Summary
Background
Data from animal studies show that antioxidants can compensate against noise-induced stress and sensory hair cell death. The aim of this study was to evaluate the otoprotection efficacy of various versions of orally administered Acuval 400® against noise damage in a rat animal model.
Material/Methods
Fifty-five Sprague Dawley rats were divided into 4 groups: A) noise-exposed animals; B) animals exposed to noise and treated with the Acuval; C) animals exposed to noise and treated with a combination of Coenzyme Q10 and Acuval; D) animals treated only with Acuval and Coenzyme Q10 and with no exposure to noise. All solutions were administered orally 5 times: 24 and 2 hrs prior to noise exposure, and then daily for 3 days. The auditory function was assessed by measuring auditory brainstem responses (ABR) in the range from 2 to 32 kHz at times =1, 7, 14 and 21 days after noise exposure.
Results
At low frequencies (click and 4 kHz) animals from both A and B groups showed significant threshold shifts in the majority of the tested frequencies and tested times. For the same frequencies, animals from group C presented threshold levels similar to those from group D. At frequencies ≥8 kHz the protective performance of the 2 Acuval groups is more clearly distinguished from the noise group A. At 32 kHz the 2 Acuval groups perform equally well in terms of otoprotection. Animals in Group D did not show any significant differences in the hearing threshold during the experiment.
Conclusions
The data of this study suggest that a solution containing Coenzyme Q10 and Acuval 400®, administered orally, protects from noise-induced hearing loss.
Keywords: Acuval 400®, Coenzyme Q10 terclatrate (QTer), antioxidant, auditory brainstem response, noise-induced hearing loss
Background
Noise-induced hearing loss (NIHL) is a significant source of hearing impairment among humans. The causative mechanisms can be attributed to: (i) direct mechanical damage; and (ii) damage caused by metabolic stress, mediated by increased oxidative metabolism in the inner ear. Oxidative stress is generally characterized by the presence of lipid peroxidation [1], a process by which reactive oxygen species (ROS) and free radicals break down lipid molecules, leading eventually to cell death [24]. ROS are oxygen-based molecules acting as free radicals such as the superoxide (O2–−), the hydroxyl (OH−), and the peroxynitrite radical (ONOO1−); or as free radical-generating molecules such as hydrogen peroxide (H2O2) or ozone (O3) [2].
Many studies have demonstrated that noise exposure can increase the production of free radicals, with subsequent cochlear damage and hearing loss [3]. Ohinata et al have shown that the pattern of noise-induced lipid peroxidation correlates well with the subsequent morphological damage in the cochlea of guinea pigs exposed to 4 kHz octave band noise for 5 h at 115 dB SPL. [4]. Ohlemiller and Dugan have found in a mouse model that the level of hydroxyl radicals in the cochlea increases more than 10-fold after continuous noise exposure [5]. Industrial workers exposed to noise levels of 95–110 dB SPL have shown high ROS levels blood after long-term noise exposure [6].
Noise-induced hearing loss can be reduced by treatment with antioxidants. Previous studies have shown that endogenous antioxidants such as glutathione (GSH) can protect the guinea pig inner ear from noise trauma or other ototoxic agents [7] by scavenging ROS [8].
Data from the literature show that exogenous antioxidant agents can reduce NIHL and sensory cell death in animal models, using a variety of free radical scavengers [1], as well as dietary antioxidants [9–12], including vitamins C (ascorbate), E (α-tocopherol) and A (retinol), and diverse polyphenols, including flavonoids and organosulfur compounds [13–15]. In addition, a combination of vitamins A, C, E and magnesium, administered 1 h before noise exposure, has provided evidence of a remarkable reduction in NIHL and cell death [16].
In animal models, antioxidants are administered locally or systemically. The administration of antioxidants locally through the round window increases the level of local glutathione and reduces the effects of NIHL [17]. The intra-peritoneal administration of acetyl-L carnitine (ALCAR) or D-methionine (D-MET) has shown protection against the noise-induced permanent threshold shift (PTS), and reduced damage to the outer and inner hair cells [18]. Other molecules such as ebselen [19], allopurinol [20] and resveratrol [21], administered systemically, have been shown to increase the endogenous antioxidant defense.
In terms of timing, Kopke et al have demonstrated that antioxidants administered prior to noise-exposure significantly reduce hearing threshold shift and hair cell death [18,22]. Additionally they have shown that a combination of antioxidant compounds [salicylate, N-L-acetylcysteine (L-NAC)] delivered post-noise exposure also reduces threshold shifts [23].
