Summary
Background
Cardiotrophin-1 (CT-1) is a member of the interleukin (IL-6) family of cytokines and is increased in various cardiovascular diseases, including chronic heart failure. The aim of the study was to determine if plasma CT-1 is associated with diastolic heart failure (DHF) and to investigate the relationship between CT-1 and echocardiographic parameters.
Material/Methods
Fifty-seven consecutive patients (mean age 57±8 years, 24 males) diagnosed with DHF in our clinic and 33 controls (mean age 55±7 years, 12 males) were included in the study. All study participants underwent echocardiographic evaluation and blood samples were obtained.
Results
CT-1 and NT-proBNP values were significantly higher in DHF subjects than in controls (11.30 [8.09–16.51] vs. 17.5 [8.95–28.74] fmol/mL, P=0.017 and 64 [27.5–95] vs. 82 [55.5–241] pg/mL, P=0.009, respectively). The mitral peak velocity of early diastolic filling (E), mean ratio of E to early diastolic mitral annular velocity (E/Em), and the pulmonary capillary wedge pressure (PCWP) estimated from E/Em measurements were all significantly higher in the patient group (62.27±14.69 vs. 75.67±18.85 cm/sec, 6.40±1.48 vs. 10.30±3.48, and 10 [9–11]vs. 14[12–16] mmHg, P≤0.001 for all). Lateral and septal Em were significantly lower in the patient group (10.69±1.87 vs. 8.69±2.00 cm/sec and 8.91±1.22 vs. 6.65±1.58 cm/sec, P<0.001 for both). CT-1 positively correlated with NT-proBNP (P=0.001, r=0.349), mean E/Em (P=0.003, r=0.307), and estimated mean PCWP (P=0.001, r=0.308).
Conclusions
CT-1 is elevated in patients with DHF and is associated with NT-proBNP and estimated left ventricular filling pressures.
Keywords: Cardiotrophin-1, diastolic heart failure, tissue Doppler echocardiography, N-terminal pro-B-type natriuretic peptide
Background
Diastolic heart failure (DHF) is a clinical syndrome characterized by the symptoms and signs of heart failure, a preserved ejection fraction and abnormal diastolic function [1]. The percentage of patients with DHF in epidemiological studies ranges from 40–71% (mean 56%), but in hospital-based cohort studies it is slightly lower, ranging from 24–55% (mean 41%) [2]. Elderliness, hypertension with left ventricular (LV) hypertrophy, pathologies such as diabetes, obesity, coronary artery disease, new onset atrial fibrillation, and others are commonly associated with DHF [2–4].
Cardiotrophin-1 (CT-1) is a member of the interleukin (IL)-6 cytokine family that shares the transmembrane signaling protein, glycoprotein (gp) 130, as a receptor [4]. CT-1 mRNA is expressed in adult human heart, skeletal muscle, ovary, colon, prostate and testis and in fetal kidney and lung [5]. CT-1 has hypertrophic and cytoprotective actions on the cardiac myocytes and may play an important role in other organ systems [6,7]. The plasma concentration of CT-1 is increased in various cardiovascular diseases such as congestive heart failure, hypertension, valvular heart disease, acute coronary syndrome, and cardiomyopathies [8–18].
Although, the prognostic importance of CT-1 in various cardiovascular diseases, including congestive heart failure, is well-known, there is limited data about CT-1 in patients with DHF. The purpose of the present study was to determine if CT-1 levels are significantly different in DHF patients compared to controls and to investigate the relationship between CT-1 and echocardiographic parameters.
Material and Methods
Study population
Fifty-seven consecutive patients (mean age 57±8 years, 24 (42%) males) diagnosed with DHF in our clinic and 33 controls (mean age 55±7 years, 12 (36%) males) were included in the study. DHF was diagnosed when symptoms (dyspnea not associated with any other cause) and signs (rale or peripheral edema) of heart failure were observed along with a preserved LV ejection fraction (LVEF) (≥50%) and evidence of diastolic dysfunction. The control group was formed from volunteer subjects admitted to our clinic who did not have heart failure symptoms and signs and who had a preserved LVEF. In order to exclude other causes of dyspnea, all patients underwent physical and laboratory examinations, including serum hemoglobin and thyroid hormones, chest radiogram and spirometry. Patients with systolic heart failure, moderate or severe valvular stenosis or regurgitation, congenital heart disease, atrial fibrillation, chronic obstructive pulmonary disease, malignancy, and other extracellular fluid-increasing diseases, such as hypothyroidism and liver cirrhosis, were excluded from the study.
