Summary
Background
Reflux esophagitis is caused mainly by excessive exposure of the mucosa to gastric contents. In the present study, we examined the effect of several amino acids on acid reflux esophagitis in rats.
Material/Methods
After 18 h of fasting, acid reflux esophagitis was induced by ligating both the pylorus and the transitional region between the forestomach and the corpus under ether anesthesia, and the animals were killed 4 h later. The severity of esophagitis was reduced by the oral administration of omeprazole, a proton pump inhibitor, or pepstatin, a specific pepsin inhibitor.
Results
The development of esophageal lesions was dose-dependently prevented by L-arginine and glycine, given intragastrically (i.g.) after the ligation, with complete inhibition obtained at 250 mg/kg and 750 mg/kg, respectively, and these effects were not influenced by the prior s.c. administration of indomethacin or L-NAME. By contrast, both L-alanine and L-glutamine given i.g. after the ligation aggravated these lesions in a dose-dependent manner. These amino acids had no effect on acid secretion but increased the pH of the gastric contents to 1.8~2.3 due to their buffering action.
Conclusions
The results confirmed an essential role for acid and pepsin in the pathogenesis of acid reflux esophagitis in the rat model and further suggested that various amino acids affect the severity of esophagitis in different ways, due to yet unidentified mechanisms; L-alanine and L-glutamine exert a deleterious effect on the esophagitis, while L-arginine and glycine are highly protective, independent of endogenous prostaglandins and nitric oxide.
Keywords: acid reflux esophagitis, rat model, pepsin, amino acid, dual effect
Background
Reflux esophagitis, an endoscopically positive gastroesophageal reflux disease, is caused mainly by excessive exposure to gastric contents due to impairments of various protective mechanisms that prevent reflux into the esophagus and resist the refluxate [1,2]. Since gastric acid plays a key role in the pathogenesis of reflux esophagitis, luminal pH control is considered important in the management of this disease [2,3]. Indeed, antisecretory drugs, such as histamine H2 receptor antagonists and proton pump inhibitors, have been shown to be effective against acid reflux esophagitis in experimental animals and humans [4–7].
In addition to acid, pepsin secreted by chief cells in the stomach is also an important component of the gastric refluxate. Since this enzyme is an acid-activated protease, the effect of acid suppressant therapy against reflux esophagitis may be accounted for, at least partly, by modulation of the proteolytic activity of pepsin. Indeed, studies have demonstrated a pathogenic role for pepsin in the development of acute esophagitis in rabbits and cats [8,9]. We also reported that pepstatin, a pepsin-inactivating pentapeptide, totally prevented the occurrence of acid reflux esophagitis, confirming an essential role for pepsin in the pathogenesis [10]. The proteolytic activity of pepsin is dependent on pH [9,10]. Thus, studying the relationship between pepsin activity and pH range is of fundamental importance to understanding the pathogenic role of pepsin in the development of esophageal lesions. We reported that orally administered L-glutamine worsened esophageal lesions induced by the dual ligation in a rat model [10]. We also showed that L-glutamine increased intragastric pH due to its buffering action. Since the proteolytic activity of pepsin is dependent on pH, with maximal activity at a pH of approximately 2.0, it is possible that L-glutamine aggravated the esophageal lesions by increasing the activity of pepsin through a shift in the intraluminal pH to 2.0 [10,11]. However, whether the same is true for other amino acids remains to be examined.
As mentioned above, acid and pepsin represent the most common damaging agents to the esophagus. Acid, by acting as a mild irritant to the esophagus, triggers mucosal defensive mechanisms including increased cell proliferation and blood supply to the esophagus [12–14]. The mechanisms of these adaptive responses, albeit remain largely unknown, may be mediated by prostaglandins (PGs), nitric oxide (NO), epidermal growth factor, and capsaicin-sensitive afferent neurons [7,12–16]. It is possible that the development of acid reflux esophagitis may well be an expression of the failure of the mechanisms responsible for the mucosal defense and that amino acids affect the mucosal integrity of the esophagus by modulating such mechanisms.
Given the above background, we examined in the present study the influence of various amino acids on the development of acid reflux esophagitis in a rat model, and demonstrated that both L-arginine and glycine are highly effective against the disease. In addition, we also investigated the mechanisms involved in the protective action of these amino acids.
Material and Methods
Animals
Male Sprague Dawley rats (200–230 g, Nippon Charles River, Shizuoka, Japan) were used. The animals were kept in individual cages with raised mesh bottoms and deprived of food but allowed free access to tap water for 18 h prior to the experiments. Studies were carried out using 4~7 rats per group. All experimental procedures employed in the present study were approved by the Experimental Animal Research Committee of Kyoto Pharmaceutical University.
