Summary
Background
Most patients with large focal skull bone loss after craniectomy are referred for cranioplasty. Reverse engineering is a technology which creates a computer-aided design (CAD) model of a real structure. Rapid prototyping is a technology which produces physical objects from virtual CAD models. The aim of this study was to assess the clinical usefulness of these technologies in cranioplasty prosthesis manufacturing.
Material/Methods
CT was performed on 19 patients with focal skull bone loss after craniectomy, using a dedicated protocol. A material model of skull deficit was produced using computer numerical control (CNC) milling, and individually pre-operatively adjusted polypropylene-polyester prosthesis was prepared. In a control group of 20 patients a prosthesis was manually adjusted to each patient by a neurosurgeon during surgery, without using CT-based reverse engineering/rapid prototyping. In each case, the prosthesis was implanted into the patient. The mean operating times in both groups were compared.
Results
In the group of patients with reverse engineering/rapid prototyping-based cranioplasty, the mean operating time was shorter (120.3 min) compared to that in the control group (136.5 min). The neurosurgeons found the new technology particularly useful in more complicated bone deficits with different curvatures in various planes.
Conclusions
Reverse engineering and rapid prototyping may reduce the time needed for cranioplasty neurosurgery and improve the prosthesis fitting. Such technologies may utilize data obtained by commonly used spiral CT scanners. The manufacturing of individually adjusted prostheses should be commonly used in patients planned for cranioplasty with synthetic material.
Keywords: cranioplasty, reverse engineering, rapid prototyping, CT
Background
Most patients with large focal skull bone loss after craniectomy are then referred for a cranioplasty. This procedure is performed not only for aesthetic reasons, but also to protect underlying neural tissue [1,2] and improve its perfusion and metabolism [3–6].
Various materials are used, including autograft, allograft or xenograft bone [7,8], metals [9,10] and nonmetallic bone substitutes.
The autograft bone flap obtained during the initial craniectomy may be stored either fresh-frozen [7], which is the preferred method, or in the abdominal wall [11].
However, in many cases this is impossible because of bone destruction after trauma or inflammation.
Metals, like titanium, cause artifacts in CT and MR examinations, limiting the value of follow-up studies; other metals, like ferromagnetic materials, even preclude performing MR.
At present, the use of synthetic materials is more common (eg, polymethyl methacrylate (PMMA) [12,13], PMMA mixed with hydroxyapatite [14], carbon fiber-reinforced plastics (CFRP) [15], knitted polypropylene-polyester [16]).
In many cases, a bone defect has a complicated shape and different curvatures in various planes. A prosthesis must, therefore, be individually adjusted by a neurosurgeon during the surgery, which increases operating time, blood loss and infection risk [12].
Reverse engineering is a form of technology which creates a computer-aided design (CAD) model of a real structure. In medicine, virtual models of body parts may be produced using data obtained from imaging modalities like CT or MR.
Rapid prototyping, in turn, is a form of technology which produces physical objects from virtual CAD models, particularly those previously obtained using reverse engineering.
Such technologies may help obtain an individually adjusted prosthesis for cranioplasty [17–23] before the surgery, reducing the above-mentioned problems.
The aim of this study was to assess the clinical usefulness of reverse engineering and rapid prototyping in cranioplasty prosthesis manufacturing by comparing the mean operating time in the group of patients with individually-adjusted, preoperative prosthesis based on CT data with that of the control group of patients who had a prosthesis adjusted manually to the full extent during the surgery.
Material and Methods
Patients
CT (Figure 1) was performed on 19 patients with focal skull bone loss after craniectomy (12 men, 7 women, 21 to 54 years old) in the Department of Radiology of the Cracow University Hospital using a 16-row scanner Somatom Sensation 16 (Siemens Healthcare, Erlangen, Germany).The patients were included into the study during the years 2008–2010 and were randomly selected by neurosurgeons from the group of patients referred for cranioplasty.
Figure 1.
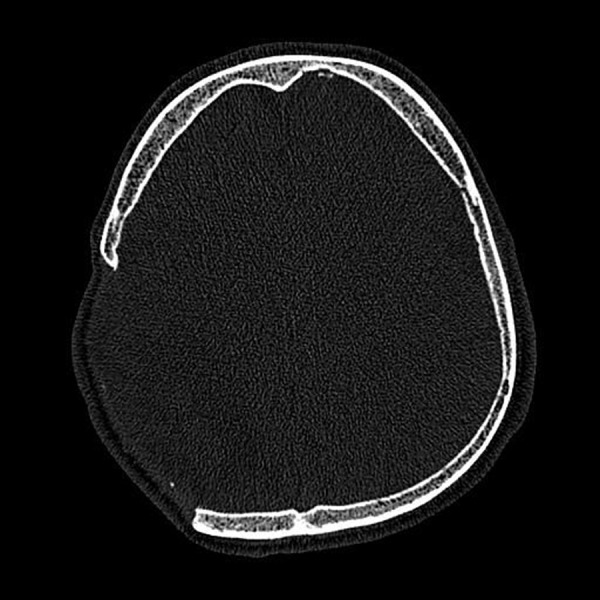
Axial image of CT in a patient with a large skull bone loss after craniectomy.