The present study was a single center study. All examinations were performed by the cardiology clinic of our hospital. The investigation conforms to the principles outlined in the Declaration of Helsinki. All subjects gave their written informed consent prior to inclusion in the study. The study protocol was approved by the ethics committee at our institution.
Echocardiographic measurements
All of the study participants underwent echocardiographic evaluation (2.5 mHz transducer, Philips EnVisor C, Bothell, Washington, USA). Standardized projections and measurements were performed for the evaluation of cardiac anatomy, ventricular function and valve competence. LVEF was measured using Simpson’s method [19]. Left ventricular mass was calculated by the formula described by Devereux et al. and LV mass index was obtained by dividing the LV mass by the body surface area [20]. The following conventional mitral inflow pulse wave Doppler parameters were measured: peak velocity of early diastolic filling (E), late filling (A), and deceleration time (DT) of the E-wave velocity and isovolumetric relaxation time (IVRT). These parameters were obtained from the apical four-chamber view with a 1 to 3 mm sample volume placed between the mitral leaflet tips during diastole. Pulmonary venous flow parameters were also measured: peak systolic velocity (Ps), peak anterograde diastolic velocity (Pd) and the Ps/Pd ratio. These parameters were obtained from the apical four-chamber view with a 2 to 3 mm sample volume placed 1 cm into the pulmonary vein. Tissue Doppler parameters were measured: peak systolic mitral annular velocity (Sm) and early diastolic mitral annular velocity (Em) and late diastolic mitral velocity (Am). These parameters were obtained from the apical four-chamber view with a 2 to 5 mm sample volume placed 1 cm within the septal and lateral insertions of the mitral leaflets. The mean of 3 or more measurements was used for analysis of the Doppler data. The ratio of mitral peak velocity of early diastolic filling to early diastolic mitral annular velocity (E/Em) was calculated for the lateral and septal annulus and the mean of the lateral and septal E/Em were also determined. As previously described, the formula (1.24×(E/Em)+1.9) was used to estimate pulmonary capillary wedge pressure (PCWP) [21]. Patients with mean E/Em ≤8 or a change in E/A ratio with the Valsalva maneuver of <0.5 were excluded from the study. Diastolic dysfunction was defined as Em <Am if Em was less than 10 cm/sec in lateral mitral annulus or less than 8 cm/sec in septal mitral annulus [22].
Biochemical measurements
Blood samples were obtained during admission for routine chemistry, including CT-1 and N-terminal pro-B-type natriuretic peptide (NT-proBNP) following an overnight fast. CT-1 values were measured with a sandwich enzyme immunoassay method (Organon Teknika Microwell System Reader 230 S, Germany) in our hospital laboratory. NT-proBNP analyses were made by the electrochemiluminescence immunoassay method (Cobas 6000 analyzer, ROCHE Diagnostics GmbH, Mannheim, Germany). The Cockcroft-Gault formula was used to calculate creatinine clearance [14].
Statistical analysis
According to Kolmogorov-Smirnov normality test, 2 independent sample T tests were used to compare the normally distributed independent variables between the 2 groups, and Mann-Whitney U Test was used to compare the non-normally distributed independent variables between the 2 groups. Normally distributed continuous data were expressed as mean ± standard deviation (SD); non-normally distributed continuous variables were presented as median and interquartile range (IQR) [quartile l to quartile 3]. Chi-square test was used for comparing the categorical data. Categorical data were expressed as count and percentages. Spearman’s correlation test was used for correlation between variables. Linear regression analyses were used to determine the effect of age, creatinine clearance, systolic blood pressure, left atrium diameter, and LV mass index on log CT-1 and log NT-proBNP. P values below 0.05 were considered statistically significant. Statistical analysis was performed by using commercial software (IBM SPSS Statistics 19, SPSS inc., an IBM Co., Somers, NY)
Results
There were no significant differences between the patient and control groups with regard to age, sex, hypertension, diabetes, CAD, smoking, medications, body mass index, fasting blood glucose, thyroid status, lipid profile, creatinine clearance, serum creatinine and hemoglobin levels (Table 1). The patient group had a higher LV posterior wall thickness and a larger left atrial size, but differences in LVEF, chamber sizes, and mass index remained insignificant (Tables 1 and 2). Arterial blood pressures were also not different between the 2 groups. CT-1 and NT-proBNP were significantly higher in the patient group (CT-1: 11.30 [8.09–16.51] fmol/ml vs. 17.5 [8.95–28.74] fmol/ml, P=0.017; NT-proBNP: 64 [27.5–95] pg/ml vs. 82 [55.5–241] pg/ml, P=0.009) (Table 1). The mitral E, lateral, septal and mean E/Em and the PCWP estimated from each of the E/Em measurements were all significantly higher, whereas Ps/Pd, lateral and septal Em and lateral and septal Em/Am were significantly lower in the patient group (Table 2).