Induction of acid reflux esophagitis
Acid reflux esophagitis was induced as described previously [10]. Under light ether anesthesia, the abdomen was incised along the midline, and the pylorus and the junction between the forestomach and corpus were ligated. The animals were killed with deep ether anesthesia 2, 3, 4, and 5 h later, and then the esophagus and stomach were removed, and treated with 2% formalin for fixation of the tissues. The area (mm2) of macroscopically visible damage in the esophagus was measured under a dissecting microscope with square grids (×10), summed per tissue, and used as a lesion score. The person measuring the lesions did not know the treatments given to the animals. Various amino acids (L-alanine, L- or D-arginine, glycine, L-glutamine: 100~1500 mg/kg) and pepstatin (a specific pepsin inhibitor: 0.3 mg/kg) were given intragastrically (i.g.) through esophageal intubation 10 min after the ligation. Omeprazole (10 mg/kg) was given orally (p.o.) 30 min before the ligation. In some cases, indomethacin (5 mg/kg), a cyclooxygenase (COX) inhibitor, or NG-nitro- L-arginine methyl ester (L-NAME: 10 mg/kg), an inhibitor of NO synthase, was given subcutaneously (s.c.) 30 min before the ligation.
Determination of gastric pH
Intragastric pH was measured in pylorus-ligated rats. Under light ether anesthesia, the abdomen was opened by a midline incision, the pylorus was ligated, and the animals were then allowed to recover from the anesthesia. Three hours later, the animals were killed under deep ether anesthesia, the stomachs were removed, and the gastric contents were collected. After centrifugation for 10 min at 3000 rpm, each sample was measured for volume and for pH using a pH meter (Horiba F-21, Kyoto, Japan). Various amino acids (250~750 mg/kg) were given i.g. immediately after the ligation.
Measurement of the buffering capacity of amino acids
The buffering capacity of various amino acids was determined in vitro by titration. L-alanine (500 mg/kg), L-arginine (250 mg/kg), L-glutamine (750 mg/kg) or glycine (750 mg/kg) was suspended or dissolved in a 0.5% CMC solution, and the changes in pH of the solution were monitored when 1 ml of each amino acid solution was titrated by the addition of 150 mM HCl.
Preparation of drugs
The drugs used were various amino acids (L-alanine, L or D-arginine, L-glutamine and glycine; Nacalai Tesque, Kyoto, Japan), pepstatin (Banyu, Tokyo, Japan), indomethacin, NG-Nitro-L-arginine methyl ester (L-NAME) (Sigma Chemicals, St. Louis, MO), omeprazole (Astra Zeneca, Möndal, Sweden) and mannitol (Nacalai Tesque). Amino acids, pepstatin and omeprazole were suspended or dissolved in a 0.5% carboxymethylcellulose solution (CMC; Wako, Osaka, Japan). Indomethacin was suspended in saline with a drop of Tween 80 (Nacalai Tesque), while L-NAME was dissolved in saline. Each drug was prepared immediately before use and administered i.g. or s.c. in a volume of 0.5 ml/100 g body weight.
Statistical analysis
Data are presented as the mean ±SE for four to seven rats per group. Statistical analyses were performed using the two-tailed Student t-test or Dunnett’s multiple comparison test, and values of P<0.05 were regarded as significant.
Results
Time-course of changes in acid reflux esophagitis and inhibition by the acid or pepsin inhibitor
Following ligation of the pylorus and forestomach, severe hemorrhagic damage developed in the proximal 3 cm of the esophagus in all animals in a time-dependent manner, and the lesion scores at 3 and 4 h after the ligation were 68.5±4.8 mm2 and 114.5±8.6 mm2, respectively (Figure 1A, B). The severity of these lesions was significantly reduced by the p.o. administration of omeprazole (10 mg/kg) 30 min before the ligation or the p.o. administration of pepstatin (0.3 mg/kg) 10 min after the ligation, the inhibition in both cases being over 95% (Figure 1A). On the basis of these results, we used the reflux esophagitis model induced by 3 h of ligation to examine the effect of various amino acids in the subsequent studies.
Figure 1.
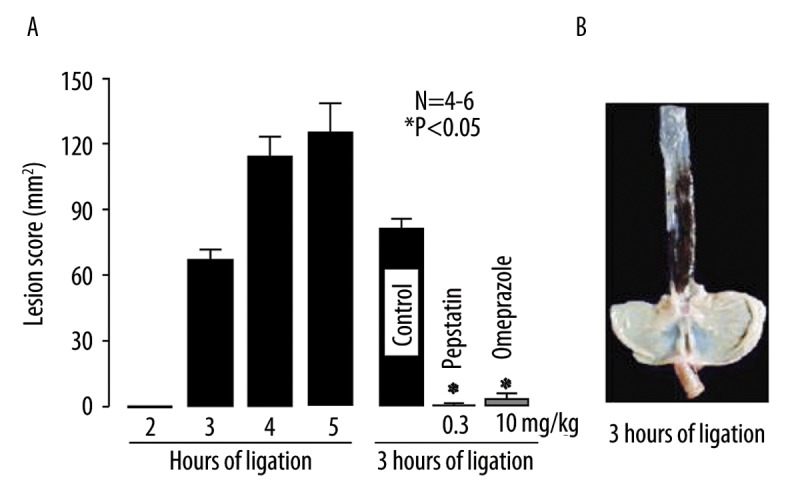
(A) Time-course of changes in acid reflux esophagitis in rats. Under ether anesthesia, both the pylorus and forestomach were ligated, and the esophageal mucosa was examined 2~5 h later. In some cases, omeprazole (10 mg/kg) or pepstatin (0.3 mg/kg) was given orally 30 min before or 10 min after the ligation, respectively, and the mucosa was examined 3 h after the ligation. Data are presented as the mean ±SE for 4~6 rats. * Significant difference from control, at P<0.05. (B) Macroscopic appearances of esophageal lesions induced by ligation of the pylorus and forestomach for 3 h.
