Summary
Background
The reaction of vascular endothelial cells to occlusion and heat in Southeast Asian Indians (SAI) compared to Caucasians (C) has not been studied, although genetic differences are found in endothelial cells between the races.
Material/Methods
Ten C and Ten SAI (<35 years old) male and female subjects participated. There was no difference in the demographics of the subjects except that the SAI group had been in the United States for 6 months; C was natives to the US. Endothelial function was assessed by the response of the circulation (BF) to local heating and the response to vascular occlusion. The effects of local heat on circulation in the skin on the forearm was assessed by applying heat for 6 minutes at temperatures, 38, 40 and 42°C on 3 separate days. On different days, vascular occlusion was applied for 4 minutes to the same arm and skin blood flow was measured for 2 minutes after occlusion; skin temperature was either 31°C or 42°C.
Results
When occlusion was applied at a skin temperature of 31°C, the BF response to occlusion was significantly lower in the SAI cohort compared to C (peak BF C=617±88.2 flux, SAE=284±73 flux). The same effect was seen at skin temperatures of 42°C. The circulatory response to heat was also significantly less in SAI compared to C at each temperature examined (p<0.05)(for temperatures of 38, 40 and 42°C, peak blood flow for C was 374.7±81.2, 551.9±91.3 and 725.9±107 flux respectively and 248.5±86.2, 361.4±104.3 and 455.3±109.7 flux respectively for SAI. (p<0.05).
Conclusions
Thus there seems to be big differences in these 2 populations in endothelial response to these stressors. The difference may be due to genetic variations between the 2 groups of subjects.
Keywords: circulation, heat, thermal, endothelial function
Background
The blood flow in the skin and other tissues is controlled by vascular endothelial cells. Studies show that different racial populations have different genes that can alter endothelial function [1]. For example, people from Thailand and other North and Southern Asians have a thrifty gene that alters their endothelial function [1,2]. This gene was developed to protect this population from starvation and alters a nuclear transmitter PPAR, which in turn, alters the response of the endothelial cells to stressors such as anoxia [2]. Little is known about the endothelial function of people from India. The response of the skin, for example, to anoxia or even local heat application has not been examined in even a younger population of Southeast Asian Indians compared to other racial groups.
A standard measure of endothelial function is the vascular response to occlusion of the circulation [3–5]. When vascular occlusion is applied for 4 minutes and then released, there is a rapid post occlusive hyperemia which returns to normal in about 2 minutes[2,6]. This is a standard test of endothelial function used in clinical medicine [2,4]. Nitric oxide inhibition only reduces the hyperemic response to occlusion slightly [7]. It is believed that the response is driven by prostaglandins, tactile sensory nerves and other unknown factors [7,8]. Ageing and diabetes reduce the blood flow response to vascular occlusion [6,9].
Local application of heat is also mediated by the vascular endothelial cells in skin blood vessels. It is well established that when heat is applied to the skin there is an increase in skin blood flow [9–11]. Initially, tactile neurons in the skin release Substance P and Calcitonin Gene Related Peptide when the skin is exposed to local heat [12,13]. This causes an increase in potassium permeability in vascular smooth muscle surrounding the endothelial cell [12,14,15]. Relaxation of vascular smooth muscle then increases blood flow. But this response only lasts a few minutes. The sustained response to increasing temperature in the skin is mediated by TRPV-4 voltage gated calcium channels in the vascular endothelial cells [16–19]. Above a temperature of 35°C, these cells cause an exponential increase in calcium influx into the endothelial cell from the interstitial space. Calcium activates the enzyme nitric oxide synthase producing endothelial nitric oxide [11]. Nitric oxide, a potent vasodilator, diffuses into the surrounding smooth muscle activating cyclic GMP which in turn increases potassium permeability and relaxes vascular smooth muscle [12,15,20–22]. Damage to endothelial cells with ageing and diabetes diminishes the response to local heat [6,9].
Both of these tests, that is occlusion and the response of the skin to local heat have been well characterized in Caucasians but little has been done to examine the responses in other races. Some studies have been conducted on Asians. For example, the response to occlusion is blunted in people from Thailand compared to Caucasians [2]. While occlusion is a useful clinical test, it is more practical to examine the response to other stressors such as to heat since heat is used as a therapeutic modality [23–25]. Many of the same genes as seen in people from Thailand are found in Southeast Asian Indians. Since people from Southeast Asia have a similar gene pool to people from Thailand, the hypothesis in the present investigation is that people from India will have a reduced response to heat and occlusion as was seen for occlusion with people from Thailand. Therefore, the present investigation tested the hypothesis that people from Southeast Asia would have a diminished response to heat and vascular occlusion.
Material and Methods
Subjects
Twenty subjects participated in these experiments. The subjects were divided into two groups; one group of people from India and one other group of Caucasians. Both were living in the United States and were students at Loma Linda University. Most students were physical therapists. The cohort from India was only in the United States for 6 months. The Caucasian cohort were all born and grew up in the United States. There were 10 subjects in each group. Subjects had no diagnosed cardiovascular disease, were not taking any medications that would affect the cardiovascular system and did not have any known peripheral circulatory diseases. The general characteristics of the subjects are shown below in Tables 1 and 2. As shown in Tables 1 and 2, there was no significant statistical difference between ages, height, weight, BMI or % body fat of the 2 groups of the subjects. However, although there was no significant statistical difference in the general demographics, when ultrasound was used to measure subcutaneous fat thickness and skin thickness, the average skin thickness for the Caucasian group was 0.04±0.01 mm with the same skin thickness in the group from south-east India. Subcutaneous fat thickness was higher at 0.11±0.03 for the Caucasian group compared to 0.08±0.03 for the Indian group. This difference was statistically significant (p<0.05). The resting heart rate and blood pressure of the 2 groups of subjects was 72±6 beats per minute and 122±8.9/77±5.6 mmHg in the C group respectively and 71±7 and 120±10.2/75±8.2 mmHg in the SAI group. There was no significant difference between the groups.
