Summary
Background
Tumor growth in multiple myeloma (MM) is regulated by the cytokine networks which are produced by myeloma cells and the microenvironment of the bone marrow. Interleukin-17 (IL-17) is implicated in the increased angiogenesis in the bone marrow of MM. Recent studies reported elevated levels of interleukin 17A (IL-17A) in the sera of patients with advanced stages according to Durie-Salmon classification.
Material/Methods
We compared the concentration of IL-17A and IL-17E in the blood serum of 34 newly diagnosed MM patients with healthy subjects’ sera. We also evaluated the concentration of IL-17A and IL-17E in the blood serum of MM patients and the relation to the percentage of plasma cells and other clinical parameters. The concentration of IL-17E and IL-17A of healthy subjects and patients with MM was assessed by enzyme-linked immunosorbent assay (ELISA).
Results
Our data confirm that IL-17A and IL-17E serum levels were significantly higher in all MM patients and also in patients with advanced stage compared with healthy subjects. We found the correlation between serum levels of IL-17A in MM patients and percentage of plasma cells. Our results also showed that if serum levels of IL-17E were higher in MM patients, the percentage of plasma cells and beta-2-microglobulin levels were lower.
Conclusions
The IL-17 family of cytokines may suppress or promote tumor growth. There seems to be some balance between the effects of IL-17A and IL-17E. The role of increased levels of IL-17E needs further investigation to understand its role in the pathobiology of MM.
Keywords: interleukin-17A, interleukin-17E, multiple myeloma
Background
Interleukin-17 (IL-17) is the founding member of a new cytokine family that has recently gained prominence due to its involvement in autoimmune and allergic diseases in both human and mouse. IL-17 family consists of 6 members including IL-17 (also called IL-17A), IL-17B, IL-17C, IL-17D, IL-17E (also called IL-25) and IL-17F [1]. The three best characterized members of the IL-17 cytokine family are IL-17A, IL-17E and IL-17F. The major function of these 3 members is chemoattractant activity through the induction of cytokines and chemokines [2]. IL-17A and IL-17F share the greatest similarity showing 50% identity at the amino acid level, whereas IL-25 is most divergent in the family [1,2]. IL-17E is less related, sharing only 16–30% identity at the primary sequence level [3]. IL-17 family cytokines are produced by a newly defined lineage of T lymphocytes, Th17 T cells [4]. The population of Th17 cells might be expanded as a result of disease activity [5]. The receptor for IL-17A is expressed in several human cell types, such as a lung epithelial cell line, foreskin fibroblasts, a B-cell line, a myelomonocytic cell line, and an embryonal kidney cell line [6]. IL-17E is a new player in regulating type 2 immunity. It is directly involved in Th2- associated allergic inflammation [2]. IL-17E not only could directly influence naïve T cell differentiation, but also enhance effectors Th2 cell expansion, and memory Th2 cell polarization [1]. IL-17E appears to be a non-T/non-B cell population, expressing class II major histocompatibility complex (MHC class II) and CD11c molecules, a typical accessory cell phenotype [7]. Both IL-17A and IL-17E are able to induce the activation of nuclear factor-kappaB (NF-κB) and similar, but not identical, panel of cytokines and chemokines [2].
New aspects of the biological role of IL-17 family cytokines in carcinoma patients have recently been indicated [3,8,9]. IL-17 is implicated in the angiogenesis of multiple myeloma (MM) [10]. IL-17 induces expression of a number of chemokines and cytokines including interleukin-6 (IL-6), transforming growth factor-beta (TGF-β), granulocyte-colony stimulating factor (G-CSF) or granulocyte, macrophage-colony stimulating factor (GM-CSF), matrix metalloproteinases (MMP) and intercellular adhesion molecule-1 (ICAM-1) in a variety of cell types, including the bone marrow stromal cells [8]. IL-6 from stromal cells may promote myeloma growth [10]. Human dendritic cells are efficient antigen-presenting cells for the induction of polyfunctional Th17-1 cells, and such cells are the dominant population of IL17 producers in the tumor bed in human myeloma [11]. A recent study suggests that increased numbers of TH17 cells along with increased levels of IL-17 in MM are an important therapeutic target for both anti-MM responses as well as to improve immune function [8]. IL-17E can inhibit the proliferation of human progenitor bone marrow cells of the granulocyte-macrophage series (CFU-GM), thus reducing the number of mature leukocytes [12]. IL-17E can also induce the secretion of cytokines and increase the recruitment of B cells [13].