Effect of L-glutamine on acid reflux esophagitis
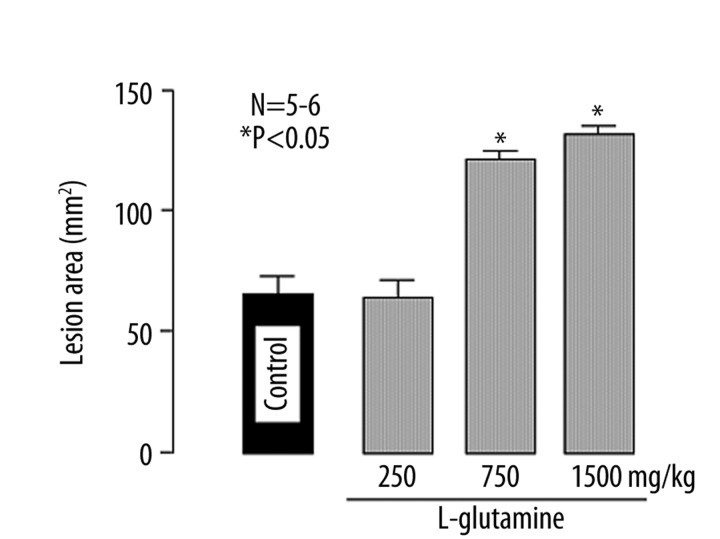
Ligation of the pylorus and forestomach for 3 h caused hemorrhagic lesions in the esophagus, the lesion score being 63.2±5.1 mm2. Intragastric administration of L-glutamine (250~1500 mg/kg) increased the severity of esophageal lesions in a dose-dependent manner, and a significant effect was observed at 750 mg/kg or greater (Figure 2). At the dose of 1500 mg/kg, damage was evident in all of the esophagus and the degree of aggravation was over 200% (Figure 2).
Figure 2.
Effect of L-glutamine on acid reflux esophagitis in rats. Under ether anesthesia, both the pylorus and forestomach were ligated, and the esophageal mucosa was examined 3 h later. L-glutamine (250, 750 and 1500 mg/kg) was given i.g. 10 min after the ligation. Data are presented as the mean±SE for five to six rats. * Significant difference from control, at P<0.05.
Effects of glycine, L-alanine and L(D)-arginine on acid reflux esophagitis
Consistent with a previous paper [10], we confirmed that L-glutamine aggravated acid reflux esophagitis. To investigate whether the same is true for other amino acids, we examined the effects of L-alanine, L- or D-arginine, and glycine on the esophagitis induced by the 3-h ligation.
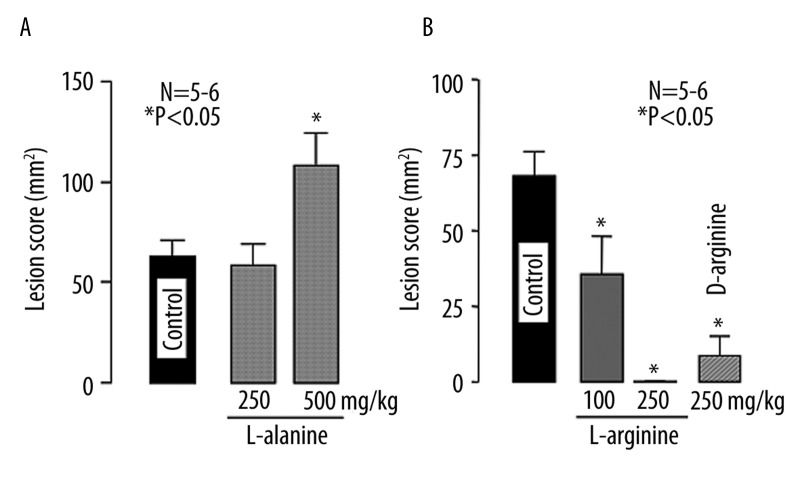
The intragastric administration of L-alanine had no effect on the development of the esophagitis at 250 mg/kg but at 500 mg/kg significantly worsened the lesions, the lesion score being 124.5±16.1 mm2, which was almost 2 times the control value (68.1±6.2 mm2) (Figure 3A). By contrast, when L-arginine (100 and 250 mg/kg) was given i.g. 10 min before the ligation of the pylorus and forestomach, this amino acid dose-dependently reduced the severity of esophageal lesions, with complete inhibition observed at 250 mg/kg (Figure 3B).
Figure 3.
Effects of L-alanine (A) and L- or D-arginine (B) on acid reflux esophagitis in rats. Under ether anesthesia, both the pylorus and forestomach were ligated, and the esophageal mucosa was examined 3 h later. L-alanine (250 and 500 mg/kg), L-arginine (100 and 250 mg/kg) or D-arginine (250 mg/kg) was given i.g. 10 min after the ligation. Data are presented as the mean ±SE for 5~6 rats. * Significant difference from control, at P<0.05.