Coenzyme Q10 (2,3-dimethoxy-5-methyl-6-decaprenyl-1, 4 benzoquinone) is a molecule involved in the proton and electron transport in the respiratory chain of the mitochondria. It is a vitamin-like antioxidant, which reacts with oxygen radicals and lipoperoxides to prevent damage of biomolecules in various tissue and cell compartments [25]. In its original form is not very soluble [26], but recently the Q10 terclatrate (Qter) version was developed which shows an improved water solubility. The latter characteristic improves the efficacy of CoQ10 against oxidative injures, lipid peroxidation and mitochondrial damage [27–29]. Qter is a terclatrate substance obtained by mechano-physical activation, a solid-state procedure that brings different substances into supramolecular contact through the administration of energy and turning a simple mixture into a multi-composite material (applicant: Patent WO/2003/097012, Actimex Srl, Italy). Qter consists of an outer case (an inactive pharmaceutical grade incipient) entrapping CoQ10 moieties (10% w/w) and an amino acid that serves as a catalyst to enable the formation of the multi-composite. In previous studies, Fetoni et al. [24] showed that in guinea pigs an intra-peritoneal administration of Qter 1 h before and then daily for 3 consecutive days prevented hair cell apoptosis after noise exposure (6 kHz for 1 h at 120 dB SPL). Similarly Yoshinobu et al. [30] have shown that guinea pigs treated with coenzyme Q10 intraperitoneally 2 h before noise exposure (130 dB SPL for 3 h) did not develop NIHL.
Acuval 400® is a food supplement multivitamin containing various vitamins (A, E, B1, B2, B6 and B12), L-Arginine, ginkgo biloba and minerals such as magnesium, selenium and zinc, as well as small amounts of Coenzyme Q10 (0.31 g/100 g). The aim of this study was to evaluate the otoprotective efficacy of various versions of Acuval (in its current composition and in a solution containing an additional quantity of coenzyme Q10), against NIHL in the Sprague-Dawley rat model. The first part of the investigation presents the data related to the tested solutions of Acuval. The second part will present data on the oral otoprotective efficacy of Coenzyme Q10 in the Sprague-Dawley rat model and the differences in the otoprotective performance between the various Acuval solutions.
Material and Methods
Animals and groups
Fifty-five male Sprague Dawley rats (150–200 g; Charles River, Italy) were used in this study. The animals were treated according to Italian guideline DL 116/92 with reference to European Economic Community directive 86–609. The animals were divided into 5 groups as follows:
noise-exposed animals (n=15),
animals exposed to noise and treated with 100 mg/kg of Acuval 400® (n=15),
animals exposed to noise and treated with 100 mg/kg of Acuval 400® and 500 mg/kg of Coenzyme Q10 (n=15),
animals treated with 100 mg/kg of Acuval 400® and 500 mg/kg of Coenzyme Q10 without any exposure to noise (n=10).
The animals from groups B, C and D were treated orally with the various solutions for a total of 5 times, following this time schedule: 24 h and 1 h prior to noise exposure and then once daily for 3 consecutive days. In all animals the auditory function was evaluated by measuring auditory brain responses (ABR) at times t=0, and 1, 7, 14 and 21 days after noise exposure.
Drugzs
Coenzyme Q10 was provided by Pharmaland (Republica di San Marino, Italy) and was manufactured using an industrially available native CoQ10 (Kaneka Pharma Europe, Brussels, Belgium).
The standard Acuval solution (treatment of group B) was prepared from diluting 100 mg/kg of Acuval 400® in 1 ml of water. The combined Acuval and Coenzyme Q10 solution (treatment of group C and D) was prepared by diluting 100 mg/kg of Acuval 400® and 500 mg/kg of coenzyme Q10 in 1 ml of water. In all treated animals the oral administration was performed using a feeding tube.
For the ABR recordings, animals were pre-treated with an anesthesia cocktail composed by 50 mg Zoletil 100 (Virbac) in 1-mL physiological saline solution in which 0.5 ml 2% Xylazine (Rompun) was added.
Noise conditioning
The animals were exposed to a 6 kHz white noise (115±3 dB SPL) for 2 hours, generated by 2 speakers placed on the top of a 1-m3 soundproof chamber. For the noise exposure, each awake animal was placed in a small wire mesh cage, permitting an equal noise exposure to both ears. The speakers were centered over the animals at a distance of 30 cm. The stimuli were generated by a dedicated software (Filtered Noise Generator by Timo Esser©), amplified by a Harman Kardon amplifier (model 3390). The stimulus levels were calibrated (Type 2209 precision sound level meter, Type 4134 microphone, Brüel and Kjær Instruments, Norcross, GA, USA) at multiple locations within the sound chamber to ensure uniformity of the stimulus. During the noise exposure, the stimulus level was monitored by a microphone positioned inside the soundproof chamber.
Measurement of evoked potentials
Auditory brainstem responses (ABR) were used to assess the auditory threshold. All ABR testing was performed in the 1-m3 soundproof chamber with the animals placed on a homoeothermic blanket to maintain a constant body temperature at 37.5°C.
The ABR responses were recorded with 3 platinum-iridium needle electrodes placed sub-dermal over the vertex (positive), the mastoid (negative), and the dorsum area (reference/ground) of the animal. A Motorola tweeter sound transducer (flat response ±1.5 dB; 4.0–35 kHz) was used and a sound delivery tube was inserted into the external ear canal. The ABR was amplified 20 000 times and filtered from 20 to 5000 Hz. Each recording was the average of 1000 individual responses. The ABR was generated in response to 4, 8, 16, and 32 kHz tone pips (1-ms rise-fall time, 10-ms plateau) in the range from100 to 30 dB. The stimulus sound intensity was varied in −5 dB intervals. Threshold was based on the visibility and reproducibility of wave III, and 2 recordings were acquired at the minimum threshold level. Threshold was defined as the lowest intensity at which a measurable ABR wave was observed in 2 averaged runs.
