Summary
Background
The aim of this study was to investigate the expression patterns of estrogen receptor (ER) β1 (wild-type ERβ) and ERβ2 (ERβcx) in papillary thyroid cancer (PTC) and nodular thyroid goiter (NTG), and to explore the reasons for the higher incidence of PTC in women of reproductive age.
Material/Methods
ERβ1 and ERβ2 expression was examined immunohistochemically on paraffin-embedded thyroid tissues from 106 patients with PTC and 30 patients with NTG.
Results
There was significant difference in the subcellular localization of ERβ1 (P<0.001), but not in the positive percentage, between PTC and NTG specimens. No significant difference was found in the positive percentage or the subcellular distribution of ERβ2 expression between PTC and NTG specimens. Both nuclear and nucleocytoplasmic ERβ1 expressions were significantly lower in PTC lesions than in NTG tissue (P<0.001 and P<0.05, respectively), while ERβ2 expression was significantly higher in the former than the latter (P<0.05). ERβ1 expression in reproductive-aged (18~45 years) female patients with PTC was lower than that in age-matched male patients (P<0.05), while ERβ2 expression had the opposite expression profile (P<0.05). There was no significant difference in ERβ1 and ERβ2 expression between reproductive-aged and advanced reproductive-aged (>45 years) female patients with PTC.
Conclusions
This preliminary study indicates that the expression patterns of ERβ1 and ERβ2 differ between malignant PTC lesions and benign NTG tissue, and their expression might be involved in the female predominance of PTC during the reproductive years. The clinical and biological significance of these results await further investigation.
Keywords: estrogen receptor β, splice variants, thyroid gland, tumors, goiter
Background
Thyroid cancer represents the most frequent endocrine malignancy, and its incidence has significantly increased in the past several decades [1]. Papillary thyroid cancer (PTC) is the most common type of thyroid cancer, accounting for 80% of all thyroid cancers. The incidence of thyroid cancer is 3 to 5 times more frequent in women than in men. This female predominance, which is greatest during reproductive age, is observed in all geographical areas and ethnic groups [2]. The use of oral contraceptives appears to result in a moderately increased risk of developing thyroid cancer [3–5]. An elevated risk was also reported in women who used estrogens for gynecological problems, but not for low-dose estrogen replacement therapy in postmenopausal women [3,6]. In fact, the incidence of thyroid cancer decreases after menopause [7]. The difference in incidence between the sexes suggests that the growth and progression of thyroid tumors is influenced by female sex hormones, particularly estrogen, which has been clearly implicated in the development and progression of breast, endometrial, and prostate cancers [8–10].
Estrogens are the predominant sex hormones in females. They exert their action in target tissues via binding to 1 of the 2 estrogen receptors (ER), ERα or ERβ. Like other steroid hormone receptors, ERs act as dimers to regulate transcriptional activation. Full transcriptional activation by ERs is mediated by synergism between 2 activation domains, Activation Function-1 (AF-1) at the N terminus and AF-2 in the ligand-binding domain. Both ERα and ERβ contain the potent AF-2 function, but unlike ERα, ERβ seems to have a weaker corresponding AF-1 function, and thus depends more on the ligand-dependent AF-2 for its transcriptional activation function [11]. Recently, several splice variants of ERβ with truncations or insertions in the C-terminal ligand-binding domain (ERβ1~ ERβ5) have been described and widely studied in breast, endometrial, and prostate cancers [8–10]. Among the exon 8 splice variants of ERβ, the expression and function of ERβ2 (ERβcx) is well-documented. It appears that ERβ2 lacks the AF-2 core region and does not bind to ERβ ligands, but does bind to estrogen responsive elements (EREs) as a homodimer and as a heterodimer with either wild-type ERα or ERβ [12,13]. ERβ2 preferentially forms a heterodimer with ERα rather than with ERβ, inhibiting DNA binding by ERα. ERβ2 shows a dominant negative activity on transactivation mediated only by ERα [12,14,15]. The possible molecular mechanism is that ERβ2 induces proteasome-dependent degradation of ERα, presumably through the formation of ERβ2/ERα heterodimers [16].