To further investigate whether the protective effect of L-arginine is due to its physicochemical properties or mediated by its biological actions, we repeated the same experiments using D-arginine. As shown in Figure 3B, D-arginine (250 mg/kg) also provided good protection against the acid reflux esophagitis, the inhibition being 89.0%. Likewise, glycine (250~750 mg/kg) dose-dependently reduced the severity of the esophagitis, and the lesion score at 250 and 500 mg/kg was 37.1±14.7 mm2 and 12.6±4.1 mm2, respectively (Figure 4A). Complete inhibition by glycine was observed at 750 mg/kg (Figure 4B).
Figure 4.
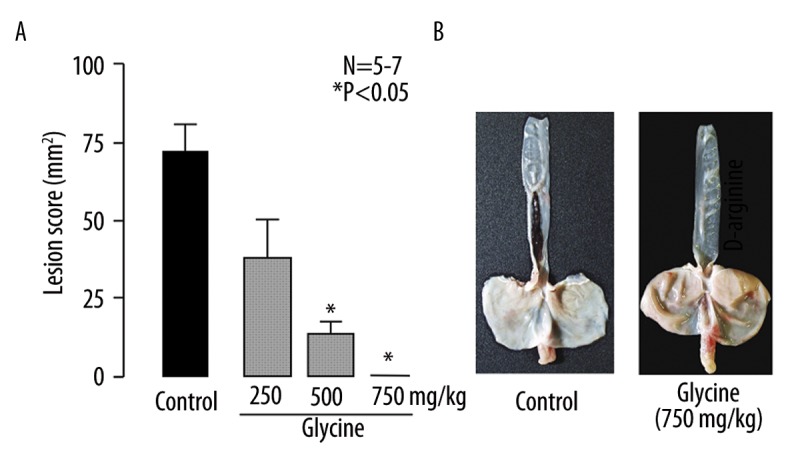
(A) Effect of glycine on acid reflux esophagitis in rats. Under ether anesthesia, both the pylorus and forestomach were ligated, and the esophageal mucosa was examined 3 h later. Glycine (250, 500 and 750 mg/kg) was given i.g. 10 min after the ligation. Data are presented as the mean ±SE for 5~7 rats. * Significant difference from control, at P<0.05. (B) Macroscopic appearance of esophageal lesions induced by the dual ligation for 3 h. Note that glycine completely inhibited the development of hemorrhagic esophageal lesions.
Effects of indomethacin and L-NAME on the protective action of L-arginine and glycine against acid reflux esophagitis
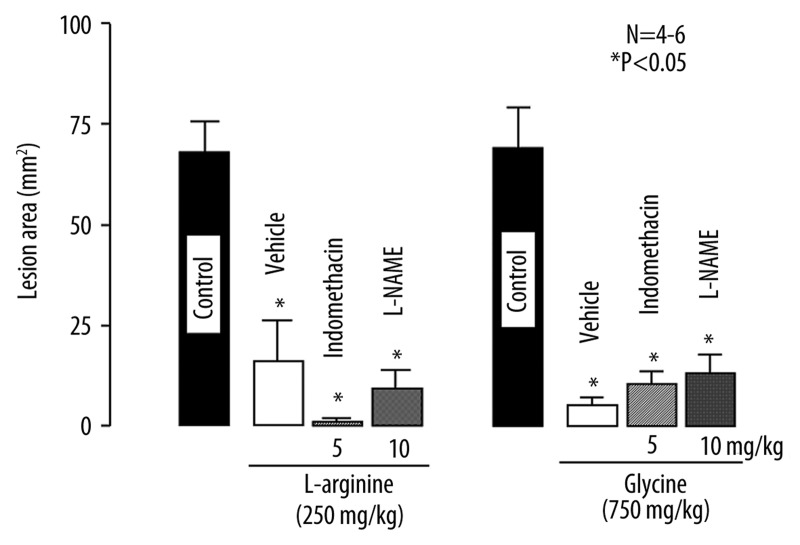
Both L-arginine and glycine were found to provide strong protection against the acid reflux esophagitis. To investigate the possible involvement of endogenous PGs and NO in the protective action, the effects of indomethacin and L-NAME on the protective action of L-arginine or glycine were examined.
The severity of acid reflux esophagitis was significantly reduced by the i.g. administration of L-arginine (250 mg/kg) or glycine (750 mg/kg), the inhibition being 75.7% and 90.5%, respectively (Figure 5). Prior s.c. administration of neither indomethacin (5 mg/kg) nor L-NAME (10 mg/kg) had any effect on the protective action of L-arginine, the degree of protection in the presence of these agents being equivalent to that in the control group. Likewise, the protective effect of glycine was not significantly affected by either of these agents, and the inhibition was about 90% even in the presence of indomethacin or L-NAME. On the other hand, mannitol given i.g. at 261 mg/kg, a concentration equimolar to that of L-arginine (250 mg/kg), had no effect the severity of esophageal lesions (not shown).
Figure 5.