For convenience, the ABR data responses are recorded in terms of stimulus attenuation. The relationship between hearing threshold and attenuation at frequency F(x) is given by the formula: Threshold at F(x) in dB SPL=110 – Attenuation at F(x).
Earplugs were used to occlude the contralateral ear to avoid possible binaural stimulation at the highest stimulus intensities.
Statistical analysis
A repeated measures model (for details see section A1 in the Appendix) which incorporated right censoring was fit to the ABR response at each frequency (click, 4K, 8K, 16K, 32K). The occasions of the repeated measures were pre-exposure, 1, 7, 14 and 21 days. One between-subjects factor, group (A, B,C, D) and 1 within-subject factor (occasion, the repeated measures factor), as well as their interaction, were included in the model. The model assumed a lognormal distribution for the response and was fit by maximum likelihood, using SAS Proc Nlmixed. Each fit was checked by a residual analysis. A p value of <0.05 was considered significant, and a p value <0.01 was considered as highly significant.
Additional details on the statistical analyses are presented in the Appendix, section A2.
Evaluation rules
In order to evaluate the possible otoprotective effects of the Acuval solutions, certain rules were established: (1) animals treated with the protector agent should present a faster recovery of hearing threshold that the un-protected animals; (2) the values of the hearing threshold level of the protected animals at day 21 should be similar to the pre-exposure hearing level values. To quantify this statement better, a 10dB criterion was established (twice as large as the tested stimulus resolution of 5 dB) in order to evaluate the hearing level of the animals at day 21, in accordance with the statistical analyses for the following rationale – if the observed difference is not statistically significant, it means that no matter how large the calculated difference, one cannot reasonably conclude that a difference exists at all since it cannot be differentiated from the natural background variation.
Results
The data in this section are presented in the following format: (i) data referring to possible ototoxic adverse effects in animals treated with Acuval and Coenzyme Q10; (ii) data from the group exposed to noise with no protection; and (iii) data from the animals exposed to noise and protected by Acuval or by Acuval and Coenzyme Q10.
Pre-treatment data
Table 1 shows the ABR responses (derived from the repeated measures model) for groups A, B, C, and D at time t=0. All animals presented normal hearing. Tests for the equality of pre-exposure means showed lack of significance in all frequencies except at click and at 32 k. In both cases the difference was due to the data from group A, which had larger values. For this reason the threshold shifts and not the threshold values were analyzed for the tested times. While this does not guarantee that the groups are comparable in all relevant aspects, it does mitigate a known source of bias.
Table 1.
Detailed data generated from the repeated measures model showing the pre-noise exposure values of groups A, B, C and D across the tested frequencies. The column titled “mean Estimate” refers to the Average threshold values in dB SPL. The last column titled “Error” presents estimates of the standard error.
| Frequency | Group and Time | Mean Estimate | Error |
|---|---|---|---|
| CLICK | A time 0 | 34.6949 | 1.3722 |
| B time 0 | 28.0347 | 1.9189 | |
| C time 0 | 30.0316 | 1.1878 | |
| D time 0 | 28.7633 | 1.4679 | |
| 4 kHz | A time 0 | 51.0671 | 2.0116 |
| B time 0 | 45.5796 | 3.1072 | |
| C time 0 | 45.4752 | 1.7913 | |
| D time 0 | 46.5358 | 2.3653 | |
| 8 kHz | A time 0 | 39.5017 | 1.7159 |
| B time 0 | 39.5845 | 2.9754 | |
| C time 0 | 35.7268 | 1.5519 | |
| D time 0 | 35.7319 | 2.0027 | |
| 16 kHz | A time 0 | 35.3546 | 1.6773 |
| B time 0 | 33.3505 | 2.7372 | |
| C time 0 | 31.6006 | 1.4992 | |
| D time 0 | 30.7574 | 1.8825 | |
| 32 kHz | A time 0 | 51.6767 | 1.4202 |
| B time 0 | 47.1562 | 2.2437 | |
| C time 0 | 46.3500 | 1.2738 | |
| D time 0 | 45.0778 | 1.5989 |
Ototoxicity assessment of the Acuval + Coenzyme Q10 treatment of group D
The repeated measures model indicated that the animals of Group D presented non-significant threshold shifts over time at each frequency except for the click stimulus, where the means at observation times 7, 14 and 21d were all lower than the corresponding means at time t=0. The issue of negative threshold shifts, mainly visible at t=7 and 21d, can be interpreted as an improvement of the hearing threshold over the initially recorded values. At time t=21d, 3 frequency bands (4, 8 and 32 kHz) showed lower threshold values than the initial hearing levels. The data are summarized in Figure 1 and the details are reported in Table 2 in the Appendix section A3.
