Summary
Background
This study aimed to investigate the role of osteopontin and its receptor, integrin αv, in gallstone formation using human tissue specimens and a guinea pig lithogenic model.
Material/Methods
The nucleation role of osteopontin was determined in patients’ and normal gallbladder bile samples in vitro. Normal gallbladder was the control, and gallstone gallbladders were divided into group I (with normal epithelia) and group II (with degenerated epithelia) based on pathology change. Immunostaining, mRNA and protein expressions of osteopontin and integrin αv were analyzed. The animals were randomly divided into a lithogenic diet group and a normal diet group; the osteopontin mRNA expression in gallbladder and liver and osteopontin concentrations were determined.
Results
Osteopontin prolonged nucleation time and inhibited the pro-nucleating role induced by calcium in human bile in vitro. Immunostaining for osteopontin and integrin αv in human gallbladder tissues showed a higher reactivity in Group I than control group and Group II. The immunostaining in Group II was weaker than control group; similar results were observed for mRNA and protein expression of osteopontin and integrin αv. In the animal assay, the mRNA expression and concentration of osteopontin in gallbladder and liver gradually increased at initial stages and decreased in later stages. The concentrations of osteopontin in bile and serum of guinea pig showed similar trends.
Conclusions
Our results suggest that osteopontin is involved in cholesterol gallstone formation, and the role of osteopontin might correlate with integrin αv and calcium.
Keywords: osteopontin, cholesterol gallstone formation, bile, lithogenic model
Background
Gallstone disease has a high prevalence rate of 10–15% in adults in the USA and Europe, but is lower in Asia (about 3–15%) [1–6]. However, the incidence of cholesterol gallstone in China has shown an increasing tendency in the past few decades [7]. It is generally accepted that lithogenic gallbladder bile, gallbladder and liver play important roles in cholesterol gallstone formation. Several low molecular weight calcium binding glycoproteins isolated from bile are involved in gallstone formation as the pro-nucleating or anti-nucleating factors [8,9].
Osteopontin (OPN) is an acidic extracellular matrix glycoprotein present in several human organs and body fluids [10–13]. It possesses a characteristic RGD (Arg-Gly-Asp) sequence and the calcium binding property [14] and exerts many of its biological effects by interacting with integrins [15]. OPN is involved in the metabolism of calcium, an important factor in gallstone formation; moreover, previous reports showed that OPN is involved in pigment gallstone formation [16,17]. However, the exact role of OPN and its receptor integrin αv in the pathogenesis of cholesterol gallstone remains unknown.
In this study, the role of osteopontin and its receptor integrin αv in gallstone formation were investigated by using human specimens and an animal model. Immunohistochemical staining of OPN and integrin αv were performed with patients’ and control gallbladders, and the messenger ribonucleic acid (mRNA) and protein expressions of OPN and integrin αv were analyzed using reverse transcriptase polymerase chain reaction (RT-PCR) and Western blotting, respectively. Furthermore, the mRNA of OPN was analyzed in gallbladder and liver tissues in guinea pigs fed a lithogenic diet. These results will help determine the involvement of OPN in gallstone formation and provide a potential target for gallstone prevention and treatment.
Material and Methods
Human tissues and biological samples
Gallstone patients scheduled for elective cholecystectomy were included in this study (trial group) and were divided into 2 sub-groups based on their pathological changes: Group I (10 females and 7 males) with normal epithelia (little or no degeneration or desquamation and without fibrosis in gallbladder wall) and Group II (5 females and 5 males) with pathological epithelia (remarkable degenerated, desquamation and fibrosis in gallbladder wall). All gallstone specimens were classified as cholesterol gallstone since all the stones contained over 65% cholesterol.
As the control group, 15 specimens were acquired from liver transplantation donors (9 males and 6 females) who presented no evidence of gallstone via ultrasonography and no cholesterol crystals via polarizing microscopy. The demographic data were comparable between the cases and control groups (Table 1). The bile samples were obtained according to the method of Strasberg [18], 2 ml of bile samples were extracted according to the methods of Bligh and Dyer [19] and stored at −70°C until further analysis, and the gallbladder tissue samples were clipped under aseptic conditions with surgical scissors, flushed with phosphate-buffered saline (PBS, pH 7.4), and stored in liquid nitrogen until RT-PCR and Western blot analyses. Partial gallbladder samples were fixed with 4% paraformaldehyde for immunohistochemical analysis.
Table 1.
The demographic data of patients and controls.
| Group I | Group II | Control | p value | |
|---|---|---|---|---|
| Gender (F/M) | 10/7 | 5/5 | 9/6 | NS |
| Age (yr) | 50.5±9.8 | 51.7±10.2 | 49.2±11.5 | NS |
| Height (cm) | 165.5±7.3 | 167.2±6.8 | 167.1±6.9 | NS |
| Weight (kg) | 68.7±8.4 | 65.9±10.2 | 64.3±10.6 | NS |
| BMI (kg·m−2) | 24.5±3.4 | 23.2±3.8 | 23.1±2.7 | NS |
Continuous variables are expressed as mean ± standard deviation and percentages are rounded to whole number.