Table 1.
Baseline characteristics and laboratory findings of patient and control groups.
| Control (n=33) | Patient (n=57) | P value | |
|---|---|---|---|
| Age (years) | 55±7 | 57±8 | 0.205 |
| Gender (Male) | 12 (36) | 24 (42) | 0.592 |
| Hypertension | 19 (58) | 43 (74) | 0.115 |
| Diabetes | 8 (24) | 16 (28) | 0.692 |
| Coronary artery disease | 6 (18) | 20 (35) | 0.088 |
| Smoking | 7 (21) | 11 (19) | 0.827 |
| RAS blockers | 11 (33) | 30 (53) | 0.076 |
| β-blockers | 7 (21) | 20 (35) | 0.166 |
| Calcium channel blockers | 7 (21) | 9 (16) | 0.517 |
| Statins | 6 (18) | 15 (26) | 0.379 |
| Acetyl salicylic acid | 11 (33) | 24 (42) | 0.411 |
| Body mass index (kg/m2) | 30.3±4.3 | 31.3±4.2 | 0.281 |
| Systolic blood pressure (mmHg) | 132±23 | 139±21 | 0.173 |
| Diastolic blood pressure (mmHg) | 80 [70–90] | 85 [80–90] | 0.530 |
| Glucose (mg/dL) | 99 [92–110] | 105 [93–127] | 0.132 |
| Thyroid stimulating hormone (μIU/mL) | 1.30 [0.85–1.70] | 1.20 [0.8–1.85] | 0.834 |
| Creatinine (mg/dL) | 0.7 [0.6–0.9] | 0.8 [0.6–0.9] | 0.990 |
| Ceatinine clearance (mL/min/1.73 m2) | 114±28 | 115±36 | 0.906 |
| Cholesterol (mg/dL) | 196±42 | 196±42 | 0.961 |
| Triglyceride (mg/dL) | 161±89 | 153±61 | 0.614 |
| LDL-cholesterol (mg/dL) | 127±36 | 125±40 | 0.979 |
| HDL-cholesterol (mg/dL) | 45±11 | 46±13 | 0.493 |
| Hemoglobin (gr/dL) | 13.7±1.6 | 13.4±1.6 | 0.475 |
| CT-1 (fmol/mL) | 11.30 [8.09–16.51] | 17.5 [8.95–28.74] | 0.002* |
| NT-proBNP (pg/mL) | 64 [27.5–95] | 82 [55.5–241] | 0.007* |
RAS – Renin-angiotensin system; LDL – Low-density lipoprotein; HDL – High-density lipoprotein; CT-1 – Cardiotrophin-1; NT-proBNP – N terminal pro B-type natriuretic peptide. Data are shown as n (%), mean ± SD, median [interquartile range].
calculated by log transformed data.
Table 2.