Figure 1.

Data (threshold shifts with 95% confidence interval error bars), from group D. The lack of significant mean differences across the tested frequencies per observation window, is an indication of lack of possible ototoxic side-effects. Negative threshold shifts can interpreted as an improvement over the pre-noise exposure values (t=0), but since these differences are not statistically significant, no final conclusions can be made.
Table 2.
Detailed data from the threshold shifts observed in group D at the tested frequencies and times. The third column titled “Estimate” shows the shifts in dB. Negative values suggest smaller shifts and are considered as “better”. The table shows that for 4, 8, 16 and 32 kHz the t=21d values are lower than the pre-noise exposure values. This could imply an improvement of the hearing threshold, but since the observed differences are not statistically significant no such conclusion can be reach.
| Frequency | Label | Estimate | Error | DF | t Value | Pr>t |
|---|---|---|---|---|---|---|
| CLICK | X1-X0 | 0.01443 | 2.0750 | 87 | 0.01 | 0.9945 |
| X7-X0 | −2.1927 | 2.3360 | 87 | −0.94 | 0.3505 | |
| X14-X0 | 1.5618 | 2.5410 | 87 | 0.61 | 0.5404 | |
| X21-X0 | 1.2870 | 3.5665 | 87 | 0.36 | 0.7191 | |
| 4 kHz | X1-X0 | −0.09368 | 3.3393 | 87 | −0.03 | 0.9777 |
| X7-X0 | −5.0618 | 3.6844 | 87 | −1.37 | 0.1730 | |
| X14-X0 | −1.1426 | 3.8930 | 87 | −0.29 | 0.7698 | |
| X21-X0 | −5.6990 | 4.9947 | 87 | −1.14 | 0.2570 | |
| 8 kHZ | X1-X0 | −0.2249 | 2.8208 | 87 | −0.08 | 0.9366 |
| X7-X0 | −0.00214 | 3.3481 | 87 | −0.00 | 0.9995 | |
| X14-X0 | −2.3812 | 3.2065 | 87 | −0.74 | 0.4597 | |
| X21-X0 | −0.8851 | 4.5977 | 87 | −0.19 | 0.8478 | |
| 16 kHz | X1-X0 | −0.5852 | 2.6342 | 87 | −0.22 | 0.8247 |
| X7-X0 | −0.7871 | 3.0951 | 87 | −0.25 | 0.7999 | |
| X14-X0 | 1.0766 | 3.2179 | 87 | 0.33 | 0.7388 | |
| X21-X0 | −0.8164 | 4.3146 | 87 | −0.19 | 0.8504 | |
| 32 kHz | X1-X0 | −0.8349 | 2.2395 | 87 | −0.37 | 0.7102 |
| X7-X0 | −1.8674 | 2.6037 | 87 | −0.72 | 0.4752 | |
| X14-X0 | −2.9909 | 2.5618 | 87 | −1.17 | 0.2462 | |
| X21-X0 | −3.6471 | 3.5021 | 87 | −1.04 | 0.3006 |
Noise treatment groups
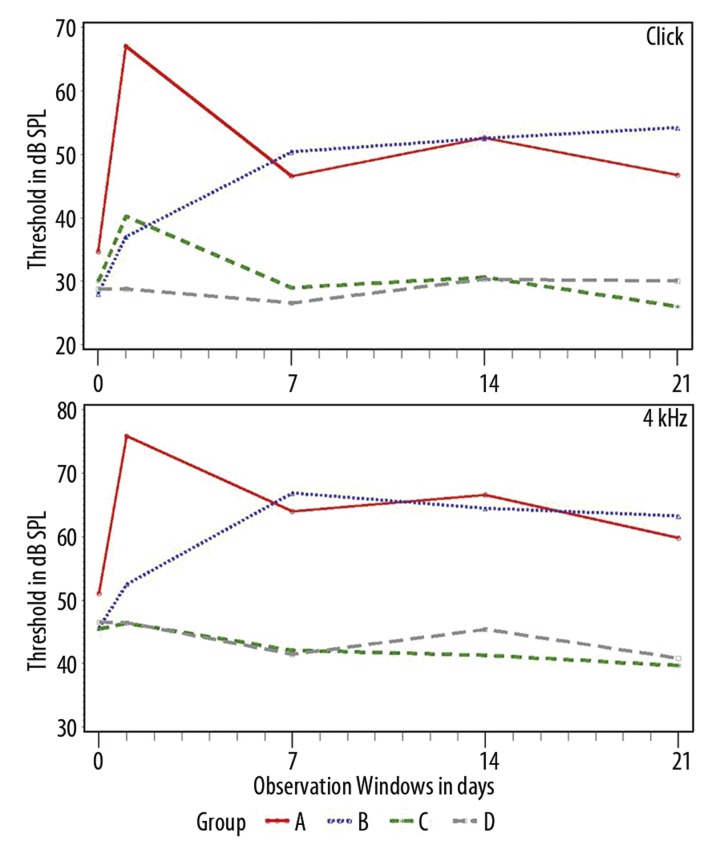
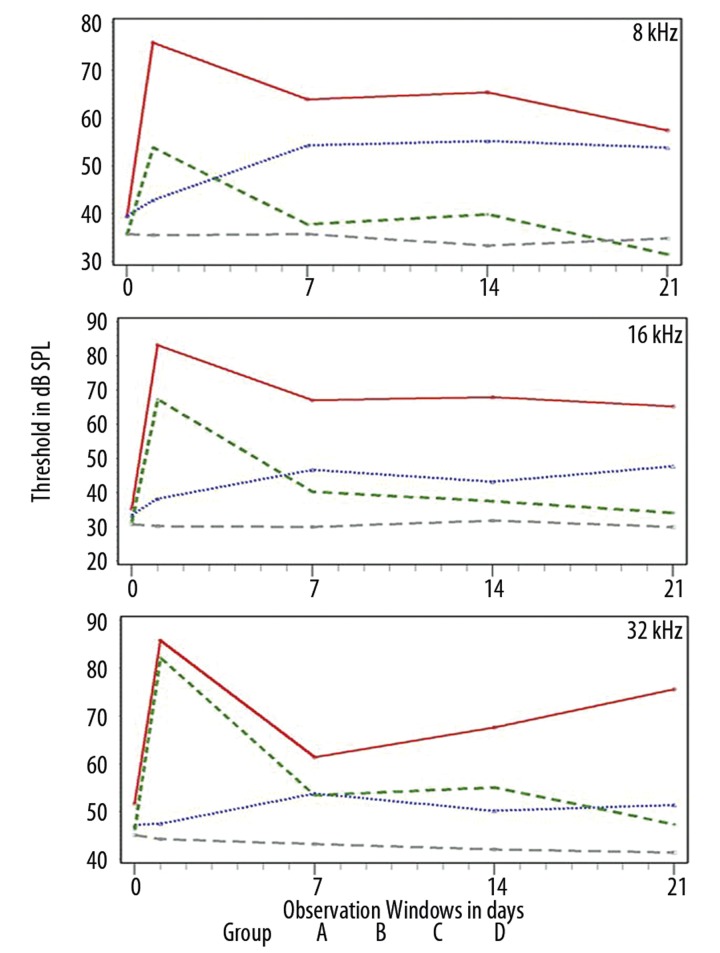
The data from Groups A, B, and C across the 4 observation windows are presented in Figures 2 and 3. Data from group D are also presented as a point of reference across time since no significant changes were observed in this group from time t=0.
Figure 2.
Data from the click and 4 kHz datasets, of all treated groups, in post-treatment observation windows from t=1 to 21d. The Y-axis depicts threshold in dB SPL, the X-axis time in days.
Figure 3.