None of the patients or controls had shown any clinical or laboratory evidence for diabetes mellitus, hyperlipoproteinemia, obesity, alcohol abuse, cancer or others conditions that could affect the function of the liver or kidneys. In addition, none of them took any medications known to affect lipid metabolism. Histological examination of removed gallbladders revealed no acute inflammation. Blood and bile samples were collected from the case and control groups. Two milliliters of peripheral vein blood was drawn and stored at −70°C for further analysis. This study was conducted in accordance with the principles of the Helsinki Declaration and the protocol was approved by the Ethics Committee of Huashan Hospital, Fudan University. Informed consent was obtained from each patient before the operation.
Immunohistochemical staining for OPN and integrin αv
Immunohistochemical staining was performed as described previously [20]. All gallbladder samples were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at 4 μm thickness. Immunohistological staining of OPN and integrin αv was performed using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s protocol. Briefly, tissue sections were deparaffinized and rehydrated. Endogenous peroxidase activity was blocked by immersing the sections in 3% H2O2 in methanol for 30 min. After non-specific binding was blocked with 10% normal goat serum in PBS for 30 min at room temperature, the reaction with the primary antibody (monoclonal antibody against human and mouse OPN and integrin av, 1:100) was carried out at 4°C overnight, followed by incubation with streptavidin-peroxidase complex [21]. Peroxidase conjugates were subsequently visualized in diaminobenzidine solution. The preparations were lightly counterstained with hematoxylin, mounted with Permount, and examined by light microscopy [22].
Reverse transcriptase-PCR Analysis
The mRNA expression of OPN and integrin αv was studied by a semi-quantitative RT-PCR analysis (RNA PCR kit; Takara, Tokyo, Japan) as described previously [23]. Total RNA was isolated from 100 mg of gallbladder tissue with Trizol (Invitrogen, Carlsbad, California, USA), following the manufacturer’s instructions [24]. The total RNA (2 μl) was employed to generate first strand complementary deoxyribonucleic acid (cDNA) with Oligo (dT)18 primers and the M-MLV reverse transcriptase (Promega, USA). Two μl of the RT reaction production product was subsequently used for the RNA reaction. The primer sequences used are listed in Table 2. The PCR conditions included 94°C for 5 min, 30 cycles at 94°C for 45s, 56°C for 30s, and 72°C for 1 min, and a final extension at 72C for 5 min. PCR products were visualized on a 1.5% agarose gel. Glyceraldehyde phosphate dehydrogenase (GAPDH) gene was used as an internal control of the analysis of gene expression.
Table 2.
PCR primer used in the study.
| Gene | Orientation Sequence (5′-3′) | bp | Tm | |
|---|---|---|---|---|
| Human | OPN | Forward GGA CTC CAT TGA CTC GAA CG | 436 | 58 |
| Reverse TAA TCT GGA CTG CTT GTG GC | ||||
| Integrin αv | Forward CTC ATC GTT TCC ATT CCA CT | 396 | 58 | |
| Reverse CTT CAG TCT CAG GGT TCT CC | ||||
| GAPDH | Forward ACC ACA GTC CAT GCC ATC AC | 450 | 56 | |
| Reverse CCA CCA CCC TGT TGC TGT AG | ||||
| Guinea pig | OPN | Forward GAC TTT GAC CTC AGT CC | 327 | 55 |
| Reverse ATC TCA GAA GCA GAA TCT | ||||
| β-actin | Forward CCT CTA TGC CAA CAC AGT GC | 211 | 57 | |
| Reverse GTA CTC CTG CTT GCT GAT CC | ||||
Western blot analysis
Western blot analysis was performed as described previously [25]. The frozen tissue samples were homogenized in lysis buffer containing 50 mM Tris-HCl (pH 7.4), 50 mM sodium chloride, 1% Triton X-100, and 1 mM EDTA, and 100 μg/mL phenylmethylsulfonyl fluoride. The homogenates were centrifuged at 15 000 g for 10 min at 4°C. Protein concentration in the supernatant was determined by the Lowry method [26]. The protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membranes (Amersham Biosciences, Piscataway, USA) by electroblotting. The membranes were blocked with 5% non-fat dried milk for 30 min at room temperature, incubated with affinity-purified anti-osteopontin and anti-integrin αv (1:1000, Abcam, Cambridge, UK) at 4°C for 24 h, and then incubated with HRP- rabbit anti-mouse IgG (1:5000) for 1 h. The membranes were developed using a substrate kit (Pierce, Rockford, USA) for 5 min. For semi-quantification of the protein expression levels, the intensity of the detected bands was quantified by using the software Smartview 2001 (Shanghai Furi Science & Technology Co. Ltd., China), using GAPDH as an internal control.