The aim of this study was to estimate the level of the IL-17A and IL-17E in the blood serum of multiple myeloma patients and healthy donor sera. We also evaluated the concentration of the IL-17A and IL-17E in the blood serum of multiple myeloma patients and correlate it to the percentage of plasma cells and other clinical parameters.
Material and Methods
Peripheral blood samples were collected from 34 newly diagnosed MM patients (23 males and 11 females; median age 70 years, range was 57 to 83). Twenty patients had stage II disease and 14 patients had stage III disease according to the Durie-Salmon classification. Twenty-three patients had monoclonal immunoglobulin IgG and 11 patients had monoclonal immunoglobulin IgA. The control group consisted of 50 healthy individuals, age- and sex-matched. Serum samples were collected from all patients prior to the initiation of any treatment. Sera collected from patients and controls were put into separate vials and stored at −70°C. The pre-treatment evaluation included a complete blood count, albumin in serum, the concentration of paraprotein in serum, immunoglobulin class subtype (Ig) and the levels of calcium, beta-2-microglobulin (β2m), lactate dehydrogenase (LDH) and C-reactive protein (CRP). Bone marrow aspirates and trephine biopsies (TB) were obtained (Table 1). Informed written consent was obtained from all participants and the Ethics Committee of the Medical University of Bialystok approved the study.
Table 1.
Multiple myeloma patients characteristics.
| All patients | Stage II | Stage III | |
|---|---|---|---|
| Mean ±SD | Mean ±SD | Mean ±SD | |
| IL 17-E [pg/ml] | 18.96±3.71 | 20.02±3.73 | 17.46±2.61 |
| IL 17-A [pg/ml] | 14.09±3.26 | 12.83±3.04 | 15.90±2.15 |
| Age | 70.82±9.90 | 70.10±10.32 | 71.86±9.97 |
| Hb [g/dl] | 10.56±2.16 | 11.57±1.91 | 9.13±1.70 |
| Serum Ca [mmol/L] | 2.46±0.29 | 2.34±0.15 | 2.64±0.36 |
| Serum M protein [g/dl] | 2.37±2.17 | 1.50±1.12 | 3.61±2.75 |
| %plasma cells in aspirate | 23.11±19.90 | 8.92±7.69 | 43.39±12.32 |
| %plasma cells in TB | 45.18±28.52 | 24.80±13.39 | 74.71±10.51 |
| IgG [mg/dl] | 3331.61±2371.16 | 2439.93±1203.81 | 5003.50±3142.27 |
| IgA [mg/dl] | 3260.64±2554.67 | 1385.40±1230.47 | 4832.33±2322.62 |
| IgM [mg/dl] | 43.67±44.82 | 57.05±50.86 | 24.56±27.46 |
| Serum albumin [g/l] | 3.94±0.64 | 4.14±0.42 | 3.64±0.81 |
| β2m [mg/l] | 5469.47±3804.62 | 2757.22±978.16 | 8181.72±3636.14 |
| CRP [mg/L] | 6.57±4.53 | 7.38±5.83 | 5.41±1.10 |
| LDH [IU/l] | 201.71±46.90 | 200.00±59.16 | 204.14±24.59 |
IL – interleukin; Hb – hemoglobin; Ca – calcium; M – monoclonal; TB – trephine biopsy, Ig – immunoglobulin; β2m – beta-2-microglobulin; CRP – C-reactive protein; LDH – lactate dehydrogenase.
The concentration of IL-17E and Il-17A in the blood serum of healthy subjects and patients with MM was assessed by enzyme-linked immunosorbent assay (ELISA). IL-17E concentrations in the blood serum were determined by the ELISA method using a PeproTech kit (Rocky Hill, USA) according to the instructions enclosed. Human recombinant IL-17E was used as a standard. IL-17A levels in the serum were measured by the ELISA method using R&D Systems kits (Minneapolis, USA) according to the instructions provided. Human recombinant IL-17A was used as a standard.
Results were expressed as mean ±SD. The correlations between the various parameters measured were calculated with Spearman’s rank correlation coefficient. The Student’s T test was used. P values less than 0.05 were considered statistically significant.