Effects of indomethacin and L-NAME on the protective action of L-arginine or glycine against acid reflux esophagitis in rats. Under ether anesthesia, both the pylorus and forestomach were ligated, and the esophageal mucosa was examined 3 h later. L-arginine (250 mg/kg) or Glycine (750 mg/kg) was given i.g. 10 min after the ligation. Indomethacin (5 mg/kg) or L-NAME (10 mg/kg) was given s.c. 30 min before the ligation. Data are presented as the mean ±SE for 4~6 rats. * Significant difference from control, at P<0.05.
Effects of various amino acids on luminal pH in pylorus-ligated stomachs
Since the amino acids used in the present study had different effects, protective or aggravative, on acid reflux esophagitis, and since the severity of the esophagitis is influenced by the pH of the gastric contents, their effects on gastric pH may also be different after i.g. administration. To test this possibility, we examined the effect of these amino acids on the pH of the gastric contents in the pylorus-ligated rats. As shown in Table 1, ligation of the pylorus for 3 h accumulated about 6 ml of gastric juice in the stomach, the pH of the contents being 1.30±0.05. None of the amino acids (L-arginine, L-alanine, L-glutamine and glycine) significantly affected the volume of gastric contents, although the volume tended to be increased by some amino acids at 500 or 750 mg/kg. On the other hand, the pH of the gastric contents was significantly increased by all amino acids at the doses used. Notably, L-alanine at 500 mg/kg and glycine at 750 mg/kg increased the pH to 2.36±0.12 and 2.38±0.07, respectively, the difference in both cases being highly significant. Likewise, both L-arginine at 250 mg/kg and L-glutamine at 750 mg/kg significantly raised the pH of the gastric contents to 1.82±0.05 and 1.73±0.09, respectively.
Table 1.
Effects of amino acids on the volume and pH of gastric contents in pylorus-ligated rats.
| Drugs | Doses (mg/kg) | No. of rats | Volume (mL) | Gastric pH |
|---|---|---|---|---|
| Control | – | 5 | 6.38±0.87 | 1.30±0.05 |
| L-arginine | 250 | 4 | 6.30±0.41 | 1.82±0.05* |
| L-alanine | 500 | 5 | 8.56±0.48 | 2.36±0.12* |
| L-glutamine | 750 | 5 | 8.66±0.81 | 1.73±0.09* |
| Glycine | 750 | 4 | 8.63±0.41 | 2.38±0.07* |
Amino acids were given orally immediately after the pylorus was ligated. The gastric contents were collected 3 h after the ligation. Following centrifugation for 10 min at 3,000 rpm, the volume of each sample was measured, and the pH was determined by using a pH meter. Values are presented as the mean ±SE for four to five rats.
Significant difference from control at P<0.05.
Acid-buffering capability of various amino acids in vitro
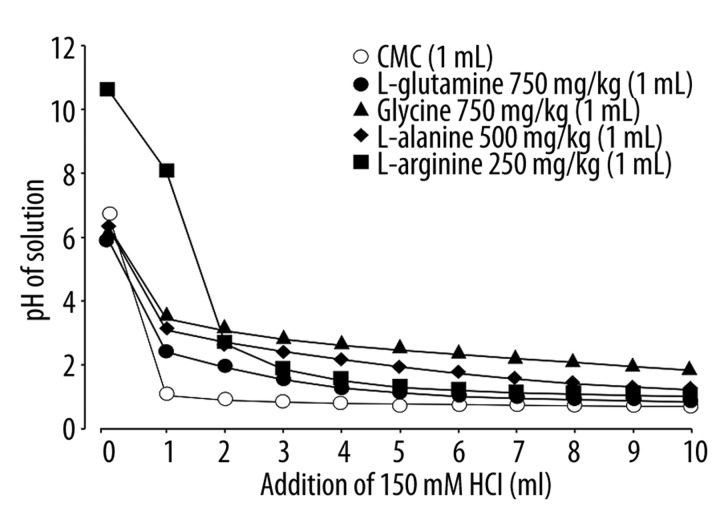
Since the proteolytic activity of pepsin is dependent on pH and maximal at approximately pH 2.0 [10] and since pepsin plays an important role in the pathogenesis of acid reflux esophagitis [8–10], the different effects of amino acids on acid reflux esophagitis may be attributable to differences in their acid-buffering capability to modify the optimal pH for the proteolytic action of pepsin. To test this possibility, we titrated the solution of amino acids on varying the pH with the addition of HCl in vitro.
Among the amino acids used in this experiment, both L-arginine (250 mg/kg) and glycine (750 mg/kg) showed all but complete protection against acid reflux esophagitis, while both L-alanine (500 mg/kg) and L-glutamine (750 mg/kg) aggravated the esophagitis. The solution of L-arginine was a strong base, pH 10.6, but that of other amino acids was neutral, pH 6~7 (Figure 6). When these solutions were titrated with the addition of 150 mM HCl, the amino acids showed a similar buffering action against HCl, although the potency was slightly different depending on the dose used. Among the amino acids used, no difference was found in their buffering capability at around pH 2.
Figure 6.
Buffering capability of various amino acids against HCl in vitro. L-glutamine, glycine, L-arginine or L-alanine (500 mg/kg), L-arginine (250 mg/kg), L-glutamine (750 mg/kg) or glycine (750 mg/kg) was suspended or dissolved in a 0.5% CMC solution, and 1 ml of these solutions was titrated by addition of 150 mM HCl. Changes in pH of the solution were determined by a pH meter.