Echocardiographic parameters of patient and control groups.
| Control (n=33) | Patient (n=57) | P value | |
|---|---|---|---|
| LVEF (%) | 69 [64–73] | 72 [65–75] | 0.380 |
| LVEDD (mm) | 46.6±6.4 | 46.1±5.0 | 0.633 |
| LVESD (mm) | 29.7±4.7 | 29.9±4.2 | 0.866 |
| IVSD (mm) | 11 [10–12] | 12 [10–13] | 0.317 |
| PWD (mm) | 9 [9–11] | 11 [10–12] | 0.003 |
| Left atrium diameter (mm) | 34.7±4.1 | 38.1±5.3 | 0.002 |
| Left ventricular mass index (gr/m2) | 96.0±19.0 | 99.5±20.4 | 0.424 |
| Ps/Pd | 1.29±0.34 | 1.07±0.30 | 0.002 |
| E (cm/sec) | 62.27±14.69 | 75.67±18.85 | 0.001 |
| A (cm/sec) | 75.48±21.95 | 83.07±22.30 | 0.121 |
| E/A | 0.79 [0.65–1.04] | 0.85 [0.76–1.00] | 0.273 |
| DT (msec) | 224±46 | 218±45 | 0.519 |
| IVRT (msec) | 88±17 | 96±25 | 0.355 |
| Lateral | |||
| Sm (cm/sec) | 9.22±2.03 | 8.92±2.17 | 0.524 |
| Em (cm/sec) | 10.69±1.87 | 8.69±2.00 | <0.001 |
| Am (cm/sec) | 11.88±2.35 | 11.74±3.19 | 0.828 |
| Em/Am | 0.87 [0.74–1.02] | 0.71 [0.63–0.86] | 0.006 |
| E/Em | 5.70 [5.05–6.83] | 8.47 [6.96–10.47] | <0.001 |
| PCWP (mmHg) | 9.00 [8.00–10.50] | 12.00 [10.50–15.00] | <0.001 |
| Septal | |||
| Sm (cm/sec) | 8.18±1.36 | 7.63±1.60 | 0.100 |
| Em (cm/sec) | 8.91±1.22 | 6.65±1.58 | <0.001 |
| Am (cm/sec) | 10.78±1.57 | 10.34±2.52 | 0.367 |
| Em/Am | 0.82 [0.70–0.91] | 0.63 [0.57–0.72] | <0.001 |
| E/Em | 7.05±1.67 | 11.90±3.84 | <0.001 |
| PCWP (mmHg) | 10.00 [9.00–12.00] | 16.00 [13.00–18.50] | <0.001 |
| E/Em mean | 6.40±1.48 | 10.30±3.48 | <0.001 |
| PCWP mean (mmHg) | 10.00 [9.00–11.00] | 14.00 [12.00–16.00] | <0.001 |
LVEF – left ventricular ejection fraction; LVEDD – left ventricular end-diastolic dimension; LVESD – left ventricular end-systolic dimension; IVSD – interventricular septal dimension; PWD – posterior wall dimension; Ps/Pd – ratio of peak systolic velocity and peak anterograd diastolic velocity of pulmonary venous flow; E – peak velocity of early diastolic filling; A – peak velocity of late filling; DT – deceleration time of the E-wave velocity; IVRT – isovolumetric relaxation time; Sm – peak systolic mitral annular velocity; Em – early diastolic mitral annular velocity; Am – late diastolic mitral annular velocity; E/Em – early mitral inflow velocity to early diastolic mitral annular velocity ratio; PCWP – pulmonary capillary wedge pressure. Data are shown as mean ± SD, median [interquartile range].
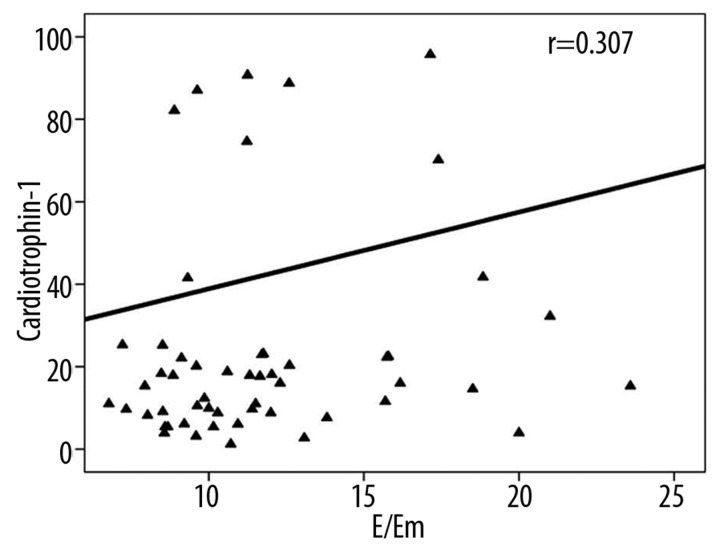
CT-1 positively correlated with NT-proBNP (P=0.001, r=0.349), mean E/Em (P=0.003, r=0.307), and estimated mean PCWP (P=0.001, r=0.308) (Figure 1). According to bivariate linear regression analyses, log CT-1 was predicted by left atrium diameter and log NT-proBNP was predicted by age, creatinine clearance, and left atrium diameter (Table 3). In multiple linear regression analysis, log CT-1 and log NT-proBNP were predicted only by left atrium diameter (Table 4).