We used a dedicated CT protocol (collimation 0.75 mm, slice thickness 0.75 mm, reconstruction increment 0.5 mm, sharp kernel H60s, reconstruction FOV adjusted to the bone defect size) to get the highest spatial resolution possible.
In the control group of 20 patients with focal skull bone loss after craniectomy (14 men, 6 women, 18–60 years of age, included into the study during the years 2008–2010), a prosthesis was individually adjusted by a neurosurgeon during the surgery, without using CT-based reverse engineering/rapid prototyping.
Informed consent was obtained from each patient in the study after the nature of the procedure had been fully explained. The protocol was approved by an institutional review board.
Cranioplasty prosthesis
The main problem in the subsequent creation of a virtual 3D model was the proper assessment of bone edges. The commonly-used computer method of edge detection is based on a threshold value, arbitrarily selected by the user to discriminate between bone and other structures. However, the bone thickness is large enough to create partial volume artifacts and generate important errors in edge detection.
Therefore, specific software was created at the Cracow University of Technology for edge detection using a luminance analysis method (Figure 2), which significantly reduced the edge detection errors.
Figure 2.
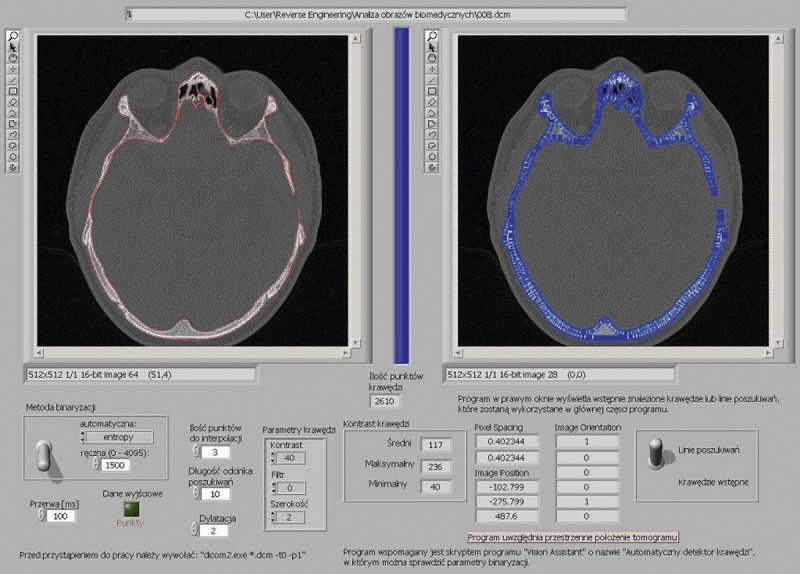
Software for edge detection using luminance analysis method created in Cracow University of Technology.
Then, in each case, a virtual model of the skull (Figure 3), based on CT data, was generated using CAD software CATIA (Dassault Systèmes, Vélizy-Villacoublay, France).
Figure 3.
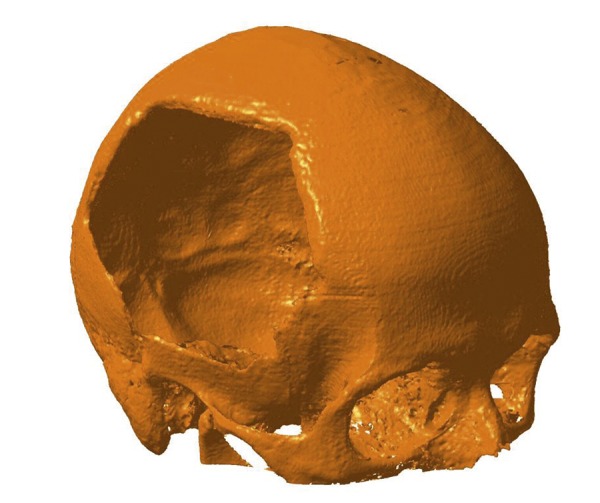
The virtual model of the skull generated using CAD software CATIA.
Such a model was the basis for designing the cranioplasty prosthesis.
For the prosthesis we used a knitted polypropylene-polyester material called Codubix by Tricomed, which is the most popular in Poland (Figure 4).
Figure 4.

Universal cranioplasty prosthesis - knitted polypropylene-polyester material Codubix by Tricomed.
In the afore-mentioned group of 19 patients, we tested 2 ways of obtaining individually adjusted prosthesis.