Analysis of nucleation time (NT)
The NT was defined as the onset time of the first cholesterol monohydrated crystal detected through polarized light microscopy. The thawed bile samples were mixed thoroughly, and NT was determined by the method of Holan et al. [27] with minor modifications [28]. Bacteria have not been observed in all samples. Then 10 μl of 20% sodium azide was added to 1 ml of bile, and 1 drop of bile was immediately used for examination of cholesterol crystals. OPN (Sigma Chemical Co., USA) was dissolved in Tris buffer to obtain appropriate concentrations, and then added in aliquots of 12.5 μl to the bile samples of 1 ml. Thereafter, the samples were flushed with nitrogen, sealed and incubated at 37°C. We obtained the NT values for the 4 groups of human gallbladder bile samples in vitro and the final concentrations and conditions were as follows: Tris-HCl (10 mM, pH 7.40) as the control group, OPN (100 μg/ml), calcium ions (10 mM), and OPN (100 μg/ml) + calcium ions (10 mM).
Statistical analysis
Data are expressed as mean ±SD. Comparisons of values among groups were made using the one-way analysis of variance (ANOVA) methods (SPSS, Chicago, IL, USA) followed with SNK-test [29]. Statistical significance was set at P<0.05.
Animal model and assays
Lithogenic guinea pig model of gallstone
Guinea pigs, weighing 200 to 300 g, were purchased from the Animal Center of Fudan University (Shanghai, China) and randomized into 2 groups. Animals in the lithogenic group were fed a lithogenic diet containing 1.2% cholesterol and 0.5% cholic acid as described by Reihner et al. [30]. Three to 5 animals were killed under ether anesthesia on days 7, 14, 28, 42, 56 and 70 after starting the diet. The control animals were fed with standard chow and killed on the same days. The gallbladder and hepatic tissues were removed as described previously [31] and stored in liquid nitrogen for subsequent use. Gallbladder bile and sera were collected as described previously [32] and stored at −70°C until analysis. The animal care and use protocol was approved by the Animal Care and Use Committee of Fudan University.
RT-PCR
RT-PCR was used to evaluate the change in OPN mRNA over time in experimental animals. The RNA isolation and RT-PCR assay were analyzed as previously described [33]. Experiments were performed in duplicates and the mRNA expression was normalized to that of β-actin. The primers are listed in Table 2.
OPN concentration in bile and blood samples
Commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Inc. Minneapolis, USA) were used for the analysis of the concentrations of OPN in bile and sera of guinea pigs.
Statistic Analysis
Data are presented as mean ± standard deviation. The statistical analyses included one-way ANOVA and the Student’s T test (two-tailed). A p-value of <0.05 was considered statistically significant.
Results
Immunohistochemistry staining for OPN and integrin αv in gallbladders from patients and controls
The OPN and integrin αv exhibited a weak and diffuse immunoreactivity in gallbladder epithelial cells and smooth muscle cells from the controls, who were liver transplantation donors. The immunohistochemical staining of OPN (Figure 1A) and integrin αv (Figure 1B) were also examined in cytoplasm of epithelial cells and smooth muscle cells in the control’s gallbladder walls, and the focal and diffused OPN and integrin αv staining were observed in epithelia.
Figure 1.
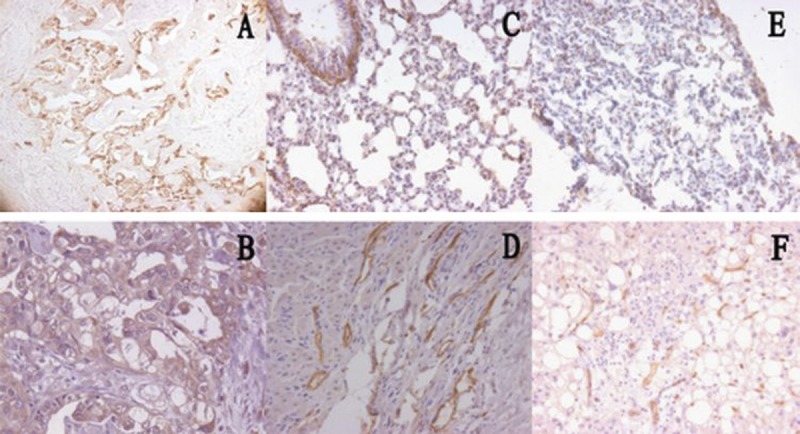
OPN and integrin αv immunostaining in experimental and control gallbladder wall: In normal gallbladder, the focal and diffused immunohistochemical staining of OPN (A) and integrin αv (B) were examined in cytoplasm of epithelial cell and smooth muscle cell. In experimental group I, intensive and strong OPN immunostaining was found in epithelial cell (C), the diffuse and strong membranous integrin αv staining was found in epithelial cytoplasm (D). In group II, immunostaining for OPN was relative weaker and diffused (E). Meanwhile, immunostaining for integrin αv was lower expression in these specimens (F). OPN and integrin αv, ×20.
In the gallstone patients, different levels of immunostaining were observed. In Group I, the immunostaining of OPN (Figure 1C) and integrin αv (Figure 1D) in epithelial cells was intensive and strong when compared with controls (Figure 1A, B). The immunoreactivity in the periphery and basal region was more intense than that in the central region in specimens of Group I. Some positive staining of integrin αv was observed in the cytoplasm of the macrophages (Figure 1D), and differential expression of OPN and integrin αv revealed that subcellular configuration exerted the differential immunoreactivity for OPN.