Results
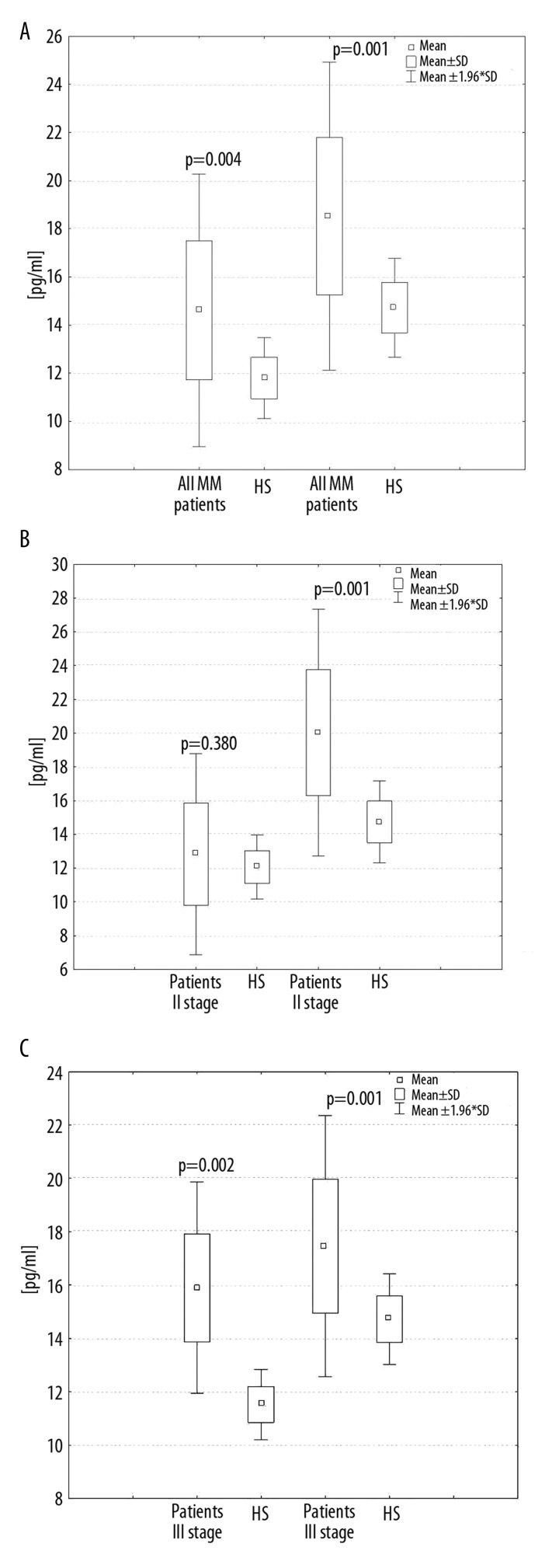
Significantly elevated serum levels and statistically significant differences of IL-17A (14.09±3.26 pg/ml, p=0.004) and IL-17E (18.96±3.71 pg/ml, p=0.001) were observed in all patients in comparison to the healthy subjects for IL-17A (11.79±0.84 pg/ml) and for IL-17E (14.74±1.02 pg/ml). Statistically significant differences were observed between IL-17E levels in patients from groups with stage II (20.02±3.73 pg/ml, p=0.001) and stage III disease (17.46±2.61 pg/ml, p=0.001) compared to the healthy subjects. Statistically significant differences were observed between IL-17A in patients from the group with stage III disease (15.90±2.15 pg/ml, p=0.002) compared to the healthy subjects, but not with stage II (12.83±3.04 pg/ml, p=0.38). These results are presented in Figure 1A–C.
Figure 1.
Serum levels of IL-17A and IL-17E in healthy subjects (HS) and all multiple myeloma (MM) patients (A) and MM patients in II stage disease (B) and MM patients in III stage disease (C) according to the Durie-Salmon classification.
The correlations between the concentrations of IL-17E and IL-17A and chosen clinical parameters in MM patients are presented in Table 2. From the various parameters we found statistical positive correlation between serum levels of IL-17A in all patients (r=0.571, p=0.017) and patients from the group with stage III (r=0.85, p=0.037) to the percentage of the plasma cells in TB. We also found a positive correlation between serum levels of IL-17A in all patients to the levels of lactate dehydrogenase (r=0.517, p=0.006). A positive correlation (r=0.927, p=0.003) between IL-17A serum levels and the concentration of IgA in patients from the group with stage III was observed as well.
Table 2.