ERs have been described in both neoplastic and non-neoplastic human thyroid tissues by immunohistochemical studies. In PTC, ERα expression is significantly higher in premenopausal women than in post-menopausal women and in men of various ages [17]. No consistent findings were reported about the correlation between ERβ expression and clinicopathological findings including age, menopausal status, sex, and/or histological type of thyroid lesions [17,18]. To the best of our knowledge, the expression of ERβ splice variants in PTC has not yet been evaluated by immunohistochemistry in the English literature.
In this paper, we used immunohistochemical method to determine the expression of ERβ1 (wild-type ERβ) and the C-terminal truncated splice variant ERβ2 (ERβcx) in benign and malignant thyroid tissues, and to further explore the reasons for the higher incidence of PTC in women of reproductive age.
Material and Methods
Clinical material
Thyroid specimens were obtained from 106 patients with PTC and 30 patients with NTG who were first admitted to our hospital with ≤3 years’ duration and who underwent a standard thyroidectomy between 2007 and 2010. Patient records were obtained from the Medical Records Department at our hospital and also from pathology reports. Their diagnoses were confirmed by histopathological examination. None of these patients had a history of familial thyroid cancer or neck external irradiation. The patients were divided into 2 groups on the basis of age: reproductive-aged patients (18~45 years) and advanced reproductive-aged patients (>45 years). Of the 106 PTC patients, there were 50 reproductive-aged female patients (mean age, 32.50±8.71 years; age range, 18~45 years), 39 advanced reproductive-aged female patients (mean age, 56.87±7.89 years; age range, 46~81 years), and 17 reproductive-aged male patients (mean age, 32.88±7.17 years; age range, 23~45 years). NTG patients comprised 30 reproductive-aged patients (mean age, 38.43±4.90 years; age range, 24~45 years).
Immunohistochemistry
Immunohistochemical staining was performed for both ERβ1 and ERβ2 to evaluate immunoreactivity in the above-mentioned tissue samples. Paraffin-embedded tissue sections fixed in formalin were used. Slides were deparaffinized, rehydrated, and subjected to microwave heat antigen retrieval in 10 mM citrate buffer (pH 6.0) for 20~25 min. After blocking endogenous peroxidase activity, sections were incubated with primary antibodies against ERβ1 (1: 20; Clone PPG5/10, Serotec) and ERβ2 (1: 400; Clone 57/3, Serotec) overnight at 4°C. After washing with PBS, staining was performed by the Elivision™ plus two-step system (Maixin Bio, China). Immunoreactivity was visualized using the chromogen 3,3′-diamino-benzidine (DAB) (Maixin Bio, China). Slides were then counterstained with hematoxylin, washed, dehydrated with alcohol and xylene, and mounted onto coverslips. Appropriate positive and negative controls were run simultaneously with the patient specimen.
Evaluation of immunohistochemistry
Immunohistochemistry staining for ERβ1 and ERβ2 was interpreted using the Allred score [19,20]. Briefly, a proportion score (PS) represented the estimated proportion of tumor cells staining positive, as follows: 0 (none); 1 (1/100); 2 (1/100 to 1/10); 3 (1/10 to 1/3); 4 (1/3 to 2/3); and 5 (>2/3). An intensity score (IS) represented the average intensity of the positive cells: 0 (none); 1 (weak); 2 (intermediate); and 3(strong). The proportion and intensity scores were then added to obtain a total score (TS), which could range from 0 to 8, and “positive” is defined as scores ≥3. Two investigators (Dong WW and Huang YH) independently evaluated the tissue sections. If the TS differed between the 2 investigators, a third investigator (Li J) evaluated the tissue sections, and a consistent result was rendered.