In group II, immunoreactivity for OPN was relative weaker and diffused when compared with Group I and controls; the discontinuous punctiform positive expression was observed in epithelial cells and several degenerated cells (Figure 1E). Meanwhile, the immunostaining for integrin αv showed lower expression in specimens of Group II; the weakly punctiform and linear positive expression was found in the basal epithelia and vacuoles changes in the gallbladder wall (Figure 1F).
The mRNA and protein expression of OPN and integrin αv in human gallbladder
The amplified PCR products for OPN, integrin αv and GAPDH were 436bp, 396bp and 450bp, respectively. The levels of the amplified mRNAs of OPN and integrin αv in gallbladders are correlated with the immunostaining results. Compared with normal controls, the mRNA expression of OPN and integrin αv was increased in gallbladder tissues in Group I (Figure 2A), but markedly decreased in Group II (Figure 2B). Based on Western blotting, OPN and integrin αv were shown in a single band at approximately 33kD and 116kD, respectively. Compared with normal tissues, the protein expression of OPN and integrin αv was significantly elevated in Group I (Figure 3A) but decreased in Group II (Figure 3B).
Figure 2.
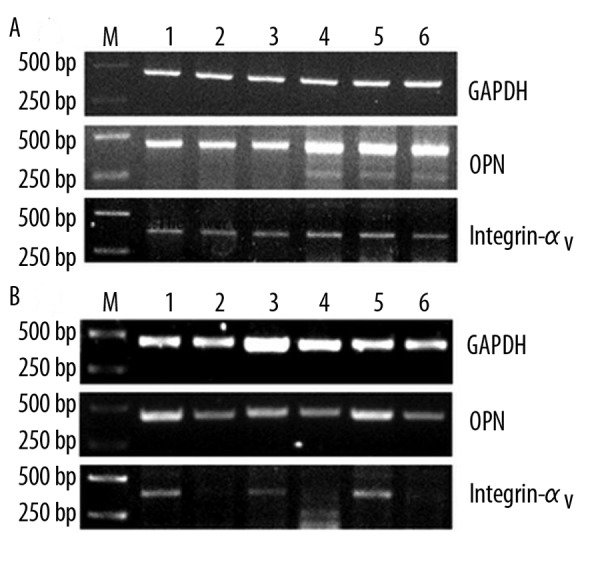
The mRNA expression of OPN in human gallbladder. Compared with control, the mRNA expression of OPN and integrin αv was increased in Group I (A). The mRNA expression of OPN and integrin αv markedly decreased in specimens of group II (B). M: marker; A: lane 1–3, control group; lane 4–6, experimental group. B: lane 1, 3, 5, control group; lane 2, 4, 6, experimental group.
Figure 3.
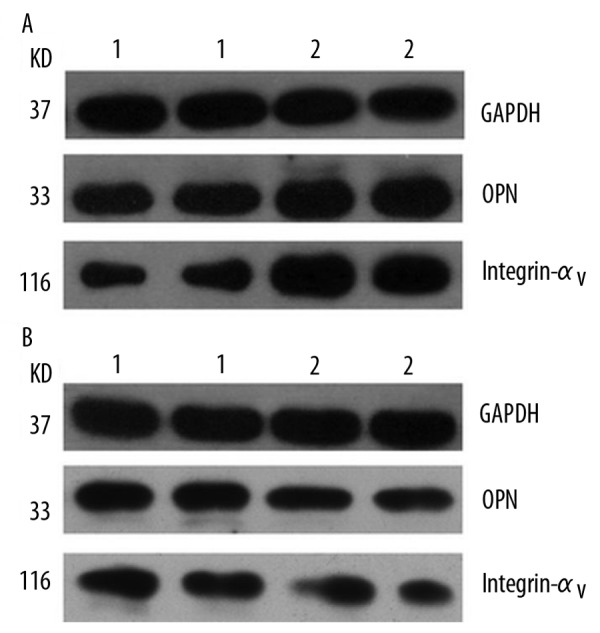
OPN protein expression in human gallbladder: Compared with control, the protein expression of OPN and integrin αv was significantly increased in Group I (A) but markedly decreased in Group II (B). 1: control group; 2: experimental group.
NT in patients and normal controls
As shown in Table 3, OPN prolonged the NT and calcium ions shortened the NT. In the patient gallbladder bile, OPN increased the NT and the spontaneous nucleation was prolonged by 79.14% (P<0.01 vs. Tris-HCl). Calcium ions decreased NT in human gallbladder (P<0.01 vs. Tris-HCl). However, in the OPN + calcium group, the nucleating effect of calcium ions was reversed by OPN and NT was prolonged by 138.78% (P<0.01 vs. calcium). Similar trends were observed in controls, but to a lesser extent. OPN inhibited the spontaneous nucleation, with NT being prolonged by 14.46% (P<0.01 vs. Tris-HCl). Meanwhile, OPN reversed the nucleating effects induced by calcium, as indicated by prolongation of NT by 31.03% (P<0.01 vs. calcium).
Table 3.