Figure 1.
Relationship between cardiotrophin-1 and mean E/Em.
Table 3.
Bivariate linear regression models for log CT-1 and log NT-proBNP.
| Dependent variable | Independent variables | β coefficient | Standard error | P value |
|---|---|---|---|---|
| Log CT-1 | Age | 0.010 | 0.007 | 0.131 |
| Creatinine clearance | −0.002 | 0.002 | 0.336 | |
| Systolic blood pressure | 0.001 | 0.003 | 0.732 | |
| Left atrium diameter | 0.024 | 0.010 | 0.025 | |
| Left ventricular mass index | 0.002 | 0.003 | 0.426 | |
| Log NT-proBNP | Age | 0.026 | 0.007 | <0.001 |
| Creatinine clearance | −0.005 | 0.002 | 0.002 | |
| Systolic blood pressure | 0.001 | 0.003 | 0.786 | |
| Left atrium diameter | 0.030 | 0.011 | 0.007 | |
| Left ventricular mass index | 0.005 | 0.003 | 0.080 |
CT-1 – Cardiotrophin-1; NT-proBNP – N terminal pro B-type natriuretic peptide.
Table 4.
Multiple linear regression models for log CT-1 and log NT-proBNP.
| Dependent variable | Independent variables | β coefficient | Standard error | P value |
|---|---|---|---|---|
| Log CT-1 | Constant | −0.060 | 0.730 | 0.934 |
| Age | 0.005 | 0.008 | 0.533 | |
| Creatinine clearance | −0.001 | 0.002 | 0.604 | |
| Systolic blood pressure | 0.001 | 0.003 | 0.564 | |
| Left atrium diameter | 0.023 | 0.011 | 0.049 | |
| Left ventricular mass index | 0.000 | 0.003 | 0.957 | |
| Log NT-proBNP | Constant | 0.315 | 0.709 | 0.658 |
| Age | 0.015 | 0.008 | 0.066 | |
| Creatinine clearance | −0.003 | 0.002 | 0.083 | |
| Systolic blood pressure | 0.001 | 0.002 | 0.721 | |
| Left atrium diameter | 0.023 | 0.011 | 0.033 | |
| Left ventricular mass index | 0.002 | 0.003 | 0.510 |
CT-1 – Cardiotrophin-1; NT-proBNP – N terminal pro B-type natriuretic peptide.
Discussion
The data of this study shows that the CT-1 is elevated in DHF patients and is associated with NT-proBNP and estimated LV filling pressures in DHF patients.
CT-1 is a cytokine that causes hypertrophic and cytoprotective effects on the cardiac myocytes [5]. The CT-1 protein expression is constitutive not only in the heart, but also in the pulmonary, renal, gastrointestinal, cerebral, and muscular tissues. CT-1 is also expressed by vascular endothelial cells and adipocytes [6]. Cardiac myocytes and cardiac fibroblasts may produce CT-1 in situations of biomechanical stress and under exposure to humoral factors such as angiotensin II and norepineprine [23–25]. Ventricular stretch caused by pressure or volume overload is thought to be the major stimulus of myocardial CT-1 release [26].
Previous studies have found that CT-1 promotes myocardial structural changes, later participating in the progression of LV remodeling, which results in LV failure in various diseases such as hypertensive heart disease, coronary artery disease, aortic stenosis, and dilated cardiomyopathy [27]. Both BNP and CT-1 have a beneficial effect not only on the myocardium but also on hemodynamic variables [28].