Table 1.
General characteristics of the 4 men and 6 women in the Caucasian group of subjects.
| Age (years) | Height (cm) | Weight (kg) | BMI | % fat | |
|---|---|---|---|---|---|
| Mean | 26.78 | 173.61 | 70.56 | 23.32 | 25.87 |
| SD | 2.64 | 10.22 | 11.28 | 2.62 | 6.53 |
Table 2.
General characteristics of the 7 men and 3 women in the Indian group of subjects.
| Age (years) | Height (cm) | Weight (kg) | BMI | % fat | |
|---|---|---|---|---|---|
| Mean | 25.2 | 170.9 | 74.2 | 25.2 | 26.8 |
| SD | 2.6 | 7.1 | 14.6 | 3.8 | 5.6 |
All protocols and procedures were approved by the Institutional Review Board of Loma Linda University and all subjects signed a statement of informed consent.
Methods
Measurement of skin blood flow
Skin blood flow was measured with a MOOR Laser Doppler imager (Moor LTD Oxford, England). The imager used a red Laser beam to measure blood flow in the skin. The Laser was used in a single spot mode. In this mode, the Laser scanned a single point continuously to measure the skin blood flow. Blood flow in a Laser Doppler imager is calculated in a unit called flux. The stated reliability from the manufacturer is ±5% from day to day. The laser was calibrated just before and in the middle and end of the study; there were no calibration changes noted.
Measurement of skin temperature
Skin temperature was measured by a thermocouple. The thermocouple is a copper constantan alloy thermocouple from Columbus Instruments (Columbus, Ohio) and was connected to an IsoThermix analysis system. The IsoThermix system was internally calibrated and measured temperature from a catheter which is small enough to fit into the tip of a 22 gauge needle. The thermocouple was calibrated at 2 different temperatures, 30 and 45 degrees centigrade before the experiments started, in the middle and at the end. The baths were verified with a standard reference thermometer.
Control of skin temperature
Skin temperature was controlled with a thermode. The thermode consisted of a plastic chamber with a port on each end so that water could pass through the interior. The chamber was approximately 5.3 cm by 2.5 cm by 2.5 cm in size. On each end of the chamber was a thermocouple such that as water circulated through the interior from a controlled temperature water bath (Biopac systems, Goleta California), the temperature difference across the chamber could be measured. Heat flow from the chamber to the skin was measured by multiplying the flow of water per minute going through the outlet times the temperature differential across the chamber. This provided the number of calories of heat being delivered to the skin. Water bath temperatures were kept at 38, 40 or 42°C. A hole through the thermode allowed the Laser to scan through the center of the thermode onto the skin. Further detail on the technique and the reliability and validity are published elsewhere [26,27].
Body fat content
Body fat content was measured by an Quantum II Body Composition Analyzer (RJL Systems, Clinton TWP, MI).
Skin thickness and subcutaneous fat thickness
Skin and subcutaneous fat thickness was assessed with a Mindray M7 Ultrasound. The probe used was a linear probe with 512 elements which could measure the thickness of skin and subcutaneous fat to a resolution of 0.01 mm. It was used at a base frequency of 10 MHz
Procedures
Subjects entered a thermally neutral room (22°C) and rested comfortably for 20 minutes in the seated position. The area used was the skin over the belly of the brachioradialus muscle. For each person, a mark was placed at the side of the flow measurement with ink so that placement was the same from one experiment to the next. Baseline blood flow was recorded for 1 minute. Five different experiments were then conducted. In 3 of these experiments, the thermode was placed on the skin and the water temperature warmed the skin to 38°C, 40°C or 42°C on 3 separate days, respectively. The thermode was left on for 6 minutes. On another day, occlusion was applied for 4 minutes by a blood pressure occlusion cuff inflated to 200 mmHg followed by 2 minutes of additional blood flow recording. Finally, on the last day, the occlusion experiments were repeated but the thermode was applied throughout the entire 6 minute period bringing skin temperature to 42°C. Skin temperature at this site was measured throughout the experimental period.
Analysis of data
Means and standard deviations were calculated using Excel and SPSS version 15. Two way ANOVA repeated measures (Scheffé test Post Hoc testing) was also calculated on SPSS with a level of significance of p<0.05.