The NT (days) study in cases and normal gallbladder bile.
| Cases | Normal | |
|---|---|---|
| Tris-HCl (10 mM/L) | 6.04±1.99 | 22.13±2.23 |
| OPN (100 μg/ml) | 10.81±1.55* | 25.33±2.41* |
| Calcium ions (10mM/L) | 3.63±1.36 | 15.47±1.51 |
| OPN + calcium ions | 8.67±1.64** | 20.27±1.53** |
P<0.01, compared with Tris-Hcl;
P<0.01, compared with calcium ions.
Guinea pig lithogenic model
In guinea pigs fed the control diet, no gallstone or cholesterol monohydrate crystals were detected. In the group fed a lithogenic diet, the gallbladder bile gradually changed to viscous and turbid from the 2nd week, the first cholesterol monohydrate crystal was observed at the 6th week (2/5) and the first macroscopically gallstone was observed at the 8th week (4/6), and the gallstone and cholesterol crystals had developed in all gallbladders in the lithogenic guinea pigs at the 10th week (12/12).
The mRNA expression of OPN in gallbladder and liver of guinea pigs
The PCR products for OPN and β-actin were 327bp and 211 bp, respectively. In the lithogenic diet fed group, the OPN mRNA was examined in the gallbladder tissue at the 4th and 6th week, and the highest OPN mRNA expression was examined at the 6th week; it was not detected at the 1st, 2nd, 8th or 10th week (Figure 4A). The OPN mRNA was detected in liver tissue at the 6th week only (Figure 4B). In the control group, the expression of OPN mRNA was very weak throughout the experimental period.
Figure 4.
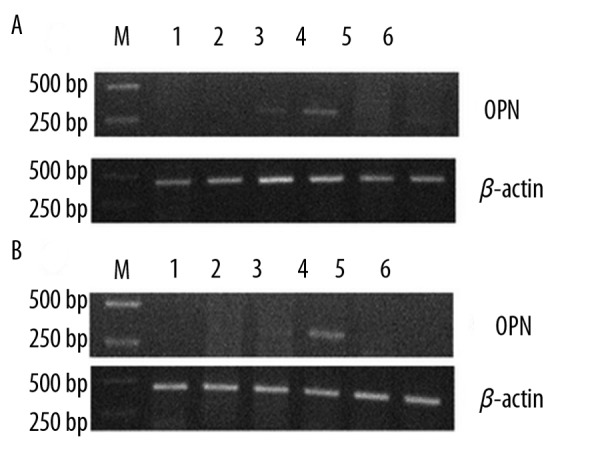
The mRNA expression of OPN in gallbladder and liver of guinea pigs: In lithogenic group, the highest level expression were found at week 6 (A). Moreover, the mRNA expression of OPN was detected in liver at week 6 only (B). In the control group, the OPN mRNA expression was weak throughout the study.
OPN content in bile and sera in guinea pigs
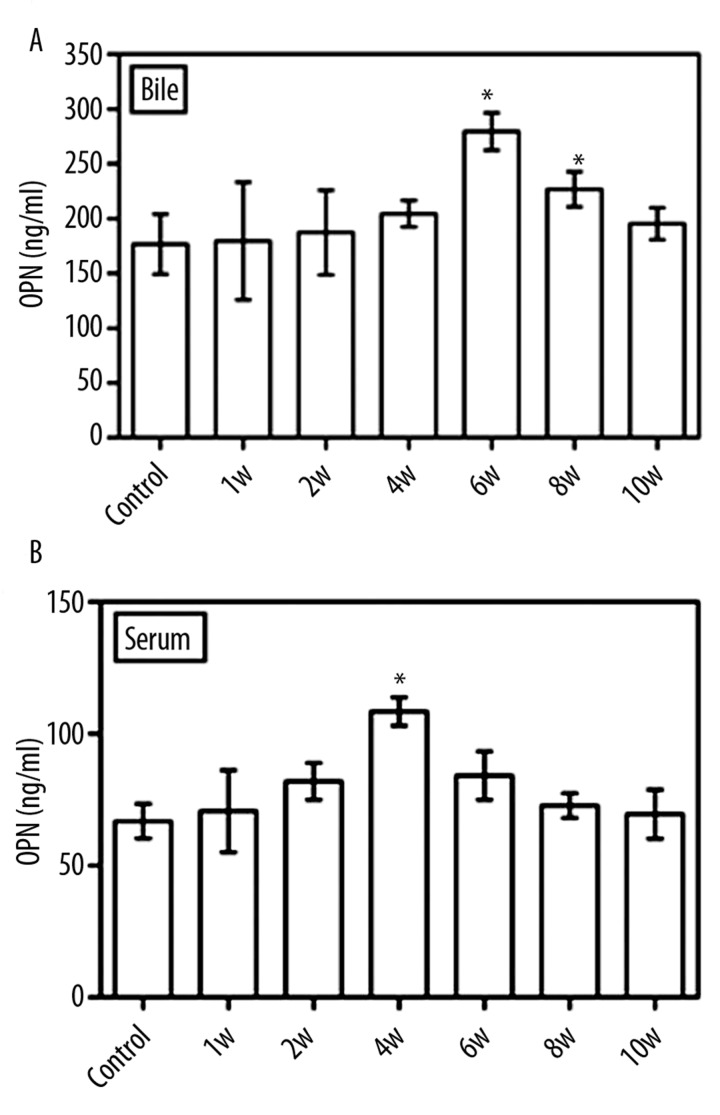
The changes in OPN contents in gallbladder bile in the lithogenic guinea pigs are illustrated in Figure 5A. Compared with controls, the OPN contents increased gradually from week 1 to week 6, peaked at week 6 (P=0.004 vs. control) and then decreased gradually, although the OPN contents were still higher than controls at week 8 (P=0.004 vs. control). At week 10 the OPN levels returned to baseline. We found the similar pattern of the changes in the OPN content in guinea pig sera (Figure 5B). The highest values of OPN were seen at week 4 (P=0.002 vs. control)
Figure 5.