Statistical analysis
Descriptive statistics were used according to the distribution of variables. The Mann-Whitney U test was used for comparison of the immunohistochemistry scores. The χ2 test or Fisher’s exact test was used for the comparison of the frequency or proportions of single variables. Analyses were performed with SPSS software, version 16.0 (SPSS Inc., Chicago, IL, USA). P values less than .05 were considered statistically significant.
Results
Expression of ERβ splice variants in reproductive-aged female patients with PTC and NTG
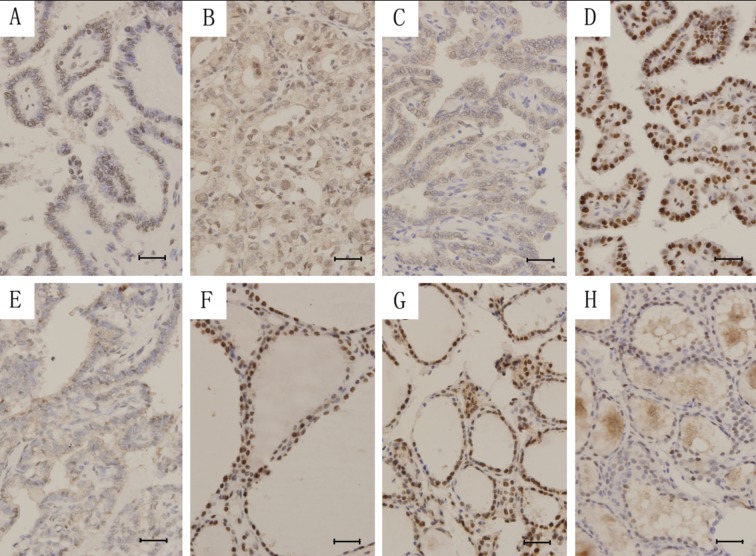
The subcellular distribution of ERβ1 and ERβ2 in PTC and NTG are shown in Table 1. Figure 1 illustrates the subcellular localization of ERβ1 and ERβ2 in PTC and NTG. There was significant difference in the subcellular localization of ERβ1 (χ2=34.069, P<0.001), but not in the positive percentage (χ2=0.281, P=0.628; Fisher’s Exact test), between PTC and NTG specimens. No significant difference was found in the positive percentage or subcellular distribution of ERβ2 between PTC and NTG specimens.
Table 1.
Subcellular distribution of ERβ1 and ERβ2 in PTC and NTG.
| ERβ1 | ERβ2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Nu | Cyto | Nu+Cyto | — | Nu | Cyto | Nu+Cyto | — | |
| NTG | 23 (76.7) | 0 (0.0) | 5 (16.7) | 2 (6.7) | 30 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PTC | 7 (14.0) | 5 (10.0) | 36 (72.0) | 2 (4.0) | 49 (98.0) | 0 (0.0) | 1 (2.0) | 0 (0.0) |
| P | 0.001* | —— | 0.014** | —— | 0.03# | —— | —— | —— |
Numbers in parentheses represent percentages. Nu – positive nuclear staining of ERβ splice variants; Cyto – positive cytoplasmic staining of ERβ splice variants; Nu+Cyto – positive nuclear and cytoplasmic staining of ERβ splice variants; ‘−’ – negative staining of ERβ splice variants.
difference in ERβ1 nuclear staining between NTG and PTC;
difference in ERβ1 nuclear and cytoplasmic staining between NTG and PTC;
difference in ERβ2 nuclear staining between NTG and PTC.
Figure 1.
The subcellular distribution of ERβ1 and ERβ2 in PTC and NTG. Nuclear (A), nucleocytoplasmic (B) and cytoplasmic (C) staining of ERβ1 and nuclear (D) and nucleocytoplasmic (E) staining of ERβ2 in PTC. Nuclear (F) and nucleocytoplasmic (G) staining of ERβ1 and nuclear (H) staining of ERβ2 in NTG. Scale bar =5 μm.