Discussion
We recently reported a simple method to produce acid-reflux esophagitis in rats by ligating both the pylorus and forestomach and demonstrated that pepsin as well as acid play an important role in the pathogenesis of this model [7,10]. In the present study, we confirmed the pathogenic importance of acid and pepsin in the occurrence of acid reflux esophagitis in this rat model and further suggested that amino acids given i.g. affected the severity of the esophagitis in different ways; L-arginine and glycine are highly effective in reducing the severity, while L-alanine and L-glutamine have an aggravating effect.
Reflux esophagitis is a chronic disease caused mainly by excessive exposure of the esophagus to the gastric contents. Since acidic gastric content plays a major role in the pathogenesis of reflux esophagitis, luminal pH control is considered important in the management of this disease [1–3]. Indeed, medical treatment has focused on acid suppression with antisecretory drugs, such as histamine H2 receptor antagonists and proton pump inhibitors, and these drugs have been shown to be effective against acid reflux esophagitis in experimental animals and humans [4–7]. We also observed in previous studies that antisecretory drugs significantly prevented esophageal damage in an acute rat model [7,10], supporting the contention that gastric acid plays a pivotal role in the development of esophageal lesions.
In addition to gastric acid, pepsin, conjugated or deconjugated bile acids, and pancreatic enzymes are also included in the refluxate [8–10,17–20]. However, it is unlikely that bile acids and pancreatic enzymes participated in the pathogenesis of esophagitis in the present model, because the esophagitis was induced in pylorus-ligated stomachs where no regurgitation of the duodenal content occurs into the stomach. Although there is no clinical evidence of a definite role for pepsin in the pathogenesis of reflux esophagitis, experimental evidence has suggested a pathogenic role for pepsin in addition to acid [9,21,22]. Consistent with our previous observation [10], the present study showed that pepstatin, a specific pepsin inhibitor, completely prevented the development of esophageal lesions, similar to omeprazole, a proton pump inhibitor. These results strongly suggest that pepsin, in addition to gastric acid, plays a major role in the pathogenesis of the reflux esophagitis model.
Of interest in the present study is the finding that intragastric administration of glycine or L-arginine potently inhibited the acid reflux esophagitis induced by the dual ligation, while L-alanine as well as L-glutamine aggravated the lesions. We previously reported that L-glutamine aggravated these esophageal lesions by increasing the proteolytic activity of pepsin in the refluxate through a shift in the intraluminal pH to around 2.0, the optimal pH for peptic activity [10]. Okabe et al. reported that L-glutamine markedly aggravated Shay ulceration in the forestomach caused by pylorus ligation in rats [11]. Because this model is caused by the corrosive actions of acid and pepsin and because the esophageal mucosa is covered by stratified squamous epitheliums, similar to the epithelium in the forestomach, it would be understandable for the mechanism aggravating these lesions to be associated with peptic activity. If the aggravation is really brought about by such a buffering capability, then other amino acids would be similarly expected to aggravate the esophageal lesions. However, the aggravation was observed on the intragastric administration of L-alanine but not other amino acids, such as L-arginine and glycine. Interestingly, the latter two amino acids did prevent the development of esophageal lesions, showing all but complete inhibition at 250 mg/kg and 750 mg/kg, respectively. We also observed in the present study that these amino acids had a potent buffering action and increased luminal pH to around 2.0, similar to L-alanine or L-glutamine. Thus, it is assumed that L-arginine and glycine exert a protective effect against acid reflux esophagitis due to yet unknown mechanisms, although they increase luminal pH and probably pepsin activity, similar to L-glutamine.
The involvement of endogenous PGs in the mucosal protection and the functional responses induced in the stomach by mild irritants has been demonstrated by many investigators [21–24]. We previously reported that acid reflux esophagitis was aggravated by indomethacin and SC-560 but not rofecoxib, suggesting the participation of COX-1/PGE2 in the mucosal defense against esophagitis [25]. Since PGE2 prevented the development of acid reflux esophagitis at a dose that had no influence on acid secretion but increased pepsin secretion, the underlying mechanism remained unknown. In addition, a previous study showed that L- or D-arginine given p.o. provided gastric cytoprotection against HCl-induced damage in rats, probably by acting as a mild irritant and mediated by endogenous PGs [26]. Thus, it is possible that L-arginine or glycine prevented the development of esophagitis via adaptive cytoprotection mediated by endogenous PGs. However, the protective effect of these amino acids was not influenced by indomethacin, excluding the possibility that endogenous PGs are involved in the protective action of these amino acids in the esophageal mucosa.