OPN content in gallbladder bile and blood of guinea pig: (A). Compared with control, the highest content of OPN was detected at week 6 (P=0.004); however, at weeks 1, 2, and 4, the OPN contents increased but without statistic difference (P>0.05). At weeks 8 and 10, the OPN contents decreased gradually. The OPN contents in animal blood exhibited similar results (B). The highest value of OPN was seen at week 4 (P=0.002 vs. control).
Discussion
The pathogenesis of cholesterol gallstones appears to be multifactorial. Although OPN has been detected in normal human gallbladder tissue and gallbladder bile [10,11], the role of OPN in gallstone formation remains unknown. In the present study, we found that OPN had differential immunostaining in patients’ gallbladders with different pathological changes; relative higher OPN expression was detected in gallbladder walls with normal epithelia (Group I). When gallbladders showed degeneration, fibrosis and atrophy (Group II), the immunostaining of OPN was weaker than the former. Moreover, integrin αv, the receptor of OPN, showed a similar differential immunostaining distribution pattern as OPN. These immunoreactivities were observed in cytoplasm and the peripheral region epithelia and other cells; the different immunostaining patterns may correlate with pathological changes in the gallbladder. Furthermore, we found that OPN and integrin αv displayed a similar change at the molecular level. Recently, Ichikawa et al. [34] demonstrated that the OPN level varied from sites of calculous gallbladder walls, suggesting that OPN plays a different role in gallstone formation. These findings suggest that the functional and structural changes in gallbladders could affect the secretion of OPN and its contents in gallbladder bile, which may be involved in gallstone formation through different pathways in different stage of gallstone disease. During the pathogenesis of gallstone formation, OPN may have biological effects through the OPN-integrin αv pathway, which might be related to calcium [35].
Calcium plays a key role in gallstone formation; the calcium binding protein OPN may be involved in gallstone formation through binding to calcium. Konikoff et al. [36] report that calcium ions and the anionic polypeptide fraction (APF) have the opposite effects on cholesterol crystallization in bile. Furthermore, APF and calcium binding protein affect cholesterol gallstone formation via precipitating cholesterol and calcium salts from the bile [37]. These proteins play a critical role in pathologic biomineralization and are involved in gallstone formation through the interaction between mucin and calcium-bound proteins [38]. In our study, we found that OPN could inhibit cholesterol nucleation as an anti-nucleating factor in human gallbladder bile, which was related to calcium. Previous reports showed that the content of OPN was decreased in bile of gallstone patients [39], and these data indicated that OPN plays a role as an anti-nucleating factor.
Therefore, if pathogenic changes have not occurred, the relatively normal gallbladder and bile would maintain the balance at pathophysiological state, while OPN and the receptors would increase to prevent the gallstone formation. While the gallbladder develops irreversible pathological changes with damaged cellular structure and function, the secretion and absorption of biliary lipids would be affected; OPN and the subsequent biological effects would be down-regulated, leading to cholesterol gallstone formation [40–42]. Animal studies have also shown that the impaired gallbladder is a fundamental defect in gallstone formation and causes an imbalance of pro- and anti-nucleating effects [43].
However, the dynamic expression and role of OPN in the pathological process of human gallstone formation is still unclear. In this study, we used a guinea pig model to investigate OPN expression during gallstone formation. Our results indicated that the OPN mRNA expression increased gradually in the gallbladder, and liver in lithogenic animals peaked at 6th week and then decreased slowly. Simultaneously, the OPN concentration in gallbladder bile and serum samples exhibited similar changes. These findings showed that gallbladder and liver might regulate OPN expression in gallbladder bile, and OPN exhibited different roles at different stage of gallstone formation. Thus, we hypothesize that OPN is involved in gallstone formation under the following mechanisms. At the initial stage, OPN prevents gallstone formation as an anti-nucleating factor. As the disease progress, the role of OPN is suppressed by certain pro-nucleating factors and/or that the effect of OPN is decreased, resulting in gallstone formation, which may be correlated with calcium and its receptor integrin αv. This hypothesis should be validated in the future. Likewise, the change of OPN and integrin αv might be a response of the gallbladder to the lithogenic state in order to prevent gallstone formation, and correlate with the inflammatory damage of the gallbladder and other factors.
Conclusions
In summary, OPN is involved in gallstone formation, and the role of OPN correlates with integrin αv and calcium. The definitive mechanism of OPN involved in gallstone formation should be further investigated using transgenic animal models and other molecular biological methods.