Nuclear ERβ1 expression in PTC was significantly lower than that in NTG (Z=−3.213, P=0.001), and nucleocytoplasmic ERβ1 expression in PTC was also lower than that in NTG (Z=−2.557, P=0.014). ERβ2 expression in PTC was higher than that in NTG (Z=−2.170, P=0.030).
Association between expression of ERβ splice variants and gender and age of patients with PTC
ERβ1 expression in reproductive-aged female patients with PTC was lower than that in corresponding male patients (Z=−2.387, P=0.017). On the contrary, ERβ2 expression in reproductive-aged female patients with PTC was higher than that in age-matched male patients (Z=−2.141, P=0.032). There was no significant difference in ERβ1 and ERβ2 expression between reproductive-aged and advanced reproductive-aged female patients with PTC.
Discussion
Unlike the traditional ERα, which is localized in the nuclei of cancer cells, ERβ splice variants have been detected in both the nucleus and cytoplasm of cancer cells [8,21]. However, the expression pattern of ERβ splice variants in thyroid cancer has not been adequately explored. Our data show nuclear and cytoplasmic expression of ERb1 and ERβ2 were found in both PTC and NTG specimens, except cytoplasmic expression of ERβ2 in NTGs. Specifically, ERβ1 was predominantly localized in the nucleus of NTG cells, and in both the nucleus and cytoplasm of PTC cells, whereas ERβ2 was primarily localized in the nucleus of both NTG and PTC cells. It has been reported that ERβ isoforms (ERβ1, ERβ2, ERβ3, and ERβ5) are detected only in the nuclei of Barrett’s metaplasia cells negative for dysplasia, and a significant number of carcinomas show cytoplasmic ERβ immunoreactivity, which is especially the case with ERβ2 [21]. It is speculated that perhaps there is a different level of expression of ERβ splice variants between normal or benign tissue and cancer tissue where certain isoforms with predominantly cytoplasmic immunoreactivity are associated with the malignant phenotype [22]. It may be ERβ1 in PTCs and ERβ2 in esophageal adenocarcinomas.
Consistent with findings in breast cancer, ERβ2 protein levels significantly increased in ductal carcinoma in situ (DCIS) and invasive breast cancer, compared to the adjacent normal gland [23]. We also found a decrease in both nuclear and nucleocytoplasmic ERβ1 expression and an increase in ERβ2 expression in PTC compared to NTG, indicating a role for ERβ2 in carcinogenesis that opposes the protective effect of wild-type ERβ1. However, the role of ERβ2 is quite the opposite in endometrioid carcinoma. Chakravarty et al reported that ERβ2 expression was decreased in endometrioid carcinoma compared to proliferative endometrium, and was decreased in higher grade tumors [24]; indicating that the roles of ERβ splice variants are specific to cancer types.
Currently, it is widely believed that ERβ has limited importance in thyroid cancer since no significant difference has been found in its expression between males and females, in benign versus malignant lesions, or in premenopausal versus postmenopausal females [18]. However, these data may reflect the fact that the presence of ERβ splice variants was not taken into consideration. In prostate cancer, no significant correlation was found between ERβ1 or ERβ2 expression and age [25]. In primary colorectal cancer, no association between sex and ERβ1 and ERβ2 protein expression was found [26]. However, a positive correlation between age and ERβ1 expression was identified in invasive breast cancer [27]. In our study we found that ERβ1 and ERβ2 expression was associated with sex but not with age, which may partly explain the female predominance of PTC during the reproductive years. Thus, the associations between ERβ splice variants with sex and age warrant further investigation.
In our study we determined the localization and expression pattern of ERβ splice variants and its significance in PTC. However, we did not study the relationship between ERβ splice variants and ERα, as all ERβ splice variants inhibit ERα transcriptional activity. We plan to study the association between ERβ splice variants and ERα in PTC in future studies.
Conclusions
This study investigated the expression pattern of ERβ splice variants in various types of thyroid lesions at the protein level using specific well-validated antibodies. Furthermore, it highlights the importance of ERβ splice variants in the development of PTC. The female predominance of PTC during the reproductive years may be associated with the expression of ERβ splice variants; thus, their clinical and biological significance merit further investigation.