Data from the 8, 16 and 32 kHz datasets, of all treated groups, in post-treatment observation windows from t=1 to 21d. The Y-axis depicts threshold in dB SPL, the X-axis time in days.
From the analysis of the data, 2 main trends were identified. (A) At low frequencies (see Figure 2) animals from both groups A and B showed significant threshold shifts in the majority of the tested frequencies and tested times. For the same frequencies, animals from group C presented threshold levels similar to those from group D. (B) At higher frequencies (see Figure 3) the protective performance of the 2 Acuval groups is more clearly distinguished from the noise group A. At 32 kHz, the 2 Acuval groups performed equally well in terms of otoprotection (see Table 3 in the section below).
Table 3.
The table shows the significant mean differences (p≤0.05) between groups A, B, C, D, at the tested frequencies and times. Differences which are not reported, were not found statistically significant. To facilitate the interpretation of the data, the table reports groups in order of decreasing mean threshold estimates (smaller shifts are better). For example at t=1d and at the click frequency the table data indicate that the mean of group A was larger than the means of groups C, B and D. It also shows that the mean of group C is larger that the mean of Group D.
| Tested frequency | T=1d | T=7d | T=14d | T=21d |
|---|---|---|---|---|
| Click | A>C, B, D; C>D | B, A>C, D | B, A>D, C | B, A>C; D>C |
| 4 kHz | A>C, D | B, A>C, D | B, A>D, C | B>D, C |
| 8 kHz | A>C, B, D; C>D | A>C, D | A>C, D | A>D, C; B>C |
| 16 kHz | A, C>B, D | A>C, D | A>B, C, D | A>C, D |
| 32 kHz | C, A>B, D | A>D | A, C>D | A>B, C, D |
In general, the data show that as frequency is increasing, the protective performance of the Acuval groups becomes more similar.
Differences between the Acuval groups
Table 3 summarizes the significant differences of mean threshold shift between the evaluated groups at all tested frequencies and times. The focus of this section is on the differences between the 2 Acuval solutions.
For the click responses, significant differences were observed at all observation windows. At 4 kHz significant differences were observed from time t=7d. At 8 kHz significant differences were observed only at t=21d. Figure 3 shows that although no differences were observed from times t=1–14d, the C group animals present smaller threshold shifts and a faster recovery of the pre-exposure values as compared to the responses of the Group D animals. At 16 and 32 kHz there is an inversion of order between the 2 groups. At t=1d the group C responses are significantly different than those of Group B. For the other observation times there are no significant differences, and according to Figure 3 the mean threshold shifts of the 2 Acuval groups are quite similar.