Acknowledgements
The authors who participated in this study declare that they did not have anything to disclose regarding funds from industries or conflicts of interest with respect to this manuscript.
Footnotes
Source of support: This project was supported by the National Natural Science Foundation of China (NO.30772111)
References
- 1.Kaechele V, Wabitsch M, Thiere D, et al. Prevalence of gallbladder stone disease in obese children and adolescents: influence of the degree of obesity, sex, and pubertal development. J Pediat Gastroenterol Nutr. 2006;42:66. doi: 10.1097/01.mpg.0000187816.31213.06. [DOI] [PubMed] [Google Scholar]
- 2.Sipos P, Krisztina H, Blázovics A, Fehér J. Cholecystitis, gallstones and free radical reactions in human gallbladder. Med Sci Monit. 2001;7(1):84–88. [PubMed] [Google Scholar]
- 3.Shaffer EA. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981–96. doi: 10.1016/j.bpg.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Yoo EH, Lee SY. The prevalence and risk factors for gallstone disease. Clin Chem Lab Med. 2009;47:795–807. doi: 10.1515/CCLM.2009.194. [DOI] [PubMed] [Google Scholar]
- 5.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–44. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Muszyski J, Konarska L, Wierzbicki Z, Kowalczyk A. Med Sci Monit. 1996;2:187–92. [Google Scholar]
- 7.Hou L, Shu XO, Gao YT, et al. Anthropometric measurements, physical activity, and the risk of symptomatic gallstone disease in Chinese women. Ann Epidemiol. 2009;19:344–50. doi: 10.1016/j.annepidem.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abeysuriya V, Deen KI, Navarathne NMM. Biliary microlithiasis, sludge, crystals, microcrystallization, and usefulness of assessment of nucleation time. Hepatobiliary Pancreat Dis Int. 2010;9(3):248–53. [PubMed] [Google Scholar]
- 9.Dixit M, Choudhuri G, Saxena R, Mittal B. Association of apolipoprotein A1-C3 gene cluster polymorphisms with gallstone disease. Can J Gastroenterol. 2007;21:569–75. doi: 10.1155/2007/329342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown LF, Berse B, Van de Water L, et al. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell. 1992;3(10):1169–80. doi: 10.1091/mbc.3.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denhardt DT, Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993;7(15):1475–82. [PubMed] [Google Scholar]
- 12.Qu H, Brown LF, Senger DR, et al. Ultrastructural immunogold localization of osteopontin in human gallbladder epithelial cells. J Histochem Cytochem. 1994;42(3):351–61. doi: 10.1177/42.3.8308252. [DOI] [PubMed] [Google Scholar]
- 13.Bazzichi L, Ghiadoni L, Rossi A, et al. Osteopontin is associated with increased arterial stiffness in rheumatoid arthritis. Mol Med. 2009;15(11–12):402–6. doi: 10.2119/molmed.2009.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh K, Deonarine D, Shanmugam V, et al. Calcium-binding properties of osteopontin derived from non-osteogenic sources. J Biochem. 1993;114(5):702–7. doi: 10.1093/oxfordjournals.jbchem.a124240. [DOI] [PubMed] [Google Scholar]
- 15.Liaw L, Skinner MP, Raines EW, et al. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J Clin Invest. 1995;95(2):713–24. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rege RV. The role of biliary calcium in gallstone pathogenesis. Front Biosci. 2002;7:e315–25. doi: 10.2741/A926. [DOI] [PubMed] [Google Scholar]
- 17.Imano M, Satou T, Itoh T, et al. An immunohistochemical study of osteopontin in pigment gallstone formation. Am Surg. 2010;76(1):91–95. [PubMed] [Google Scholar]
- 18.Strasberg SM, Harvey PR, Hofmann AF. Bile sampling, processing and analysis in clinical studies. Hepatology. 1990;12:176S–80S. discussion 180S–82S. [PubMed] [Google Scholar]
- 19.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–17. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 20.Wang P, Xu TY, Guan YF, et al. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann Neurology. 2011;69(2):360–74. doi: 10.1002/ana.22236. [DOI] [PubMed] [Google Scholar]
