nevoid basal cell carcinoma (Gorlin) syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:10–13. doi: 10.1016/j.tripleo.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 29.Maroto MR, Porras JLB, Saez RS, et al. The role of the orthodontist in the diagnosis of Gorlin’s syndrome. Am J Orthod Dentofacial Orthop. 1999;115:89–98. doi: 10.1016/s0889-5406(99)70321-5. [DOI] [PubMed] [Google Scholar]
- 30.Dahl E, Kreiborg S, Jensen BL. Craniofacial morphology in the nevoid basal cell carcinoma syndrome. Int J Oral Surg. 1976;5:300–10. doi: 10.1016/s0300-9785(76)80031-2. [DOI] [PubMed] [Google Scholar]
- 31.Lambrecht JT, Kreusch T. Examine your orofacial cleft patients for Gorlin-Goltz syndrome. Cleft Palate Craniofac J. 1997;34:342–50. doi: 10.1597/1545-1569_1997_034_0341_eyocpf_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 32.al-Anazy FH, Zakzouk SM. Otolaryngological manifestation of Gorlin Goltz syndrome. J Laryngol Otol. 1997;111:286–89. doi: 10.1017/s0022215100137119. [DOI] [PubMed] [Google Scholar]
- 33.Leonardi R, Sorge G, Caltabiano M. Bilateral hyperplasia of the mandibular coronoid processes assiociated with the nevoid basla cell carcinoma syndrome in an Italian boy. Br Dent J. 2001;190(7):349–50. doi: 10.1038/sj.bdj.4800970. [DOI] [PubMed] [Google Scholar]
- 34.Wang XX, Zhang J, Wei FC. Familial multiple odontogenic keratocysts. J Dent Child. 2007;74:140–42. [PubMed] [Google Scholar]
- 35.Gorlin RJ. Nevoid basal cell carcinoma syndrome. Dermatol Clin. 1995;13:113–25. [PubMed] [Google Scholar]
- 36.Doede T, Seidel J, Riede FT, et al. Occult, life-threatening, cardial tumor in syndactylism in Gorlin Goltz syndrome. J Pediatr Surg. 2004;39:17–19. doi: 10.1016/j.jpedsurg.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 37.Morse CB, McLaren JF, Roy D, et al. Ovarian preservation in a young patient with Gorlin syndrome and multiple bilateral ovarian masses. Fertil Steril. 2011;96:47–50. doi: 10.1016/j.fertnstert.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 38.Ball A, Wenning J, Van Eyk N. Ovarian Fibromas in Pediatric Patients With Basal Cell Nevus (Gorlin) Syndrome. J Pediatric Adolescent Gynecol. 2011;24:5–7. doi: 10.1016/j.jpag.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Ramaglia L, Morgese F, Pighetti M, Saviano R. Odontogenic keratocyst and uterus bicornis in nevoid basal cell carcinoma syndrome: case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:217–19. doi: 10.1016/j.tripleo.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 40.van der Geer S, Krekels GAM, Verhaegh ME. Treatment of the Patient with Nevoid Basal Cell Carcinoma Syndrome in a Megasession. Dermatol Surg. 2009;35:709–13. doi: 10.1111/j.1524-4725.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 41.Dębski T, Lembas L, Jethon J. Basal cell carcinoma. Current views (Part II). Diagnostics and treatment. Postępy Nauk Medycznych. 2009;XXII(9):706–13. [Google Scholar]
- 42.Kopera D, Cerroni L, Fink-Puches R, Kerl H. Different treatment modalities for the management of a patient with the nevoid basal cell carcinoma syndrome. J Am Acad Dermatol. 1996;34:937–39. doi: 10.1016/s0190-9622(96)90085-7. [DOI] [PubMed] [Google Scholar]
- 43.Kamińska-Budzińska G, Brzezińska-Wcisło L, Wyględowska-Kania M, Lis A. Współczesne kierunki w leczeniu raków skóry. Przegląd Dermatol. 2002;2:127–31. [in Polish] [Google Scholar]
- 44.Mougel F, Debarbieux S, Ronger-Savlé S, et al. Methylaminolaevulinate photodynamic therapy in patients with multiple basal cell carcinomas in the setting of Gorlin-Goltz syndrome or after radiotherapy. Dermatology. 2009;219:138–42. doi: 10.1159/000228316. [DOI] [PubMed] [Google Scholar]