Discussion
This study evaluated the otoprotective efficacy of various solutions of the Acuval 400 compound against noise-induced hearing loss (NIHL) in a Sprague-Dawley rat animal model. Two Acuval solutions were evaluated, 1 corresponding to its original composition and 1 in which an additional amount of 500 mg Coenzyme Q10 was added. The data showed that the second solution yields the best otoprotection results.
The present study is the first to demonstrate the otoprotective potential of an orally administrated agent based on Coenzyme Q10 against NIHL in animals. Previous studies by Fetoni et al. [24] and Yoshinobu et al. [27] have shown that in guinea pigs an intraperitoneal administration of coenzyme Q10 before and after noise exposure may prevent NIHL by protecting the cochlea from oxidative stress.
Data from group D (animals treated with Acuval and Coenzyme Q10, but with no exposure to noise), showed not only lack of any ototoxic adverse effects in an observation period of 21 days, but also an improvement of the hearing threshold. In this context, the drug combination for the treatment of the group C animals was shown to be safe for testing. Since the repeated measures model did not detect any significant differences between the pre-exposure and the t=21d values in the group D animals, there is no direct evidence that the improvement of hearing threshold is related to the use of both drugs and remains to be elucidated.
The analysis of the ABR data (Table 3) with a repeated measures model showed that there are significant differences in the recovery of hearing threshold between the un-treated and protected animals (ie, group A vs. Groups B and C). Due to differences in the pre-exposure data between groups at click and 32 kHz, the statistical analysis was based on threshold shifts and not on absolute threshold values.
The data from the un-protected and noise exposed group A verify previous findings from this laboratory [32,33], showing a temporary threshold shift (TTS) in frequencies ≥ 8 kHz of approximately 35–40 dB SPL, which at t=21d is transformed into a permanent threshold shift (PTS) of 25 dB SPL. The data from group A suggest that the Sprague-Dawley rat has an innate capability to recover 15–20 dB of TTS, utilizing systemic antioxidants.
The data from Group B, receiving the standard Acuval solution, showed otoprotection only at high frequencies (≥8 kHz). The data from group C showed consistent otoprotection across the tested frequencies and observation windows except in 3 cases – at 32 kHz and t=1d (p=0.7394), at 32 kHz and t=7d (p=0.5091), and at 16 kHz, and t=1d (p=0.0628).
In terms of recovery vs. the pre-exposure values, the group C data show faster rates or recovery (ie, smaller threshold shifts). In terms of the evaluation rules and the established 10 dB SPL criterion, the animals from group C at t=21d presented mean threshold values across all frequencies that were lower than the pre-exposure values (the differences were negative). The data cannot discriminate the causes of these differences, since the observed differences were not significant. However, several hypotheses are possible, such as (i) inherent data variability or (ii) the ability of Acuval + Coenzyme Q10 to improve the hearing threshold, observed as well in Group D animals. In contrast to the data from Group C, Group B did not meet the criteria of the evaluation rules, mainly due to lack of otoprotective performance at the low frequencies.
The animal model used has produced good results with other otoprotectors, such as N-Acetylcysteine (NAC). Previous findings with this model [32,33] indicate that animals treated with 4 dosages of 375 mg/kg NAC prior to and after noise exposure present ABR threshold shifts <10 dB at 8 and 16 kHz. The animals in these experiments were treated intraperitoneally and observed for only 7 days, therefore direct comparisons with the present study’s data cannot be made. Nevertheless, the NAC-treated animals presented a lower initial threshold shift, equal recovery rates (with those of group C), and at t=7 the observed threshold shifts were within the 10 dB criterion, but with higher values than the group C animals. For more detailed comparisons on the differences between the 2 otoprotectors (NAC vs. Acuval + Co Q10), additional studies are necessary.
In this study the otoprotective agents were administered before and after noise exposure, but the effective therapeutic strategy would be to effectively treat the cochlear trauma after the noise exposure. Numerous reports suggest that a post-exposure therapeutic strategy might be possible. Yamashita et al. [33] have recently found reactive oxygen species and reactive nitrogen species formation in the ear for 7–10 days following noise exposure. Kopke et al. [18] reported that treatment with antioxidants salicylate and L-NAC, delivered immediately after noise exposure, reduced threshold shift with lesser efficacy than pre-treatment, but did not reduce hair cell damage. Yamashita et al. [33] demonstrated that treatment with salicylate and Trolox, 3 days after noise exposure, significantly reduced auditory brainstem response deficits, hair cell damage, and decreased ROS formation. Considering these data, the efficacy of a post-exposure protocol based on 2 factors, the protector dosage and the time of administration, remains to be elucidated.