Results
Response to vascular occlusion
Caucasian group
The results of the determination of skin blood flow after 4 minutes of vascular occlusion are shown in Figures 1 (Caucasians) and 2 (Southeast Indians) respectively. As shown in Figure 1, when the subjects sat in a thermally neutral room, the average skin blood flow at rest was 102.1±33.1 flux. During the occlusion, there was some skin blood flow but this low blood flow, which was seen in both Figures 1 and 2 for both Caucasian and Indians, is due to molecular motion in the arm and not due to actual blood flow. Laser Doppler imaging measures molecular movement and hence, can only show 0 flux under the condition that all molecular motion stops. As shown in Figure 1, for Caucasians, after the occlusion was released at 240 seconds, there was a rapid increase in blood flow to a maximum value of 617.3±88.2 flux within 30 seconds after the occlusion cuff was removed. The skin blood flow for this group of 10 subjects reduced rapidly over the next 2 minutes towards the initial resting flow of approximately a 100 flux. The final blood flow 2 minutes after vascular occlusion in an environmentally neutral room was 208.7±81.2 flux. This was not the case when the arm was heated during vascular occlusion. When the arm was heated as shown in Figure 1, with a thermode at 42°C, both during and after the occlusion was removed, blood flow increased significantly higher, increasing to 711.3±101.4 flux at the peak after the cuff was removed but then plateaued at an average of 641±6 flux for the remaining 2 minute period. In Figure 1, there was no significant difference in the blood flow at rest. After vascular occlusion was released, blood flow was significantly different (p<0.01) for flows recorded for all time periods after occlusion was released.
Figure 1.
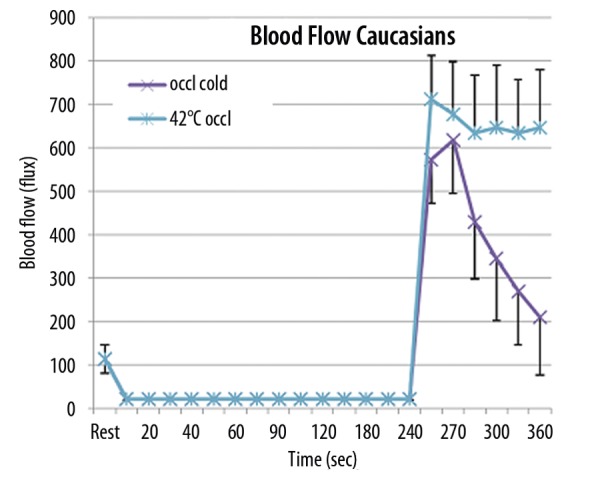
Illustrated here is the relationship between blood flow (flux) obtained on a Laser Doppler Imager and the time that the blood flow was collected in seconds on Caucasian subjects. Each point represents the mean of the results of the 10 subjects ± the respective standard deviation under 2 conditions. In the first condition, occlusion was applied for the arm for 4 minutes in a thermally neutral room. Blood flow was then recorded throughout the 4 minute period and the 2 minute period following the release of the occlusion cuff. In the second set of experiments, occlusion was also applied for 4 minutes but with the arm warmed to 42°C with the thermode during the occlusion and for 2 minutes following the occlusion.
Figure 2.
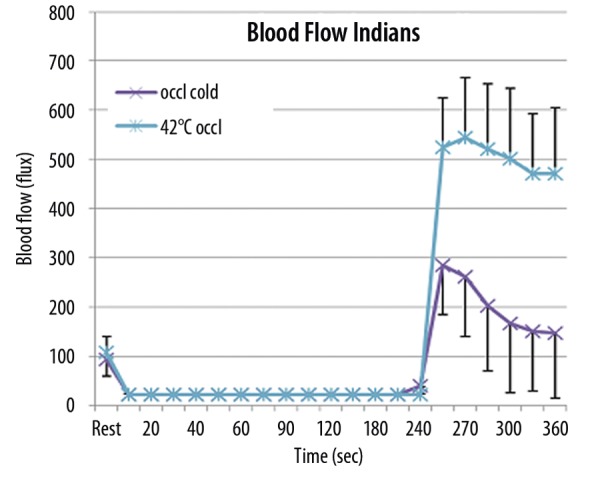
Illustrated here is the relationship between blood flow (flux) obtained on a Laser Doppler Imager and the time that the blood flow was collected in seconds in South East Asian Indians. Each point represents the mean of the results of the 10 subjects ± the respective standard deviation under 2 conditions. In the first condition, occlusion was applied for the arm for 4 minutes in a thermally neutral room. Blood flow was then recorded throughout the 4 minute period and the 2 minute period following the release of the occlusion cuff. In the second set of experiments, occlusion was also applied for 4 minutes but with the arm warmed to 42°C with the thermode during the occlusion and for 2 minutes following the occlusion.
Asian Indians
For the group of subjects from Southeast Asia, skin blood flow, at rest, averaged 101.2±24.3 flux. This was not significantly different from the resting blood flow in the Caucasians (P>0.05). In the thermally neutral environment, when the occlusion was removed, skin blood flow immediately increased during the post-occlusion reactive hyperemia to a maximum of 284.5±73.2 flux as shown in this figure. The average skin blood flow then decreased exponentially to a final value of 147.3±64.3 flux at the end of the 2 minute period. In contrast, when the skin was warmed, there was a much greater increase in the circulation, skin blood flow increasing to a maximum of 543.8±101.3 flux after the occlusion cuff was released and then plateauing at 471.1±104.5 flux at 2 minutes after the occlusion. In Figure 2, there was no significant difference in the blood flow at rest. After vascular occlusion was released, blood flow was significantly different (p<0.01) for flows recorded for all time periods after occlusion was released.