Therefore, ongoing stimulation of BNP and CT-1 caused by ventricular stretch and circulating cytokines, promote structural remodeling and may become maladaptive with the progression of heart failure [17]. CT-1 levels correlate with the severity of the heart failure and has been shown to be an independent predictor of mortality in chronic heart failure [9,27]. Plasma CT-1 has high diagnostic efficacy for heart failure (at concentration of 68 fmol/ml, sensitivity and specificity were 95% and 82.5%, respectively) [29]. Talwar et al found that plasma CT-1 levels measured shortly after an acute myocardial infarction serve as a strong independent predictor of LV systolic dysfunction [16].
In another study, Talwar et al. found that changes in CT-1 levels may reflect early changes in ventricular physiology that occur in the early part of the heart failure process before they can be detected echocardiographically [12]. It was also found that CT-1 is significantly increased in patients with moderate to severe mitral regurgitation despite having normal LV systolic function and no hypertrophy. They were unable to demonstrate a similar increase in subjects with tricuspid or aortic regurgitation. In our study, we showed CT-1 elevation in a group of DHF patients with normal LV systolic function and no significant hypertrophy, but having more dilated left atrium than controls. Considering these results, it may be speculated that left atrial wall stretch is a more important and earlier stimulation for CT-1 secretion rather than ventricular wall stretch. It has already been shown that CT-1 mRNA was detected in both atria and ventricles [5,30].
In a series of studies performed by Lopez et al, they concluded that CT-1 is a more sensitive and specific biomarker than NT-pro BNP for detection of the inappropriateness of LV mass and LV dysfunction in hypertension, and NT-pro BNP remains a useful diagnostic tool for hypertensive heart disease only when LV systolic dysfunction is present [11,31]. It was also shown that elevated CT-1 levels represent an earlier stage of the same neurohumoral cascade that results in elevated plasma BNP [12]. CT-1 increases BNP secretion from cardiomyocytes in vitro[23]. In our study, both CT-1 and NT-pro BNP were found to be more elevated in DHF patients than in control subjects, and there was a positive correlation between CT-1 and NT-pro BNP. Our findings are consistent with the previous studies.
Previous studies show that elevated NT-proBNP values is diagnostic for DHF and associated with elevated LV filling pressures [32,33]. It has been found that there is a strong correlation between NT-proBNP and E/Em, and a threshold of 269.1 pg/mL of NT-proBNP predicted an E/Em >15 with 90% sensitivity and 73% specificity in DHF [34]. The positive correlation between elevated CT-1 and NT-proBNP, E/Em and PCWP in our study implies that pressure overload represented as increases in LV filling pressures could be the main underlying mechanism for CT-1 secretion in patients with DHF.
There is limited data about CT-1 in patients with DHF. A series of studies performed by Lopez et al concluded that CT-1 is associated with systolic and diastolic dysfunction in hypertensive patients [11]. In the other study they found that the ratio of peak velocity of early diastolic filling to peak velocity of late filling of mitral inflow (E/A) is the only parameter that differed in the hypertensive group, while DT and IVRT remained unchanged [31]. Significant association was found between normalization of CT-1 and regression of LV hypertrophy and increment of E/A [10]. In their studies they only used conventional Doppler parameters in order to evaluate diastolic function, and E/A was the only parameter found to be different from the control group. In our study we evaluated diastolic functions with conventional and tissue Doppler parameters. CT-1 was significantly higher in the DHF group and also correlated with these parameters.
Chronic CT-1 treatment resulted in the development of insulin resistance as judged by a decrease in insulin-stimulated glucose uptake [35]. We found high glucose levels in DHF patients, but this difference was insignificant, probably due to small sample size.
There are potential limitations of this study. First, the sample size was relatively small. Second, despite the evidences that E/Em is an effective noninvasive predictor of LV filling pressures, we did not measure LV pressures directly. Third, both of our study groups were obese. It was already known that adipose tissue can be recognized as a source of CT-1, which could account for the high circulating levels of CT-1 in patients with metabolic syndrome [36]. A study with lean subjects may reveal the true association between CT-1 and DHF. Finally, CT-1 mRNA is expressed in other organs, and potential additional sources of circulating CT-1 cannot be excluded in patients with DHF [5].