Acknowledgements
We thank Dr. Kelie Reece from Vanderbilt University for English proofreading and corrections.
Footnotes
Source of support: This research was supported by the Key Scientific and Technological Project from Liaoning Province (JH2) (No: 2008225007), the Innovation Team Project of Education Department of Liaoning Province (No: LT2010102) and the Liaoning Millions of Talents Project (No: 2010921070)
References
- 1.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–7. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 2.Memon A, Darif M, Al-Saleh K, Suresh A. Epidemiology of reproductive and hormonal factors in thyroid cancer: evidence from a case-control study in the Middle East. Int J Cancer. 2002;97:82–89. doi: 10.1002/ijc.1573. [DOI] [PubMed] [Google Scholar]
- 3.Ron E, Kleinerman RA, Boice JD, Jr, et al. A population-based case-control study of thyroid cancer. J Natl Cancer Inst. 1987;79:1–12. [PubMed] [Google Scholar]
- 4.Preston-Martin S, Bernstein L, Pike MC, et al. Thyroid cancer among young women related to prior thyroid disease and pregnancy history. Br J Cancer. 1987;55:191–95. doi: 10.1038/bjc.1987.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McTiernan AM, Weiss NS, Daling JR. Incidence of thyroid cancer in women in relation to previous exposure to radiation therapy and history of thyroid disease. J Natl Cancer Inst. 1984;73:575–81. [PubMed] [Google Scholar]
- 6.Persson I, Yuen J, Bergkvist L, Schairer C. Cancer incidence and mortality in women receiving estrogen and estrogen-progestin replacement therapy – long-term follow-up of a Swedish cohort. Int J Cancer. 1996;67:327–32. doi: 10.1002/(SICI)1097-0215(19960729)67:3<327::AID-IJC4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Sioka C, Fotopoulos A. Effects of I-131 therapy on gonads and pregnancy outcome in patients with thyroid cancer. Fertil Steril. 2011;95:1552–59. doi: 10.1016/j.fertnstert.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Yan M, Rayoo M, Takano EA, Fox SB kConFab Investigators. Nuclear and cytoplasmic expressions of ERβ1 and ERβ2 are predictive of response to therapy and alters prognosis in familial breast cancers. Breast Cancer Res Treat. 2011;126:395–405. doi: 10.1007/s10549-010-0941-9. [DOI] [PubMed] [Google Scholar]
- 9.Collins F, MacPherson S, Brown P, et al. Expression of oestrogen receptors, ERalpha, ERbeta and ERbeta variants, in endometrial and cancers and evidence that prostaglandin F may play a role in regulating expression of ER alpha. BMC Cancer. 2009;9:330. doi: 10.1186/1471-2407-9-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung YK, Lam HM, Wu S, et al. Estrogen receptor beta2 and beta5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocr Relat Cancer. 2010;17:675–89. doi: 10.1677/ERC-09-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delaunay F, Pettersson K, Tujague M, Gustafsson JA. Functional differences between the amino-terminal domains of estrogen receptors alpha and beta. Mol Pharmacol. 2000;58:584–90. doi: 10.1124/mol.58.3.584. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa S, Inoue S, Watanabe T, et al. Molecular cloning and characterization of human estrogen receptor βcx: a potential inhibitor ofestrogen action in human. Nucleic Acids Res. 1998;26:3505–12. doi: 10.1093/nar/26.15.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Critchley HO, Henderson TA, Kelly RW, et al. Wild-type estrogen receptor (ERβ1) and the splice variant (ERβcx/β2) are both expressed within the human endometrium throughout the normal menstrual cycle. J Clin Endocrinol Metab. 2002;87:5265–73. doi: 10.1210/jc.2002-020502. [DOI] [PubMed] [Google Scholar]