Comparing Caucasians and Indians, the skin blood flow after the occlusion cuff was removed was significantly higher immediately after and for first minute and half after occlusion was removed (during the reactive hyperemia) comparing Caucasians to Indians (p<0.01) when occlusion was applied in the thermally neutral room. This was also true when heat was applied, except that here, skin blood flow after the occlusion cuff was removed was significantly higher for the Caucasians at every measuring point throughout the entire 2 minute post-occlusive hyperemia period (p<0.01).
Response to local heat
The change in skin temperature associated with 6 minutes of passive heating for Caucasians and Southeast Indians are shown in Figures 3 and 4 respectively. As illustrated for the mean results (average of all subjects in each group) in these 2 figures, for both groups of subjects, skin temperature increase continuously over the 6 minute period. However, as might be expected, the greatest skin temperature increase was after exposure to the 42°C thermode for both groups of subjects. However, there are differences between the groups.
Figure 3.
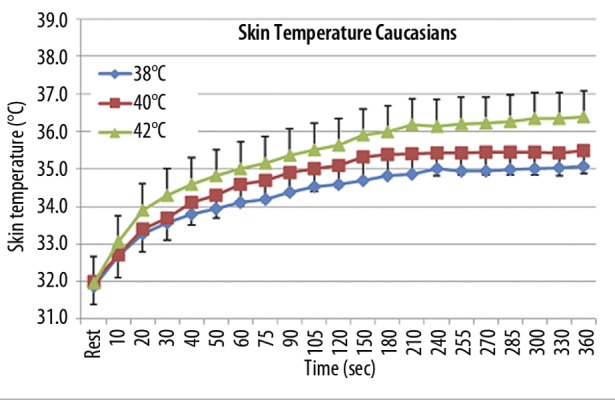
Illustrated here is the skin temperature (°C) recorded throughout the exposure to a 38, 40, 42°C for period of 360 seconds(X-axis) on 10 Caucasian subjects. Each point illustrates the mean ± respective standard deviation.
Figure 4.

Illustrated here is the skin temperature (°C) recorded throughout the exposure to a 38, 40, 42°C thermode applied to the skin for period of 360 seconds(X-axis) on 10 Southeast Asian Indian subjects. Each point illustrates the mean ± respective standard deviation.
Skin temperature in Caucasians
For the Caucasian subjects (Figure 3), the average skin temperature started at 32.6±0.6°C and increased to a maximum after exposure to the 38°C thermode of 35.0±0.9°C, after the 40°C thermode to 35.5±0.9°C and after the 42°C thermode to 36.2±1.1°C 6 minutes after the thermode was applied to the skin. The skin temperature after the 38, 40 and 42°C thermodes were significantly greater than each other and higher with the greater thermode temperatures starting at 20 seconds after the thermode was applied (ANOVA, p<0.01). For the first 20 seconds there was no significant difference in skin temperature for the 38 and 40 degree baths but the 42 degree bath showed a high skin temperature starting at 10 seconds compared to the other two experiments (p<0.05).
Southeast Asia Indians
In contrast, while the average skin temperature at rest, averaged 32.5±0.5°C for the subjects from Southeast Asia (Figure 4) was not significantly different from that of the Caucasians (p>0.05), there was a difference in the maximum temperature at the end of the 6 minutes after exposure to the 3 different thermode temperatures. The skin temperature after the 38, 40 and 42°C thermodes were significantly greater than each other and higher with the greater thermode temperatures starting at 20 seconds after the thermode was applied (ANOVA, p<0.01). For the first 20 seconds there was no significant difference in skin temperature for the 38 and 40 degree baths but the 42 degree bath showed a high skin temperature starting at 10 seconds compared to the other two experiments (p<0.05).
While the general trend was the same for Indians and Caucasians as shown in Figures 3 and 4, showing a continuous increase in the temperature of the skin to final values after thermode exposure at 38, 40 and 42°C of 36.3±0.8, 37.0±0.9 and 37.7±0.9°C respectively, the temperature in the skin in Indian subjects was generally higher than that of the Caucasian subjects. All temperature measurements for the skin starting at 30 seconds after the thermode was placed on the skin at all 3 thermode temperatures were significantly higher in the Southeast Asians compared to the Caucasians (p<0.05). For the first 30 seconds, the difference was no significant. There was also a difference in the number of calories absorbed from the thermode in the Caucasian vs. subjects from Southeast Asia.
Calories transferred during heating in Caucasians and Asians
When the number of calories to warm the skin was calculated for each of the 3 bath temperatures (38, 40, 42°C), for the Caucasians, over the 6 minute period the average was 8.1±1.3, 6.4±0.7 and 3.8±0.6 calories per minute over the 6 minute period that was transferred from the thermode into the skin. The number of calories transferred at each temperature was significantly different from each other (ANOVA, p<0.01). For the subjects, who were Southeast Asian Indians, the average number of calories was significantly less at each temperature for the thermodes than for the Caucasians (p<0.05) averaging 5.9±0.6, 4.1±0.6, 1.9±0.3 calories per minute.
Skin blood flow during local heat exposure
The results of the determination of skin blood flow during 6 minutes exposure taken after exposure to thermode temperatures of 38, 40, 42°C are shown in Figures 5 (Caucasians) and 6 (Southeast Asian Indians).
Figure 5.