Correlations between laboratory and clinical variables in multiple myeloma patients. Results with p<0.05 are bolded.
| All patients | Stage II | Stage III | ||||
|---|---|---|---|---|---|---|
| Spearman R | p | Spearman R | p | Spearman R | p | |
| IL17E & Hb | 0.444 | 0.074 | 0.539 | 0.108 | 0.018 | 0.969 |
| IL17E & serum Ca | −0.154 | 0.554 | 0.576 | 0.082 | −0.214 | 0.645 |
| IL17E & serum M protein | −0.165 | 0.526 | −0.523 | 0.121 | 0.571 | 0.180 |
| IL17E &%plasma cells in aspirate | −0.428 | 0.087 | 0.030 | 0.934 | −0.286 | 0.535 |
| IL17E &%plasma cells in TB | −0.546 | 0.023 | −0.253 | 0.480 | −0.883 | 0.008 |
| IL17E & IgG | 0.377 | 0.135 | 0.285 | 0.425 | 0.107 | 0.819 |
| IL17E & IgA | −0.056 | 0.830 | −0.212 | 0.556 | −0.214 | 0.645 |
| IL17E & IgM | 0.376 | 0.136 | 0.285 | 0.425 | −0.162 | 0.728 |
| IL17E & serum albumin | 0.215 | 0.408 | 0.462 | 0.179 | −0.286 | 0.535 |
| IL17E & β2m | −0.779 | 0.001 | −0.600 | 0.285 | −0.900 | 0.037 |
| IL17E & CRP | −0.028 | 0.916 | −0.061 | 0.868 | 0.000 | 1.000 |
| IL17E & LDH | 0.088 | 0.736 | 0.188 | 0.603 | 0.179 | 0.702 |
| IL17A & Hb | −0.300 | 0.243 | 0.109 | 0.763 | −0.156 | 0.738 |
| IL17A & serum Ca | 0.383 | 0.129 | 0.097 | 0.789 | 0.255 | 0.582 |
| IL17A & serum M protein | 0.393 | 0.118 | −0.019 | 0.960 | 0.182 | 0.696 |
| IL17A &%plasma cells in aspirate | 0.460 | 0.063 | −0.268 | 0.454 | 0.727 | 0.064 |
| IL17A &%plasma cells in TB | 0.571 | 0.017 | 0.183 | 0.613 | 0.785 | 0.037 |
| IL17A & IgG | −0.301 | 0.241 | −0.170 | 0.638 | −0.618 | 0.139 |
| IL17A & IgA | 0.041 | 0.877 | 0.012 | 0.973 | 0.927 | 0.003 |
| IL17A & IgM | −0.372 | 0.141 | −0.018 | 0.960 | 0.422 | 0.346 |
| IL17A & serum albumin | −0.457 | 0.065 | −0.152 | 0.674 | −0.673 | 0.098 |
| IL17A & β2m | 0.550 | 0.099 | −0.359 | 0.553 | 0.103 | 0.870 |
| IL17A & CRP | 0.359 | 0.158 | 0.429 | 0.215 | 0.624 | 0.135 |
| IL17A & LDH | 0.517 | 0.006 | 0.695 | 0.001 | −0.273 | 0.554 |
IL – interleukin; Hb – hemoglobin; Ca – calcium; M – monoclonal protein; TB – trephine biopsy; Ig – immunoglobulin; β2m – beta-2-microglobulin; CRP – C-reactive protein; LDH – lactate dehydrogenase.
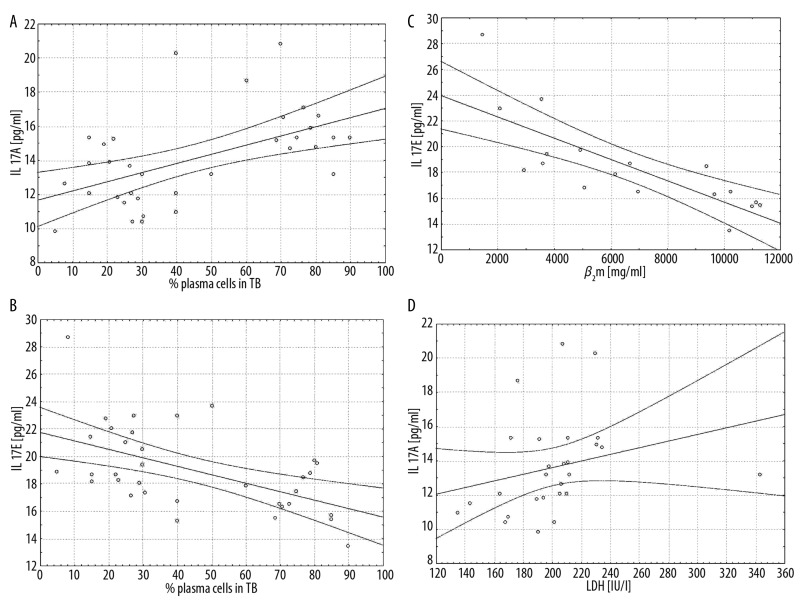
We found a negative correlation between serum levels of IL-17E in all patients (r=−0.546, p=0.023) and patients from the group with stage III (r=−0.883, p=0.008) to percentage of the plasma cells in TB. We also found a negative correlation between serum levels of IL-17E in all patients (r=−0.779, p=0.001) and patients from the group with stage III (r=−0.900, p=0.037) to the β2m levels (Figure 2A–D).