- 14.Omoto Y, Eguchi H, Yamamoto-Yamaguchi Y, Hayashi S. Estrogen receptor (ER) β1 and ERβcx/β2 inhibit ERα function differently in breast cancer cell line MCF7. Oncogene. 2003;22:5011–20. doi: 10.1038/sj.onc.1206787. [DOI] [PubMed] [Google Scholar]
- 15.Peng B, Lu B, Leygue E, Murphy LC. Putative functional characteristics of human estrogen receptor-β isoforms. J Mol Endocrinol. 2003;30:13–29. doi: 10.1677/jme.0.0300013. [DOI] [PubMed] [Google Scholar]
- 16.Zhao C, Matthews J, Tujague M, et al. Estrogen receptor beta2 negatively regulates the transactivation of estrogen receptor alpha in human breast cancer cells. Cancer Res. 2007;67:3955–62. doi: 10.1158/0008-5472.CAN-06-3505. [DOI] [PubMed] [Google Scholar]
- 17.Kawabata W, Suzuki T, Moriya T, et al. Estrogen receptors (alpha and beta) and 17beta- hydroxysteroid dehydrogenase type 1 and 2 in thyroid disorders: possible in situ estrogen synthesis and actions. Mod Pathol. 2003;16:437–44. doi: 10.1097/01.MP.0000066800.44492.1B. [DOI] [PubMed] [Google Scholar]
- 18.Vaiman M, Olevson Y, Habler L, et al. Diagnostic value of estrogen receptors in thyroid lesions. Med Sci Monit. 2010;16(7):BR203–7. [PubMed] [Google Scholar]
- 19.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immuno-histochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–81. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 20.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–68. [PubMed] [Google Scholar]
- 21.Liu L, Chirala M, Younes M. Expression of estrogen receptor-beta isoforms in Barrett’s metaplasia, dysplasia and esophageal adenocarcinoma. Anticancer Res. 2004;24:2919–24. [PubMed] [Google Scholar]
- 22.Witte D, Chirala M, Younes A, et al. Estrogen receptor beta is expressed in human colorectal adenocarcinoma. Hum Pathol. 2001;32:940–44. doi: 10.1053/hupa.2001.27117. [DOI] [PubMed] [Google Scholar]
- 23.Esslimani-Sahla M, Kramar A, Simony-Lafontaine J, et al. Increased Estrogen Receptor Bcx Expression during Mammary Carcinogenesis. Clin Cancer Res. 2005;11:3170–74. doi: 10.1158/1078-0432.CCR-04-2298. [DOI] [PubMed] [Google Scholar]
- 24.Chakravarty D, Srinivasan R, Ghosh S, et al. Estrogen receptor beta1 and the beta2/betacx isoforms in nonneoplastic endometrium and in endometrioid carcinoma. Int J Gynecol Cancer. 2007;17:905–13. doi: 10.1111/j.1525-1438.2006.00851.x. [DOI] [PubMed] [Google Scholar]
- 25.Fujimura T, Takahashi S, Urano T, et al. Differential expression of estrogen receptor beta (ERbeta) and its C-terminal truncated splice variant ERbetacx as prognostic predictors in human prostatic cancer. Biochem Biophys Res Commun. 2001;289:692–99. doi: 10.1006/bbrc.2001.6038. [DOI] [PubMed] [Google Scholar]
- 26.Wong NA, Malcomson RD, Jodrell DI, et al. ERbeta isoform expression in colorectal carcinoma: an in vivo and in vitro study of clinicopathological and molecular correlates. J Pathol. 2005;207:53–60. doi: 10.1002/path.1807. [DOI] [PubMed] [Google Scholar]
- 27.Pfeiler G, Treeck O, Wenzel G, et al. Correlation of body mass index and menopausal status with the intra-tumoral estrogen system in invasive breast cancer. Gynecol Endocrinol. 2009;25:183–87. doi: 10.1080/09513590802549825. [DOI] [PubMed] [Google Scholar]