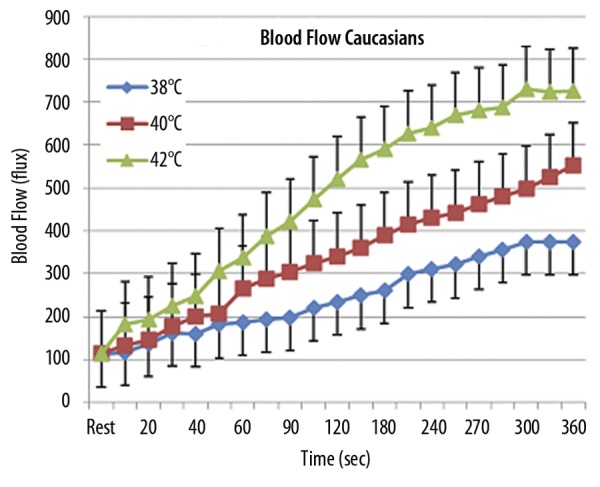
Illustrated here is the blood flow (flux) measured during the exposure to heat with the forearm heat by a thermode at 38, 40, or 42°C in 10 Caucasian subjects at rest and over a period of 360 seconds. Each point illustrates the mean of the 10 subjects ± the standard deviation for exposure to each of the 3 conditions on separate days.
Caucasians
For the Caucasian cohort, after exposure to all 3 thermode temperatures, skin blood flow increased continuously as shown in Figure 5. The greatest skin blood flow seen was after 6 minutes exposure to a thermode of 42°C. Circulation was much less in the skin of the forearm for thermode temperatures of 40 and 38°C respectively. The average resting blood flow of the forearm was 113.38±48.2 flux. From this initial value, the final blood flows after 6 minutes of exposure to the thermode at 38, 40 and 42°C were 374.7±81.2, 551.9±91.3 and 725.9±107 flux respectively. The blood flow, was significantly higher at 42 then 40 then 38°C at any point during the heat exposure (ANOVA, p<0.01) after 30 seconds of heat exposure. For rest and 10 seconds there was no significant difference and at 20 and 30 seconds only the 42 degree thermode showed a significantly higher blood flow response.
Southeast Asian Indians
For the Southeast Asian Indians, the average resting blood flow was not significantly different than that of the Caucasians averaging 96.0±37.1 flux. The final blood flow after the exposure to the thermode at 38, 40 and 42°C was 248.5±86.2, 361.4±104.3 and 455.3±109.7 flux respectively for the 3 temperatures. As was the case with the Caucasians, the skin blood flow increased above rest at each point in time for the whole 6 minutes but the magnitude of the blood flow response was significantly higher for the thermode at 42 compared to 40°C and 38°C (ANOVA, p<0.01) after 90 seconds of placement of the thermode. For the first 20 seconds, there was no significant difference in the response at any thermode temperature. From 30 to 80 seconds, only the 42 degree thermode flux response was significantly higher than the other 2 temperatures (p<0.05). Comparing Caucasians to Indians, for each temperature, that is 38, 40, 42°C that the thermode was at, Caucasians had significantly higher skin blood flow than was seen for Southeast Indian subjects (p<0.01) after 90 seconds of thermode placement. In the first 40 seconds, there was no significant difference and from 50 to 80 it was significant (p<0.05).
Discussion
The gold standard for measuring vascular endothelial function is by applying 4 minutes of occlusion to the limb and then measuring the reactive hyperemia either in the brachial artery of the forearm or the skin after the occlusion is released [2,5,6,28,29]. The test has been used in numerous studies to assess vascular endothelial function to show cardiovascular disease[30], vascular damage elicited by diabetes [6,31,32], and to assess the effect of ageing on vascular endothelial function [33]. The implication of this test is that reduced vascular endothelial function affects the blood flow to organs and impairs organ function. However, while vascular endothelial function as assessed by occlusion or even the effect of local heat on skin circulation has been well documented in Caucasians, it has not been well documented in Southeast Asian Indians.
In the present investigation, 10 young Caucasian subjects and 10 young subjects who were Southeast Asian Indians were examined. First, occlusion was applied in a thermally neutral room to assess vascular endothelial function. In these young subjects, there was a marked difference in the vascular endothelial response in the Southeast Asian Indians examined in this study compared to Caucasians. Whereas the time course of the reactive hyperemia was approximately the same in both cohorts, the blood flow response after occlusion was approximately twice as high in Caucasians as in the subjects from India. This would seem to imply reduced vascular endothelial function in this Indian population. Numerous studies have pointed to genetic differences between Asians, Southwest Asians, and people from India and Caucasians [1]. For example, Asians have a gene which has been called the “thrifty gene”[2]. This gene, which was developed historically to allow this population to survive on low caloric diets for long periods of time, with a modern diet, has resulted in endothelial dysfunction [1]. As Indians have moved from an agrarian to a Western industrialized economy, these same genes have caused one of the highest increase in diabetes and heart disease than any other population in the world [1,4,5]. The difference in the blood flow response between the 2 groups may be linked to either a genetic difference in endothelial function or, to the effect of modern diet on Indian subjects in the United States vs. Caucasians. On an Indian Americanized diet, it is possible that these subjects have enhanced oxidative stress in their diet that has reduced their vascular endothelial function. Even a single high fat meal can reduce endothelial function and the response to anoxia [2,34,35]. The mechanism of this response has been linked to increased production of free radicals from a higher fat diet than the thrifty gene normally allows Asians to consume. Increased free radical production then causes the bio-conversion of nitric oxide in vascular endothelial cells to peroxynitrite, reduces the vascular endothelial response to a stress such as anoxia [2].