Conclusions
This study evaluated the otoprotective performance of 2 Acuval 400® solutions against NIHL in the Sprague Dawley rat. The data indicated that animals who have received oral doses of 100 mg/kg Acuval 400® and 500 mg/kg Coenzyme Q10 presented faster recovery rates and significantly better hearing thresholds than the untreated animals exposed to noise. The 2 groups that were treated with Acuval 400® and Coenzyme Q10 also presented better hearing thresholds that their corresponding pre-noise exposure values.
Acknowledgments
The authors would like to thank Dr. Giorgio Manini (Scharper S.p.A.) for assistance during the project.
Appendix
A1: Repeated Measures Design
A repeated measures design [34,35] refers to studies in which the same measures are collected multiple times for each subject but under different conditions. For instance, repeated measures are collected in a longitudinal manner where change over time is assessed. The primary strengths of the repeated measures design is that it makes an experiment more efficient and helps keep the variability low. This helps to keep the validity of the results higher, while still allowing for smaller than usual subject groups. A disadvantage to the repeated measure design is that it may not be possible for each participant to be in all conditions of the experiment (i.e. time constraints, location of experiment, etc.).
The analysis of repeated measures differs from that of independent observations in that it “must account for” the within-subject correlations induced by taking repeated measurements on the same subject.
A2: ABR data treatment prior to the analysis
The ABR responses presented a strong left skewness, therefore for the statistical analysis, a transformation from “attenuation” to “threshold” was necessary. As the attenuation value of 110 was the upper limit that the ABR acquisition device could register, values of 110 were treated as “right censored”. Censored values appear when the acquisition apparatus provides a fixed response independently of the probable response value. This is common in hearing threshold detection for which above a certain limit i.e. 110 dB SPL no auditory response is detected. For the uncensored observations, a normal model seemed to fit the logarithms of the observations well, so a lognormal distribution was used for the model fit.”
A3: Group D ototoxicity assessment
The analytical data from group D are presented in the Table 2, below. Not only all differences were found as non-statistically significant but no border-line probabilities were observed.
Footnotes
Source of support: Departmental sources
References
- 1.Yamane H, Nakai Y, Takayama M, et al. Appearance of free radicals in the guinea pig inner after noise-induced acoustic trauma. Eur Arch Otorhinolaryngol. 1995;252:504–8. doi: 10.1007/BF02114761. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B, Gutteridge J. Free Radicals in Biology and Disease. Oxford: Oxford University Press; 1999. [Google Scholar]
- 3.Le Prell GG, Yamashita D, Minami SB, et al. Mechanism of noise-induced hearing loss indicate multiple methods of prevention. Hear Res. 2007b;226:22–43. doi: 10.1016/j.heares.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohinata Y, Miller JM, Altschuler RA, Schact J. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000a;878(1–2):163–73. doi: 10.1016/s0006-8993(00)02733-5. [DOI] [PubMed] [Google Scholar]
- 5.Ohlemiller KK, Dugan LL. Elevation of reactive oxygen species following ischemia-reperfusion in mouse cochlea observed in vivo. Audiol Neurootol. 1999;5:219–28. doi: 10.1159/000013845. [DOI] [PubMed] [Google Scholar]
- 6.Kaygusuz I, Ozturk A, Ustundag B, Yalcin S. Role of free oxygen radicals in noise-related hearing impairment. Hear Res. 2001;162(1–2):43–47. doi: 10.1016/s0378-5955(01)00365-3. [DOI] [PubMed] [Google Scholar]
- 7.Clerici WJ, Hensley K, DiMartin DL, Butterfield DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res. 1996;98:116–24. doi: 10.1016/0378-5955(96)00075-5. [DOI] [PubMed] [Google Scholar]
- 8.Ohinata Y, Yamasoba T, Schacht J, Miller JM. Glutathione limits noise-induced hearing loss. Hear Res. 2000;146:28–34. doi: 10.1016/s0378-5955(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 9.Biesalski HK, Wellner U, Weiser H. Vitamin A deficiency increases noise susceptibility in guinea pigs. J Nutr. 1990;120:726–37. doi: 10.1093/jn/120.7.726. [DOI] [PubMed] [Google Scholar]
- 10.Rabinowitz PM, Pierce Wise J, Hur Mobo B, et al. Antioxidant status and hearing function in noise exposed workers. Hear Res. 2002;173:164–71. doi: 10.1016/s0378-5955(02)00350-7. [DOI] [PubMed] [Google Scholar]
- 11.Derekoy FS, Koken T, Yilmaz D, et al. Effects of ascorbic acid on oxidative system and transient evoked otoacoustic emissions in rabbits exposed to noise. Laryngoscope. 2004;114:1775–79. doi: 10.1097/00005537-200410000-00019. [DOI] [PubMed] [Google Scholar]