NO is known to regulate various biological processes in the body including the alimentary tract [27,28]. Since L-arginine is a substrate for NO production, it is possible that the protective effect of this amino acid is partly mediated by NO. However, the role of NO in the pathogenesis of esophagitis remains controversial [15,29,30]. Ozel et al. [29] reported that the rabbit esophageal mucosa shows mucosal adaptation to acid and pepsin, at least partly mediated by a NO-dependent mechanism. By contrast, Ishiyama et al. [30] showed that exogenous luminal NO exacerbates tissue damage in a reflux esophagitis model of rats. A recent study also showed that NO increased pepsinogen secretion in the rat stomach via the stimulation of guanylyl cyclase [31]. In the present study, we found that the protective effect of L-arginine was not significantly antagonized by L-NAME, a NO synthase inhibitor. Likewise, the effect of glycine was also unaffected by L-NAME. Thus, it is unlikely that these amino acids afford protection against acid reflux esophagitis mediated by a NO-dependent mechanism. This idea is also supported by the finding that D-arginine had a similar protective effect to L-arginine at the same dose, because the former amino acid can not be used as a substrate for NO production. Additionally, since the effect of L-arginine was not mimicked by the intragastric administration of mannitol at an equimolar concentration, it is assumed to be brought about by the amino acid’s chemical properties and not simply to be due to its osmolarity.
Recent studies demonstrated that the esophagus has mechanisms to defend against damage from the refluxate, particularly gastric acid and pepsin, including an antireflux barrier (the lower esophageal sphincter), luminal clearance and tissue resistance, increased cell replication, and increased blood supply to the esophagus [12–15]. Hence, it is assumed that the reflux esophagitis is due to impairments of epithelial defense against acid-pepsin contact [20,32]. The mechanisms of these phenomena are not well defined, yet might be mediated, at least partly, by endogenous PGs and NO as well as capsaicin-sensitive afferent neurons [7,13,15,16,25]. Since both L-arginine and glycine exhibited protection against acid reflux esophagitis in the presence of indomethacin or L-NAME, it is unlikely that such protective actions are mediated by endogenous PGs or NO. Notwithstanding, it is possible that these amino acids prevent acid reflux esophagitis via the amelioration of the defensive mechanisms that are mediated by factors other than PGs and NO. In the present study, we have not examined the relation of the protective actions of amino acids with the sensory neurons. Further study should certainly be necessary to examine this point.
Conclusions
Given the findings of the present study, we confirmed the involvement of pepsin in the development and exacerbation of esophagitis, and further suggested that amino acids given orally affect the severity of acid reflux esophagitis in different ways, due to yet unidentified mechanisms; L-arginine and glycine are highly protective, while L-alanine and L-glutamine are deleterious. The aggravating effect of L-alanine and L-glutamine may be explained by the increase in pepsin activity due to their buffering capability. Further studies are, however, necessary to elucidate the mechanism underlying the protective action of L-arginine or glycine, despite that both amino acids show a strong buffering capability, similar to L-glutamine. The present findings may lead to the development of a novel therapeutic approach in the treatment of reflux esophagitis, in addition to acid suppressant therapy.
Footnotes
Source of support: This research was supported in part by the “Open Research” Program from the Ministry of Education, Science and Culture of Japan
References
- 1.Orlando RC. Pathogenesis of reflux esophagitis and Barrett’s esophagus. Med Clin North Am. 2005;89:219–41. doi: 10.1016/j.mcna.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Hunt RH. Importance of pH control in the management of GERD. Arch Intern Med. 1999;159:649–57. doi: 10.1001/archinte.159.7.649. [DOI] [PubMed] [Google Scholar]
- 3.Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology. 2008;134:1842–60. doi: 10.1053/j.gastro.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Bell NJ, Burget D, Howden CW, et al. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 1992;51(Suppl 1):59–67. doi: 10.1159/000200917. [DOI] [PubMed] [Google Scholar]
- 5.Hamamoto N, Hashimoto T, Adachi K, et al. Comparative study of nizatidine and famotidine for maintenance therapy of erosive esophagitis. J Gastroenterol Hepatol. 2005;20:281–86. doi: 10.1111/j.1440-1746.2004.03546.x. [DOI] [PubMed] [Google Scholar]
- 6.Inatomi N, Nagaya H, Takami K, et al. Effects of a proton pump inhibitor, AG-1749 (lansoprazole), on reflux esophagitis and experimental ulcers in rats. Jpn J Pharmacol. 1991;55:437–51. doi: 10.1254/jjp.55.437. [DOI] [PubMed] [Google Scholar]
- 7.Nagahama K, Yamato M, Kato S, Takeuchi K. Protective effect of lafutidine, a novel H2 receptor antagonist on acid reflux esophagitis in rats through capsaicin-sensitive afferent neurons. J Pharmacol Sci. 2003;93:55–61. doi: 10.1254/jphs.93.55. [DOI] [PubMed] [Google Scholar]