But other factors may be involved. The Indian cohort studied in these experiments was all medical professionals. Due to industrialization, another factor in this population, due to dark skin, is severe depression of vitamin D [4,5,36,37]. Physical therapists, due to indoor work, have the lowest vitamin D levels in their blood of all Indians studied in a recent publication[36]. Low Vitamin D seems to be related to poor vascular endothelial function in response to occlusion[4]. This population may be atypical of other Indian Cohorts, although most people in India, do have severely depressed vitamin D [4]. Additional studies would need to be conducted on other Indian cohorts in the United States and in India to confirm if the depressed endothelial function seen here is common to other Indian populations.
When the skin was warmed during and after occlusion, for the Caucasian subjects, skin blood flow was higher than that seen in the Indian cohort, increasing to 700 flux and being maintained at this high level after the occlusion was over. This is presumably due to the effect of local heating on the skin plus the occlusion, a double stressor [38]. In the subjects from India, however, the blood flow response during heating and occlusion, while being less than that seen in the Caucasians, was much greater than for occlusion in a thermally neutral room. However, the response was still approximately 1/3rd lower than that seen for Caucasian subjects.
Apparently, by combining heat with occlusion, then, the vascular response was closer in Indians to that of Caucasians. However, another factor must also be considered when sustained heat was applied without occlusion for the same period of 6 minutes. When examining skin temperature without occlusion, the skin temperature response seen in Indian subjects was 1.5°C higher than that in Caucasians. This greater increase in the skin temperature in people from India associated with same heat stress, 42°C, may be responsible for part of the greater increase in the blood flow with occlusion and heat together. Blood flow is proportional to the skin temperature. Thus, the warmer skin with occlusion should demand a greater blood flow response in the Indian cohort. However, this may only partially explain the greater proportional skin blood flow response to occlusion in heat. As shown in Figures 3–6, comparing blood flow and skin temperature in Indians vs. Caucasians, even with higher skin temperatures, people from India had significantly less skin blood flow in response to any thermode temperature applied to the skin than in the Caucasian cohort. If the blood flow is compared in the 2 cohorts at the same skin temperature (since temperatures were higher in the Indian cohort), the circulatory response was even less then shown in these figures. When Indian subjects were exposed to a thermode at 42°C, for example, skin temperature was 37°C at 120 seconds after exposure to the 42°C thermode. The skin blood flow was only 300 flux. Caucasians, even at the skin temp of 36.5°C, the highest temperature recorded in these experiments after 6 minutes exposure to thermode at 42°C, had a blood flow of over 700 flux. If skin was cooler, the Indian cohort blood flow response would be even less. In this respect, the results are comparable with the occlusion series.
Figure 6.
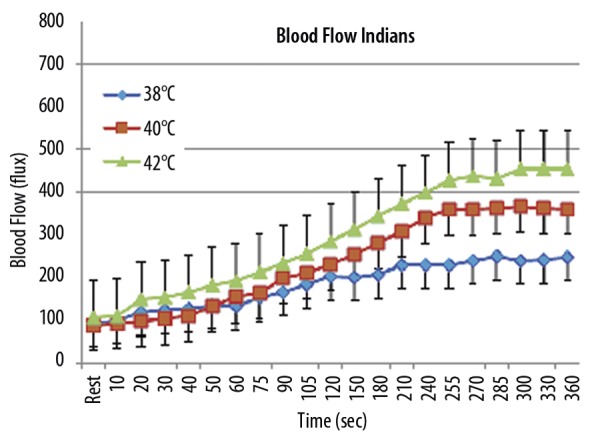
Illustrated here is the blood flow (flux) measured during the exposure to heat with the forearm heat by a thermode at 38, 40, or 42°C in 10 Southeast Asian Indian subjects at rest and over a period of 360 seconds. Each point illustrates the mean of the 10 subjects ± the standard deviation for exposure to each of the 3 conditions on separate days.
Another possibility causing the difference between the groups is related to the different distribution of males and females in the 2 cohorts. The menstrual cycle does alter the blood flow response to heat and occlusion [39,40]. There were more women in one cohort than the other but since the experiments were spread out randomly over an entire menstrual cycle, this should only cause more variance in the data but not alter the means.
Body fat distribution differences between the 2 races may also alter the responses reported here. But the key factor is the thickness of the subcutaneous fat in determining how fast skin temperature rises to a heat load [10,21,41,42]. Here, the Indian population had less subcutaneous fat than the Caucasians; as such their skin temperature should rise less and not more to a heat load. The likely cause of the increase in skin temperature in the Indians is the lower blood flow, keeping the skin from losing heat.
The blood flow response to both occlusion and heat in this young population was impaired. It is tempting to look at older populations and populations with diabetes. Old age and diabetes are associated with an increase production of free radicals and increased endothelial dysfunction [3,6,19]. It might be anticipated then that for older subjects from India with diabetes, the blood flow response would be a small fraction of that seen here. Lower blood flows in response to occlusion and heat may make this population more susceptible to skin damage and burns. Certainly, recent studies show that burns and skin damage are major problems in India [43]. But direct comparisons to Caucasian populations due to socio economic differences make causal relationships difficult to establish. Further investigation is wanted.