- 12.McFadden SL, Woo JM, Michalak N, Ding D. Dietary vitamin C supplementation reduces noise-induced hearing loss in guinea pigs. Hear Res. 2005;202:200–8. doi: 10.1016/j.heares.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Esposito E, Rotilio D, Di Matteo V, et al. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol Aging. 2002;23:719–35. doi: 10.1016/s0197-4580(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 14.Jovanovic SV, Simic MG. Antioxidants in nutrition. Ann NY Acad Sci. 2000;899:326–34. doi: 10.1111/j.1749-6632.2000.tb06197.x. [DOI] [PubMed] [Google Scholar]
- 15.Quiles JL, Huertas JR, Battino M, et al. Antioxidant nutrients and adriamycin toxicity. Toxicology. 2002;180:79–95. doi: 10.1016/s0300-483x(02)00383-9. [DOI] [PubMed] [Google Scholar]
- 16.Le Prell CG, Hughes LF, Miller JM. Free radical scavengers vitamins A,C and E plus magnesium reduce noise trauma. 2007a;42:1454–63. doi: 10.1016/j.freeradbiomed.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hight NG, McFadden SL, Henderson D, et al. Noise-induced hearing loss in chinchillas pre-treated with glutathione monoethylester and R-PIA. Hear Res. 2003;179:21–32. doi: 10.1016/s0378-5955(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 18.Kopke RD, Coleman JK, Liu J, et al. Candidate’s thesis: enhancing intrinsic cochlear stress defenses to reduce noise-induced hearing loss. Laryngoscope. 2002;112:1515–32. doi: 10.1097/00005537-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Lynch ED, Gu R, Pierce C, Kil J. Ebselen-mediated protection from single and repeated noise exposure in rat. Laryngoscope. 2004;114:333–37. doi: 10.1097/00005537-200402000-00029. [DOI] [PubMed] [Google Scholar]
- 20.Seidman MD, Shivapuja BG, Quirk WS. The protective effects of allopurinol and superoxide dismutase on noise-induced cochlear damage. Otolaryngol Head Neck Surg. 1993;109:1052–56. doi: 10.1177/019459989310900613. [DOI] [PubMed] [Google Scholar]
- 21.Seidman M, Babu S, Tang W, Naem E, Quirk WS. Effects of resveratrol on acoustic trauma. Otolaryngol Head Neck Surg. 2003;129:463–70. doi: 10.1016/S0194-59980301586-9. [DOI] [PubMed] [Google Scholar]
- 22.Hu BH, Zheng XY, McFadden SL, et al. R-phenylisopropyladenosine attenuates noise-induced hearing loss in the chinchilla. Hear Res. 1997;113:198–206. doi: 10.1016/s0378-5955(97)00143-3. [DOI] [PubMed] [Google Scholar]
- 23.Kopke RD, Weisskopf PA, Boone JL, et al. Reduction of noise-induced hearing loss using L-NAC and salicylate in the chinchilla. Hear Res. 2000;149:138–46. doi: 10.1016/s0378-5955(00)00176-3. [DOI] [PubMed] [Google Scholar]
- 24.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 25.Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–79. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell P. Proton motive redox mechanisms of cytochrome b-cl complex in the respiratory chain: proton motive ubiquinone cycle. FEBS Lett. 1975;56:1–6. doi: 10.1016/0014-5793(75)80098-6. [DOI] [PubMed] [Google Scholar]
- 27.Fetoni AR, Piacentini R, Fiorita A, et al. Water-soluble Coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL) Brain Res. 2009;1257:108–16. doi: 10.1016/j.brainres.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Corvi Mora P, Canal T, Fortuna F, Ruzzier F. An Innovative Technology For Improving Solubility And Antioxidant Properties Of Coenzyme Q10. Oxygen Society Of California Congress “Oxidants And Antioxidants In Biology”; Alba. 7–10 September 2005. [Google Scholar]
- 29.Corvi Mora P, Canal T, Fortuna F, Ruzzier F. WO/2007/009997 Composition containing micronutrients with improved anti-oxidant activity and use there of 2007.
- 30.Yoshinobu H, Sugahara T, Takefumi M, et al. Effect of water soluble coenzyme Q10 on noise-induced hearing loss in guinea pigs. Acta Otolaryngol. 2008;128:1071–76. doi: 10.1080/00016480801891694. [DOI] [PubMed] [Google Scholar]
- 31.Theneshkumar S, Lorito G, Giordano P, et al. Effect of Noise on cisplatin induced. Ototoxicity Med Sci Monit. 2009;15(7):BR173–77. [PubMed] [Google Scholar]
- 32.Lorito G, Giordano P, Petruccelli J, Martini A, Hatzopoulos S. Different strategies of treating Noise-Induced hearing Loss with N- Acetylcystine Med Sci Monit. 2008;14(8):BR159–64. [PubMed] [Google Scholar]
- 33.Yamashita D, Jiang H-Y, Le Prell CG, et al. Post-exposure treatment attenuates noise-induced hearing loss. Neuroscience. 2005a;134:633–42. doi: 10.1016/j.neuroscience.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Vonesh, Edward F, Chinchilli, Vernon G. Linear and Nonlinear Models for the Analysis of Repeated Measurements. London: Chapman and Hall; 1997. (Comprehensive treatment of theory and practice) [Google Scholar]
- 35.Conaway M. Repeated Measures Design. Retrieved February 18, 2008, from http://biostat.mc.vanderbilt.edu/twiki/pub/Main/ClinStat/repmeas.