Conclusions
CT-1 values were found to be increased in patients with DHF. This increase was associated with NT-proBNP and estimated LV filling pressures in DHF patients. Our results suggest that diastolic dysfunction and subsequent pressure increase at the left side of the heart may be responsible for CT-1 increase in DHF patients. However, further studies are required to elucidate the underlying mechanism for the CT-1 increase in these patients.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–93. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 2.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–27. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 3.El-Waseef MM, Taha S, Elgindi H. Left ventricular diastolic abnormalities and the impact of hepatitis C virus infection in multitransfused Egyptian children. Arch Med Sci. 2010;6(1):96–99. doi: 10.5114/aoms.2010.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee K, Massie B. Systolic and diastolic heart failure: differences and similarities. J Card Fail. 2007;13:569–56. doi: 10.1016/j.cardfail.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Stejskal D, Ruzicka V. Cardiotrophin-1. Review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152:9–19. doi: 10.5507/bp.2008.002. [DOI] [PubMed] [Google Scholar]
- 6.Jougasaki M. Cardiotrophin-1 in cardiovascular regulation. Adv Clin Chem. 2010;52:41–76. doi: 10.1016/s0065-2423(10)52002-x. [DOI] [PubMed] [Google Scholar]
- 7.Pennica D, Wood WI, Chien KR. Cardiotrophin-1: a multifunctional cytokine that signals via LIF receptor-gp 130 dependent pathways. Cytokine Growth Factor Rev. 1996;7:81–91. doi: 10.1016/1359-6101(96)00007-x. [DOI] [PubMed] [Google Scholar]
- 8.Talwar S, Squire IB, Downie PF, et al. Elevated circulating cardiotrophin-1 in heart failure: relationship with parameters of left ventricular systolic dysfunction. Clin Sci (Lond) 2000;99:83–88. [PubMed] [Google Scholar]
- 9.Tsutamoto T, Asai S, Tanaka T, et al. Plasma level of cardiotrophin-1 as a prognostic predictor in patients with chronic heart failure. Eur J Heart Fail. 2007;9:1032–37. doi: 10.1016/j.ejheart.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez A, Lopez B, Martin-Raymondi D, et al. Usefulness of plasma cardiotrophin-1 in assessment of left ventricular hypertrophy regression in hypertensive patients. J Hypertens. 2005;23:2297–304. doi: 10.1097/01.hjh.0000184406.12634.f9. [DOI] [PubMed] [Google Scholar]
- 11.Lopez B, Castellano JM, Gonzalez A, et al. Association of increased plasma cardiotrophin-1 with inappropriate left ventricular mass in essential hypertension. Hypertension. 2007;50:977–83. doi: 10.1161/HYPERTENSIONAHA.107.098111. [DOI] [PubMed] [Google Scholar]
- 12.Talwar S, Squire IB, Davies JE, Ng LL. The effect of valvular regurgitation on plasma Cardiotrophin-1 in patients with normal left ventricular systolic function. Eur J Heart Fail. 2000;2:387–91. doi: 10.1016/s1388-9842(00)00105-7. [DOI] [PubMed] [Google Scholar]
- 13.Talwar S, Downie PF, Squire IB, et al. Plasma N-terminal pro BNP and cardiotrophin-1 are elevated in aortic stenosis. Eur J Heart Fail. 2001;3:15–19. doi: 10.1016/s1388-9842(00)00074-x. [DOI] [PubMed] [Google Scholar]
- 14.Talwar S, Squire IB, Downie PF, et al. Plasma N terminal pro-brain natriuretic peptide and cardiotrophin 1 are raised in unstable angina. Heart. 2000;84:421–24. doi: 10.1136/heart.84.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan SQ, Kelly D, Quinn P, et al. Cardiotrophin-1 predicts death or heart failure following acute myocardial infarction. J Card Fail. 2006;12:635–40. doi: 10.1016/j.cardfail.2006.06.470. [DOI] [PubMed] [Google Scholar]
- 16.Talwar S, Squire IB, O’brien RJ, et al. Plasma cardiotrophin-1 following acute myocardial infarction: relationship with left ventricular systolic dysfunction. Clin Sci (Lond) 2002;102:9–14. [PubMed] [Google Scholar]