Conclusions
Southeast Asian Indians display both a diminished blood flow response of the skin to local heat and to 4 minutes of vascular occlusion compared to Caucasians. Since these groups were matched for age, height and weight, the differences between these groups may be due to genetic differences in the endothelial cell metabolism through what has been termed the “thrifty” found in Asians and Southeast Asians. This gene, believed to protect this population from starvation, has been shown to cause endothelial dysfunction even 2 hours after a high fat meal and may be responsible for the reduced blood flow seen here.
Footnotes
Source of support: Departmental sources
Referenes
- 1.Pemberton TJ, et al. Prevalence of common disease-associated variants in Asian Indians. BMC Genet. 2008;9:13. doi: 10.1186/1471-2156-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bui C, et al. Acute effect of a single high-fat meal on forearm blood flow, blood pressure and heart rate in healthy male Asians and Caucasians: a pilot study. Southeast Asian J Trop Med Public Health. 2010;41(2):490–500. [PMC free article] [PubMed] [Google Scholar]
- 3.Petrofsky J, et al. The effect of rosiglitazone on orthostatic tolerance during heat exposure in individuals with type II diabetes. Diabetes Technol Ther. 2007;9(4):377–86. doi: 10.1089/dia.2006.0028. [DOI] [PubMed] [Google Scholar]
- 4.Witham MD, et al. The effect of vitamin D replacement on markers of vascular health in stroke patients – A randomised controlled trial. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Witham MD, et al. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010;53(10):2112–19. doi: 10.1007/s00125-010-1838-1. [DOI] [PubMed] [Google Scholar]
- 6.Petrofsky J, Lee S. The effects of type 2 diabetes and aging on vascular endothelial and autonomic function. Med Sci Monit. 2005;11(6):CR247–54. [PubMed] [Google Scholar]
- 7.Wong BJ, et al. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol. 2003;95(2):504–10. doi: 10.1152/japplphysiol.00254.2003. [DOI] [PubMed] [Google Scholar]
- 8.Larkin SW, Williams TJ. Evidence for sensory nerve involvement in cutaneous reactive hyperemia in humans. Circ Res. 1993;73(1):147–54. doi: 10.1161/01.res.73.1.147. [DOI] [PubMed] [Google Scholar]
- 9.Petrofsky J, et al. Effects of contrast baths on skin blood flow on the dorsal and plantar foot in people with type 2 diabetes and age-matched controls. Physiother Theory Pract. 2007;23(4):189–97. doi: 10.1080/09593980701209295. [DOI] [PubMed] [Google Scholar]
- 10.Petrofsky JS, Laymon M. Heat transfer to deep tissue: the effect of body fat and heating modality. J Med Eng Technol. 2009;33(5):337–48. doi: 10.1080/03091900802069547. [DOI] [PubMed] [Google Scholar]
- 11.Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV-1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol. 2010;588(Pt 21):4317–26. doi: 10.1113/jphysiol.2010.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charkoudian N, Fromy B, Saumet JL. Reflex control of the cutaneous circulation after acute and chronic local capsaicin. J Appl Physiol. 2001;90(5):1860–64. doi: 10.1152/jappl.2001.90.5.1860. [DOI] [PubMed] [Google Scholar]
- 13.Farage MA, Miller KW, Maibach HI. Textbook of Aging Skin. Springer-Verlag; Berlin Heidelberg: 2010. [Google Scholar]
- 14.Charkoudian N, et al. Effects of chronic sympathectomy on locally mediated cutaneous vasodilation in humans. J Appl Physiol. 2002;92(2):685–90. doi: 10.1152/japplphysiol.00758.2001. [DOI] [PubMed] [Google Scholar]
- 15.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91(4):1619–26. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe H, et al. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277(49):47044–51. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe M, et al. Assessment of mechanical and thermal thresholds of human C nociceptors during increases in skin sympathetic nerve activity. Clin Neurophysiol. 2002;113(9):1485–90. doi: 10.1016/s1388-2457(02)00159-1. [DOI] [PubMed] [Google Scholar]
- 18.Petrofsky J. The effect of the subcutaneous fat on the transfer of current through skin and into muscle. Med Eng Phys. 2008;30(9):1168–76. doi: 10.1016/j.medengphy.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Alderton F, et al. G-protein-coupled receptor stimulation of the p42/p44 mitogen-activated protein kinase pathway is attenuated by lipid phosphate phosphatases 1, 1a, and 2 in human embryonic kidney 293 cells. J Biol Chem. 2001;276(16):13452–60. doi: 10.1074/jbc.M006582200. [DOI] [PubMed] [Google Scholar]
- 20.Charkoudian N, Rabbitts JA. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin Proc. 2009;84(9):822–30. doi: 10.4065/84.9.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrofsky J, et al. Dry heat, moist heat and body fat: are heating modalities really effective in people who are overweight? J Med Eng Technol. 2009;33(5):361–69. doi: 10.1080/03091900802355508. [DOI] [PubMed] [Google Scholar]