- 8.Roberts NB. Review article: human pepsins – their multiplicity, function and role in reflux disease. Aliment Pharmacol Ther. 2006;24:2–9. doi: 10.1111/j.1365-2036.2006.03038.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg HI, Dodds WJ, Gee S, et al. Role of acid and pepsin in acute experimental esophagitis. Gastroenterology. 1969;56:223–30. [PubMed] [Google Scholar]
- 10.Nagahama K, Yamato M, Nishio H, Takeuchi K. Essential role of pepsin in pathogenesis of acid reflux esophagitis in rats. Dig Dis Sci. 2006;51:303–9. doi: 10.1007/s10620-006-3129-8. [DOI] [PubMed] [Google Scholar]
- 11.Okabe S, Takeuchi K, Takata Y, et al. Effects of L-glutamine on various gastric lesions in rats and guinea pigs. Digestion. 1976;14:325–31. doi: 10.1159/000197948. [DOI] [PubMed] [Google Scholar]
- 12.De Backer A, Haentjens P, Willems G. Hydrochloric acid. A trigger of cell proliferation in the esophagus of dogs. Dig Dis Sci. 1994;9:884–90. doi: 10.1007/BF01309520. [DOI] [PubMed] [Google Scholar]
- 13.Hollwarth ME, Smith ME, Kvietys RP, Granger DN. Esophageal blood flow in the cat: Normal distribution and effects of acid perfusion. Gastroenterology. 1986;90:622–28. doi: 10.1016/0016-5085(86)91116-9. [DOI] [PubMed] [Google Scholar]
- 14.Jankowski J, Coghill G, Tregaskis B, et al. Epidermal growth factor in the esophagus. Gut. 1992;33:1448–53. doi: 10.1136/gut.33.11.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanas AI, Blas JM, Ortego J, et al. Adaptation of esophageal mucosa to acid- and pepsin-induced damage: Role of nitric oxide and epidermal growth factor. Dig Dis Sci. 1997;42:1003–12. doi: 10.1023/a:1018837003196. [DOI] [PubMed] [Google Scholar]
- 16.Konturek SJ, Konturek PC, Brzozowski T, Bubenik GA. Role of melatonin in upper gastrointestinal tract. J Physiol Pharmacol. 2007;58:23–52. [PubMed] [Google Scholar]
- 17.Tack J. Review article: the role of bile and pepsin in the pathophysiology and treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2006;24:10–16. doi: 10.1111/j.1365-2036.2006.03040.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Hunt RH. Precise role of acid in non-erosive reflux disease. Digestion. 2008;78:31–41. doi: 10.1159/000151253. [DOI] [PubMed] [Google Scholar]
- 19.Orlando RC. Reflux esophagitis: overview. Scand J Gastroenterol. 1995;30:36–37. doi: 10.3109/00365529509090267. [DOI] [PubMed] [Google Scholar]
- 20.Orlando RC. Pathogenesis of gastroesophageal reflux disease. Am J Med Sci. 2003;326:274–78. doi: 10.1097/00000441-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Robert A, Nezamis IE, Lancaster C, et al. Mild irritant prevent gastric necrosis through adaptive cytoprotection mediated by prostaglandins. Am J Physiol. 1983;245:G113–21. doi: 10.1152/ajpgi.1983.245.1.G113. [DOI] [PubMed] [Google Scholar]
- 22.Barnett K, Bell CJ, McKnight W, et al. Role of cyclooxygenase-2 in modulating gastric acid secretion in the normal and inflamed rat stomach. Am J Physiol. 2000;279:G1292–97. doi: 10.1152/ajpgi.2000.279.6.G1292. [DOI] [PubMed] [Google Scholar]
- 23.Takeeda M, Hayashi Y, Yamato M, et al. Roles of endogenous prostaglandins and cyclooxygenase isozymes in mucosal defense of inflamed rat stomach. J Physiol Pharmacol. 2004;55:193–205. [PubMed] [Google Scholar]
- 24.Poplawski C, Sosnowski D, Szaflarska-Popławska A, et al. Role of bile acids, prostaglandins and COX inhibitors in chronic esophagitis in a mouse model. World J Gastroenterol. 2006;12:1739–42. doi: 10.3748/wjg.v12.i11.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamato M, Nagahama K, Kotani T, et al. Biphasic effect of prostaglandin E2 on a rat model of esophagitis mediated by EP1 receptors: Relation to pepsin secretion. Digestion. 2005;72:109–18. doi: 10.1159/000088365. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi K, Ohuchi T, Kato S, Okabe S. Cytoprotective action of L-arginine against HC1-induced gastric injury in rats: involvement of nitric oxide? Jpn J Pharmacol. 1993;61:13–21. doi: 10.1254/jjp.61.13. [DOI] [PubMed] [Google Scholar]
- 27.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 28.Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41–60. doi: 10.1053/j.gastro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 29.Ozel SK, Dagli TE, Yuksel M, et al. The roles of free oxygen radicals, nitric oxide, and endothelin in caustic injury of rat esophagus. J Pediatr Surg. 2004;39:1381–85. doi: 10.1016/j.jpedsurg.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Ishiyama F, Iijima K, Asanuma K, et al. Exogenous luminal nitric oxide exacerbates esophagus tissue damage in a reflux esophagitis model of rats. Scand J Gastroenterol. 2009;44:527–37. doi: 10.1080/00365520802699260. [DOI] [PubMed] [Google Scholar]
- 31.Ito Y, Okuda S, Ohkawa F, et al. Dual role of nitric oxide in gastric hypersecretion in distended stomach: Inhibition of acid secretion and stimulation of pepsin secretion. Life Sci. 2008;83:886–92. doi: 10.1016/j.lfs.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Castell DO, Murray JA, Tutuian R, et al. The pathophysiology of gastro-oesophageal reflux disease: oesophageal manifestations. Aliment Pharmacol Ther. 2004;20(Suppl 9):14–25. doi: 10.1111/j.1365-2036.2004.02238.x. [DOI] [PubMed] [Google Scholar]