Figure 2.
Correlations between serum levels of IL-17A and plasma cells percentage in trephine biopsy (TB), p=0.017 (A) and serum levels of IL-17E and plasma cells percentage in TB, p=0.023 (B) and serum levels of IL-17E and beta-2-microglobulin (β2m), p=0.006 (C) and serum levels of IL-17A and lactate dehydrogenase (LDH) p=0.006 (D) in all multiple myeloma patients.
Discussion
Immune dysfunction in MM is observed. Tumor growth is regulated by the cytokine networks, which are produced by myeloma cells and the microenvironment of the bone marrow. IL-17-producing TH17 cells play an important role in the pathobiology of MM. It belongs to the indirect angiogenic factors, which promote tumor growth in vivo via the enhancement of angiogenesis [8,10]. Among other factors, IL-17 controls the myeloma change from avascular to vascular phase. Promotion of angiogenesis by IL-17 may result from enhancement of the action of the basic fibroblast (bFGF), the hepatocyte (HGF), and the vascular endothelial-cell (VEGF) growth factors [12]. The IL-17 family of cytokines may also play a role in bone resorption and lead to osteolytic fractures [3]. The Th17 T cell phenotype is a key predictor of lytic bone disease in MM [15].
Alexandrakis et al. [10] reported increasing serum levels of IL-17 in association with higher disease stage. They found the correlation of IL-17 with angiogenic factors VEGF and tumor necrosis factor-alfa (TNF-α) and with microvessel density (MVD) in newly diagnosed MM patients. They also found that the serum levels of IL-17 in MM patients were higher than in controls, although the difference did not reach statistical difference. Prabhala et al. [8] also demonstrated that a number of TH17-associated cytokines, including IL-17, are significantly elevated in myeloma compared with healthy donors. In our study higher serum levels of IL17A and IL17E were observed in all patients in comparison to the control subjects. Statistically significant differences were observed between IL17E levels in patients from group with stage II and stage III disease compared to the control subjects. The statistical difference between IL17A serum levels in the control subjects was observed only in patients from the group with stage III disease.
Prabhala et al. [8] observed that IL-17 promotes myeloma tumor cell growth in a CB-17 severe combined immunodeficient (SCID) mouse model. They showed that MM cell lines and primary cells express IL-17 receptor, providing the biologic mechanism for IL-17 effects on MM cells. In our study we found a correlation between serum levels of IL-17A in all patients and patients from the group with stage III to the plasma cells in TB, suggesting that IL-17A may promote myeloma growth in the advanced stage of MM.
The lactate dehydrogenase is one of the major independent prognostic factors in MM [16]. We did not find significant elevated levels of LDH, but we did find a correlation between the serum levels of IL-17A and LDH in all patients. Higher levels of both IL-17A and LDH could be associated with a poor outcome.
The role of IgA isotype in prognosis is unclear. Some reports suggest that IgA isotype is a poor prognostic factor [17]. In our study, in the III stage patient group we observed a correlation between IL-17A serum levels and the concentration of IgA, which additionally may suggest that IL-17A can be associated with a poor prognosis.
The data on the role of IL-17 E in the neoplastic diseases is limited [4,7]. The increase in the production of IL-17E by leukocytes observed in the group of patients may counterbalance the effects induced by IL-17A over-expression and inhibit cellular response (eg, by stimulating the production of suppressive IL-10) [3]. We confirmed that IL-17E does not have the same activity as IL-17A because in our study we found a negative correlation between serum levels of IL-17E in all patients and patients from the group with stage III to the plasma cells in trephine biopsy. We also found a negative correlation between levels of IL17E in all patients and patients from the group with stage III to the β2m levels. The protein β2m is a strong and useful prognostic parameter because it correlates with the mass of a tumor [18]. Our results showed that if serum levels of IL-17E were higher, the percentage of the plasma cells and β2m levels were lower. Both negative correlations between IL-17E to the plasma cells and IL-17E to the β2m levels indicate that IL-17E may suppress tumor growth.