- 21.Zuo H, Liu Z, Liu X, et al. CD151 gene delivery after myocardial infarction promotes functional neovascularization and activates FAK signaling. Mol Med. 2009;15:307–15. doi: 10.2119/molmed.2009.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan JM, Hake PW, Denenberg A, et al. Phosphorylation of extracellular signal-regulated kinase (ERK)-1/2 Is associated with the downregulation of peroxisome proliferator-activated receptor (PPAR)-γ during polymicrobial sepsis. Molecular Medicine. 2010;16(11–12):491–97. doi: 10.2119/molmed.2010.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraga-Silva RA, Sorg BS, Wankhede M. ACE2 activation promotes antithrombotic activity. Molecular Medicine. 2010;16(5–6):210–215. doi: 10.2119/molmed.2009.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatt K, Zhou L, Mi QS, et al. MicroRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. Molecular Medicine. 2010;16(9–10):409. doi: 10.2119/molmed.2010.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schierbeck H, Wahamaa H, Andersson U, Harris HE. Immunomodulatory drugs regulate HMGB1 release from activated human monocytes. Mol Med. 2010;16:343–51. doi: 10.2119/molmed.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 27.Holan KR, Holzbach RT, Hermann RE, et al. Nucleation time: a key factor in the pathogenesis of cholesterol gallstone disease. Gastroenterology. 1979;77:611–17. [PubMed] [Google Scholar]
- 28.Sahlin S, Ahlberg J, Angelin B, et al. Nucleation time of gall bladder bile in gall stone patients: influence of bile acid treatment. Gut. 1991;32(12):1554–57. doi: 10.1136/gut.32.12.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin K, Liao D, Tang C. ATP-binding membrane cassette transporter A1 (ABCA1): a possible link between inflammation and reverse cholesterol transport. Molecular Medicine. 2010;16(9–10):438–49. doi: 10.2119/molmed.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reihner E, Stahlberg D. Lithogenic diet and gallstone formation in mice: integrated response of activities of regulatory enzymes in hepatic cholesterol metabolism. Br J Nutr. 1996;76(5):765–72. doi: 10.1079/bjn19960082. [DOI] [PubMed] [Google Scholar]
- 31.Gosiewska A, Wilson S, Kwon D, Peterkofsky B. Evidence for an in vivo role of insulin-like growth factor-binding protein-1 and -2 as inhibitors of collagen gene expression in vitamin C-deficient and fasted guinea pigs. Endocrinology. 1994;134(3):1329–39. doi: 10.1210/endo.134.3.7509738. [DOI] [PubMed] [Google Scholar]
- 32.Shiesh SC, Chen CY, Lin XZ, et al. Melatonin prevents pigment gallstone formation induced by bile duct ligation in guinea pigs. Hepatology. 2000;32(3):455–60. doi: 10.1053/jhep.2000.16332. [DOI] [PubMed] [Google Scholar]
- 33.Mahmoodian F, Gosiewska A, Peterkofsky B. Regulation and properties of bone alkaline phosphatase during vitamin C deficiency in guinea pigs. Arch Biochem Biophys. 1996;336(1):86–96. doi: 10.1006/abbi.1996.0535. [DOI] [PubMed] [Google Scholar]
- 34.Ichikawa H, Imano M, Takeyama Y, et al. Involvement of osteopontin as a core protein in cholesterol gallstone formation. J Hepatobiliary Pancreat Surg. 2009;16(2):197–203. doi: 10.1007/s00534-009-0043-4. [DOI] [PubMed] [Google Scholar]
- 35.Farach-Carson MC, Xu Y. Microarray detection of gene expression changes induced by 1,25(OH)(2)D(3) and a Ca(2+) influx-activating analog in osteoblastic ROS 17/2.8 cells. Steroids. 2002;67(6):467–70. doi: 10.1016/s0039-128x(01)00168-4. [DOI] [PubMed] [Google Scholar]
- 36.Konikoff FM, Lechene de la Porte P, Laufer H, et al. Calcium and the anionic polypeptide fraction (APF) have opposing effects on cholesterol crystallization in model bile. J Hepatol. 1997;27(4):707–15. doi: 10.1016/s0168-8278(97)80088-8. [DOI] [PubMed] [Google Scholar]
- 37.Lafont H, Domingo N, Groen A, et al. APF/CBP, the small, amphipathic, anionic protein(s) in bile and gallstones, consists of lipid-binding and calcium-binding forms. Hepatology. 1997;25(5):1054–63. doi: 10.1002/hep.510250502. [DOI] [PubMed] [Google Scholar]
- 38.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368(9531):230–39. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Chen JH, Cai D, et al. Osteopontin plays an anti-nucleation role in cholesterol gallstone formation. Hepatology Research. 2011;41(5):437–45. doi: 10.1111/j.1872-034X.2011.00790.x. [DOI] [PubMed] [Google Scholar]
- 40.Shoda J, He BF, Tanaka N, et al. Increase of deoxycholate in supersaturated bile of patients with cholesterol gallstone disease and its correlation with de novo syntheses of cholesterol and bile acids in liver, gallbladder emptying, and small intestinal transit. Hepatology. 1995;21(5):1291–302. [PubMed] [Google Scholar]
- 41.Dray-Charier N, Paul A, Combettes L, et al. Regulation of mucin secretion in human gallbladder epithelial cells: predominant role of calcium and protein kinase C. Gastroenterology. 1997;112(3):978–90. doi: 10.1053/gast.1997.v112.pm9041261. [DOI] [PubMed] [Google Scholar]
- 42.Holzbach RT, Busch N. Nucleation and growth of cholesterol crystals. Kinetic determinants in supersaturated native bile. Gastroenterol Clin North Am. 1991;20(1):67–84. [PubMed] [Google Scholar]
- 43.Xiao ZL, Chen Q, Amaral J, et al. Defect of receptor-G protein coupling in human gallbladder with cholesterol stones. Am J Physiol Gastrointest Liver Physiol. 2000;278(2):G251–58. doi: 10.1152/ajpgi.2000.278.2.G251. [DOI] [PubMed] [Google Scholar]