- 22.Alderton F, Fan TP, Humphrey PP. Somatostatin receptor-mediated arachidonic acid mobilization: evidence for partial agonism of synthetic peptides. Br J Pharmacol. 2001;132(3):760–66. doi: 10.1038/sj.bjp.0703862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrofsky J, et al. The Ability of Different Areas of the Skin to Absorb Heat from a Locally Applied Heat Source: The Impact of Diabetes. Diabetes Technol Ther. 2011;13(3):365–72. doi: 10.1089/dia.2010.0161. [DOI] [PubMed] [Google Scholar]
- 24.Petrofsky J, et al. The ability of the skin to absorb heat; the effect of repeated exposure and age. Med Sci Monit. 2011;17(1):CR1–8. doi: 10.12659/MSM.881315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer JM, et al. Continuous low-level heat wrap therapy for the prevention and early phase treatment of delayed-onset muscle soreness of the low back: a randomized controlled trial. Arch Phys Med Rehabil. 2006;87(10):1310–17. doi: 10.1016/j.apmr.2006.07.259. [DOI] [PubMed] [Google Scholar]
- 26.Petrofsky J, et al. The contribution of skin blood flow in warming the skin after the application of local heat; the duality of the Pennes heat equation. Med Eng Phys. 2011;33(3):325–29. doi: 10.1016/j.medengphy.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Petrofsky JS. A device to measure heat flow through the skin in people with diabetes. Diabetes Technol Ther. 2010;12(9):737–43. doi: 10.1089/dia.2010.0043. [DOI] [PubMed] [Google Scholar]
- 28.Perko D, et al. Endothelium-dependent vasodilatation in migraine patients. Cephalalgia. 2011;31(6):654–60. doi: 10.1177/0333102410390396. [DOI] [PubMed] [Google Scholar]
- 29.Soltesz P, et al. Comparative assessment of vascular function in autoimmune rheumatic diseases: Considerations of prevention and treatment. Autoimmun Rev. 2011;10(7):416–25. doi: 10.1016/j.autrev.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, et al. Effect of orlistat-assisted weight loss on endothelium-dependent vasodilation in obese Chinese subjects with hypertension. Clin Exp Hypertens. 2010;32(6):395–99. doi: 10.3109/10641961003667906. [DOI] [PubMed] [Google Scholar]
- 31.Silva AM, et al. Insulin therapy does not interfere with venous endothelial function evaluation in patients with type 2 diabetes mellitus. Clinics (Sao Paulo) 2010;65(11):1139–42. doi: 10.1590/S1807-59322010001100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva PM. From endothelial dysfunction to vascular occlusion: role of the renin-angiotensin system. Rev Port Cardiol. 2010;29(5):801–24. [PubMed] [Google Scholar]
- 33.Lesniewski LA, et al. Salicylate Treatment Improves Age-Associated Vascular Endothelial Dysfunction: Potential Role of Nuclear Factor {kappa}B and Forkhead Box O Phosphorylation. J Gerontol A Biol Sci Med Sci. 2011;66(4):409–18. doi: 10.1093/gerona/glq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimabukuro M, et al. Effects of dietary composition on postprandial endothelial function and adiponectin concentrations in healthy humans: a crossover controlled study. Am J Clin Nutr. 2007;86(4):923–28. doi: 10.1093/ajcn/86.4.923. [DOI] [PubMed] [Google Scholar]
- 35.Giannattasio C, et al. Acute effect of high-fat meal on endothelial function in moderately dyslipidemic subjects. Arterioscler Thromb Vasc Biol. 2005;25(2):406–10. doi: 10.1161/01.ATV.0000152231.93590.17. [DOI] [PubMed] [Google Scholar]
- 36.Babu US, Calvo MS. Modern India and the vitamin D dilemma: evidence for the need of a national food fortification program. Mol Nutr Food Res. 2010;54(8):1134–47. doi: 10.1002/mnfr.200900480. [DOI] [PubMed] [Google Scholar]
- 37.Verma S. 14th Annual Meeting, Cosmetic Dermatology Society of India, Mumbai, September 2010. Int J Dermatol. 2011;50(1):123–24. doi: 10.1111/j.1365-4632.2010.04878_1.x. [DOI] [PubMed] [Google Scholar]
- 38.McLellan K, et al. Multiple stressors and the response of vascular endothelial cells: the effect of aging and diabetes. Diabetes Technol Ther. 2009;11(2):73–79. doi: 10.1089/dia.2008.0026. [DOI] [PubMed] [Google Scholar]
- 39.Petrofsky J, Al Malty A, Suh HJ. Isometric endurance, body and skin temperature and limb and skin blood flow during the menstrual cycle. Med Sci Monit. 2007;13(3):CR111–17. [PubMed] [Google Scholar]
- 40.Petrofsky JS, et al. Isometric strength and endurance during the menstrual cycle. Eur J Appl Physiol Occup Physiol. 1976;35(1):1–10. doi: 10.1007/BF00444652. [DOI] [PubMed] [Google Scholar]
- 41.Petrofsky JS, Lind AR. Insulative power of body fat on deep muscle temperatures and isometric endurance. J Appl Physiol. 1975;39(4):639–42. doi: 10.1152/jappl.1975.39.4.639. [DOI] [PubMed] [Google Scholar]
- 42.Petrofsky JS, Lind AR. The relationship of body fat content to deep muscle temperature and isometric endurance in man. Clin Sci Mol Med. 1975;48(5):405–12. doi: 10.1042/cs0480405. [DOI] [PubMed] [Google Scholar]
- 43.Gupta AK, et al. A clinico-epidemiologic study of 892 patients with burn injuries at a tertiary care hospital in Punjab, India. J Emerg Trauma Shock. 2011;4(1):7–11. doi: 10.4103/0974-2700.76820. [DOI] [PMC free article] [PubMed] [Google Scholar]