Conclusions
Our data confirm that higher IL-17A and IL-17E serum levels are significantly elevated in myeloma patients compared with healthy subjects, and are associated with advanced stage of MM. The members of the IL-17 family of cytokines may suppress or promote tumor growth. There seems to be some balance between the effects of IL-17A and IL-17E. The role of increased levels of serum IL-17E remains unclear, and further investigations are needed to understand its role in MM.
Footnotes
Source of support: Departmental sources
References
- 1.Pappu BP, Angkasekwinai P, Dong C. New lessons learned from interleukin 17 family cytokines. Pharm Ther. 2008;117:374–83. doi: 10.1016/j.pharmthera.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawaguchi M, Adachi M, Oda N, et al. IL-17 cytokine family. J Allergy Clin Immunol. 2004;114(6):1265–73. doi: 10.1016/j.jaci.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–74. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 4.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17- producing CD4+ effector T cells develop via lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 5.Hamzaoui K, Bouali E, Ghorbel I, et al. Expression of Th-17 and RORgt mRNA in Behçet’s Disease. Med Sci Monit. 2012;18(1):CR227–34. doi: 10.12659/MSM.881720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao Z, Fanslow WC, Seldin MF, et al. Herpesvirus samiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–21. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 7.Fort MM, Cheng J, Yen D, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2- associated pathologies in vivo. Immunity. 2001;15:985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 8.Prabhala RH, Pelluru D, Fulciniti M, et al. Elevated IL-17 produced by TH17 cells promotes myeloma growth and inhibits immune function in multiple myeloma. Blood. 2010;115(26):5385–92. doi: 10.1182/blood-2009-10-246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garley M, Jabłońska E, Grabowska SZ, Piotrowski L. IL-17 family cytokines in neutrophils of patients with oral epithelial squamous cell carcinoma. Neoplasma. 2009;56(2):96–100. doi: 10.4149/neo_2009_02_96. [DOI] [PubMed] [Google Scholar]
- 10.Alexandrakis MG, Pappa CA, Miyakis S, et al. Serum interleukin- 17 and its relationship to angiogenic factors in multiple myeloma. Eur J Intern Med. 2006;17(6):412–16. doi: 10.1016/j.ejim.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Dhodapkar KM, Barbuto S, Matthews P, et al. Dendritic cells mediate the induction of polyfunctional human IL-17 producing cells (Th17-1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112(7):2878–85. doi: 10.1182/blood-2008-03-143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broxmeyer HE, Starnes T, Ramsey H, et al. The IL-17 cytokine family members are inhibitors of human hematopoietic progenitor proliferation. Blood. 2006;108(2):770. doi: 10.1182/blood-2006-01-0292. [DOI] [PubMed] [Google Scholar]
- 13.Kim MR, Manoukian R, Yeh R, et al. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100(7):2330–40. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi H, Numasaki M, Lotze MT, Sasaki H. Interleukin-17 enhances bFGF, HGF- and VEGF- induced growth of vascular endothelial cells. Immunol Lett. 2005;98:189–93. doi: 10.1016/j.imlet.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Noonan K, Marchionni L, Anderson J, et al. A novel role of IL-17 producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood. 2010;116(18):3554–63. doi: 10.1182/blood-2010-05-283895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimopoulos MA, Barlogie B, Smith TL, Alexanian R. High serum lactate dehydrogenase level as a marker for drug resistance and short survival in mulitiple myeloma. Ann Intern Med. 1991;115:931–35. doi: 10.7326/0003-4819-115-12-931. [DOI] [PubMed] [Google Scholar]
- 17.Sirohi B, Powles R, Kulkarni S, et al. Comparison of new patients with Bence-Jones, IgG and IgA myeloma receiving sequential therapy: the need to regard these immunologic subtypes as separate disease entities with specific prognostic criteria. Bone Marrow Transplant. 2001;28:29–37. doi: 10.1038/sj.bmt.1703093. [DOI] [PubMed] [Google Scholar]
- 18.Durie BG, Stock-Novack D, Salmon SE, et al. Prognostic value of pretreatment serum beta 2 microglobulin in myeloma: a Southwest Oncology Group Study. Blood. 1990;75:823–30. [PubMed] [Google Scholar]