- 45.Friedrich RE. Diagnosis and treatment of patients with nevoid basal cell carcinoma syndrome [Gorlin-Goltz syndrome (GGS)] Anticancer Res. 2007;27:1783–87. [PubMed] [Google Scholar]
- 46.Ferreres JR, Macaya A, Jucglà A, et al. Hundreds of basal cell carcinomas in a Gorlin-Goltz syndrome patient cured with imiquimod 5% cream. J Eur Acad Dermatol Venereol. 2006;20:877–78. doi: 10.1111/j.1468-3083.2006.01552.x. [DOI] [PubMed] [Google Scholar]
- 47.Evans DG, Farndon PA. GeneReviews™. Nevoid Basal Cell Carcinoma Syndrome. [Internet] [Google Scholar]
- 48.Heretsch P, Tzagkaroulaki L, Giannis A. Modulators of the hedgehog signaling pathway. Bioorg Med Chem. 2010;18:6613–24. doi: 10.1016/j.bmc.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 49.Mendes RA, Carvalho JF, van der Waal I. Characterization and management of the keratocystic odontogenic tumor in relations to its histopathological and biological features. Oral Oncology. 2010;45:219–25. doi: 10.1016/j.oraloncology.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Ribeiro O, Jr, Borba AM, Alves CAF, Guimaraes J., Jr Carnoy’s solution over the inferior alveolar nerve as a complementary treatment for keratocystic odontogenic tumors. Rev Clin Pesq Odontol. 2007;3:199–202. [Google Scholar]
- 51.Stoelinga PJW. The treatment of odontogenic keratocysts by excision of the overlying, attached mucosa, enucleation, and treatment of the bony defect with Carnoy solution. J Oral Maxillofac Surg, 2005. 1988;63:1662–66. doi: 10.1016/j.joms.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Wilson C, Murphy M. Conservative management of multiple keratocystic odontogenic tumours in a child with Gorlin-Goltz syndrome: a case report. Eur J Paediatr Dent. 2008;9:195–98. [PubMed] [Google Scholar]
- 53.Dixit S, Acharya S, Dixit PB. Multiple odontogenic keratocysts associated with Gorlin-Goltz syndrome. Kathmandu Univ Med J (KUMJ) 2009;7:414–18. doi: 10.3126/kumj.v7i4.2765. [DOI] [PubMed] [Google Scholar]
- 54.Su CW, Lin KL, Hou JW, et al. Spontaneous recovery from a medulloblastoma by a female with Gorlin-Goltz syndrome. Pediatr Neurol. 2003;28:231–34. doi: 10.1016/s0887-8994(02)00618-5. [DOI] [PubMed] [Google Scholar]
- 55.Lopes NN, Caran EM, Lee ML, et al. Gorlin-Goltz syndrome and neoplasms: a case study. J Clin Pediatr Dent. 2010;35:203–6. doi: 10.17796/jcpd.35.2.x01248284w166485. [DOI] [PubMed] [Google Scholar]
- 56.Wallin JL, Tanna N, Misra S, et al. Sinonasal carcinoma after irradiation for medulloblastoma in nevoid basal cell carcinoma syndrome. Am J Otolaryngol. 2007;28:360–62. doi: 10.1016/j.amjoto.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Walter AW, Pivnick EK, Bale AE, Kun LE. Complications of the Nevoid Basal Cell Carcinoma Syndrome: A Case Report. J Pediatr Hematol Oncol. 1997;19(3):258–62. doi: 10.1097/00043426-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki R, Miyashita T, Matsumoto N, et al. Multiple keratocystic odontogenic tumors with nevoid basal cell carcinoma syndrome having distinct PTCH1 mutations: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:e41–46. doi: 10.1016/j.tripleo.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 59.Dębski T, Jethon J. Zespół Gorlina-Goltza-opis przypadku. Pol Merk Lek. 2010;XXVIII(168):466–69. [PubMed] [Google Scholar]
- 60.Díaz-Fernández JM, Infante-Cossío P, Belmonte-Caro R, et al. Basal cell nevus syndrome. Presentation of six cases and literature review. Med Oral Patol Oral Cir Bucal. 2005;10:57–66. [PubMed] [Google Scholar]