- 17.Tsutamoto T, Wada A, Maeda K, et al. Relationship between plasma level of cardiotrophin-1 and left ventricular mass index in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2001;38:1485–90. doi: 10.1016/s0735-1097(01)01576-5. [DOI] [PubMed] [Google Scholar]
- 18.Monserrat L, López B, González A, et al. Cardiotrophin-1 plasma levels are associated with the severity of hypertrophy in hypertrophic cardiomyopathy. Eur Heart J. 2011;32:177–83. doi: 10.1093/eurheartj/ehq400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–89. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–58. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 21.Nagueh SF, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 22.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Kuwahara K, Saito Y, Harada M, et al. Involvement of cardiotrophin-1 in cardiac myocyte-nonmyocyte interactions during hypertrophy of rat cardiac myocytes in vitro. Circulation. 1999;100:1116–24. doi: 10.1161/01.cir.100.10.1116. [DOI] [PubMed] [Google Scholar]
- 24.Sano M, Fukuda K, Kodama H, et al. Autocrine/Paracrine secretion of Interleukin-6 family of cytokines causes angiotensin II-induced delayed STAT3 activation. Biochem Biophys Res Commun. 2000;269:798–802. doi: 10.1006/bbrc.2000.2364. [DOI] [PubMed] [Google Scholar]
- 25.Funamoto M, Hishinuma S, Fujio Y, et al. Isolation and characterization of the murine cardiotrophin-1 gene: expression and norepinephrine-induced transcriptional activation. J Mol Cell Cardiol. 2000;32:1275–84. doi: 10.1006/jmcc.2000.1161. [DOI] [PubMed] [Google Scholar]
- 26.Pemberton CJ, Raudsepp SD, Yandle TG, et al. Plasma cardiotrophin-1 is elevated in human hypertension and stimulated by ventricular stretch. Cardiovasc Res. 2005;68:109–17. doi: 10.1016/j.cardiores.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Calabro P, Limongelli G, Riegler L, et al. Novel insights into the role of cardiotrophin-1 in cardiovascular diseases. J Mol Cell Cardiol. 2009;46:142–48. doi: 10.1016/j.yjmcc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Jin H, Yang R, Ko A, et al. Effects of cardiotrophin-1 on haemodynamics and cardiac function in conscious rats. Cytokine. 1998;10:19–25. doi: 10.1006/cyto.1997.0241. [DOI] [PubMed] [Google Scholar]
- 29.Ng LL, O’Brien RJ, Demme B, Jennings S. Non-competitive immunochemiluminometric assay for cardiotrophin-1 detects elevated plasma levels in human heart failure. Clin Sci (Lond) 2002;102:411–16. [PubMed] [Google Scholar]
- 30.Jougasaki M, Tachibana I, Luchner A, et al. Augmented cardiac cardiotrophin-1 in experimental congestive heart failure. Circulation. 2000;101:14–17. doi: 10.1161/01.cir.101.1.14. [DOI] [PubMed] [Google Scholar]
- 31.López B, González A, Lasarte JJ, et al. Is plasma cardiotrophin-1 a marker of hypertensive heart disease? J Hypertens. 2005;23:625–32. doi: 10.1097/01.hjh.0000160221.09468.d3. [DOI] [PubMed] [Google Scholar]
- 32.Dahlström U. Can natriuretic peptides be used for the diagnosis of diastolic heart failure? Eur J Heart Fail. 2004;6:281–87. doi: 10.1016/j.ejheart.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Tuğcu A, Yildirimtürk O, Aytekin S. The diagnostic value of N-terminal B-type natriuretic peptide in diastolic heart failure: comparison with echocardiographic findings. Turk Kardiyol Dern Ars. 2009;37:112–21. [PubMed] [Google Scholar]
- 34.Krzych LJ, Liszka L. No improvement in studies reporting the diagnostic accuracy of B-type natriuretic peptide. Med Sci Monit. 2009;15(5):SR5–14. [PubMed] [Google Scholar]
- 35.Zvonic S, Hogan JC, Arbour-Reily P, et al. Effects of cardiotrophin on adipocytes. J Biol Chem. 2004;279:47572–79. doi: 10.1074/jbc.M403998200. [DOI] [PubMed] [Google Scholar]
- 36.Natal C, Fortuńo MA, Restituto P, et al. Cardiotrophin-1 is expressed in adipose tissue and upregulated in the metabolic syndrome. Am J Physiol Endocrinol Metab. 2008;294:E52–60. doi: 10.1152/ajpendo.00506.2007. [DOI] [PubMed] [Google Scholar]