In 10 patients a material model of the focal skull bone deficit (Figure 5) was produced, using Computer Numerical Control (CNC) Milling Arrow 500 (Cincinnati Milacron, Cincinnati, Ohio, USA) and polyurethane resin Prolab 60 (Axon Technologies, Cergy Cedex, France). This model was then used by a neurosurgeon for the individual adjustment of a prosthesis before surgery (Figure 6). The adjusted prosthesis was sterilized before implantation.
Figure 5.

Skull focal bone deficit material model produced using Rapid Prototyping.
Figure 6.

individul adjustment of a universal prosthesis before surgery – Case A
In 9 patients with more complicated bone deficits (different curvatures in various planes, preventing the use of an adjusted universal prosthesis), apart from the material model of bone deficit as above, a material form to profile the prosthesis was produced (Figure 7) using the same method and aluminum-silicon alloy PA6. The manufacturer, using the form, delivered the already-sterilized prosthesis with proper curvatures and margins adjusted.
Figure 7.

material form produced using Rapid prototyping to profile the prosthesis by a manufacturer – Case B
Implantation
In each case, the prosthesis was implanted into the patient during neurosurgery in the Neurosurgery and Neurotraumatology Department of Cracow University Hospital (Figures 8, 9).
Figure 8.
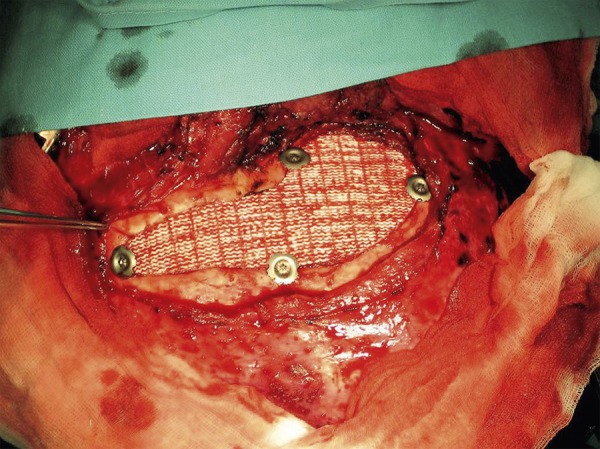
Prosthesis implanted into patient during neurosurgery – Case A.
Figure 9.
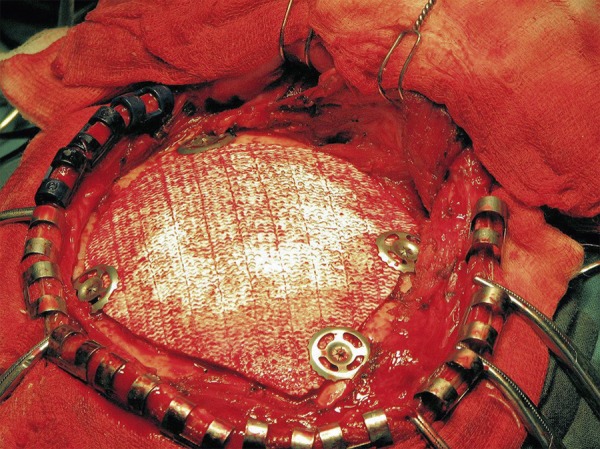
Prosthesis implanted into patient during neurosurgery – Case B.
Data analysis
We compared the mean operating time in the group of 19 patients with reverse engineering/rapid prototyping-based cranioplasty with that of the control group of 20 patients.
In the statistical analysis we used the Shapiro-Wilk normality test, Levene’s homogeneity of variance test and Student’s independent 2-sample T test.
Results
In the group of 19 patients with reverse engineering/rapid prototyping-based cranioplasty, the mean operating time was 120.3 minutes (minimum 110 minutes, maximum 140 minutes, SD 7.9 minutes).
In the control group of 20 patients, the mean operating time was 136.5 minutes (minimum 120 minutes, maximum. 150 minutes, SD 10.5 minutes).
In both groups, normal distribution was confirmed using the Shapiro-Wilk test (p>0.05) and homogeneity of variance was found using Levene’s test (p>0.05), so Student’s T test could be used. The difference between the mean values in the groups examined was found to be statistically significant (t=–5.43, p=0.000004).
The neurosurgeons found reverse engineering/rapid prototyping-based cranioplasty particularly useful in more complicated bone deficits, with different curvatures in various planes. In their opinion, in such cases individually-adjusted prostheses resulted in a much better cosmetic effect, mainly because of accurate skull symmetry achieved.
Discussion
Until now the universal prosthesis for cranioplasty was manually adjusted to a skull bone defect by a surgeon during the operation. This was a universal prosthesis which, unfortunately, meant that in many cases its fit was not good enough. In some cases, a surgeon even had to start with another prosthesis because of excessive cutting during the manual adjustment. It could thus unnecessarily extend the operation time.
After the application of reverse engineering technology to properly adjust the prosthesis before the surgery, the time needed for the operation was substantially reduced.
This has already been reported by other authors.
Lee et al. [12] compared 3 groups of cranioplasty patients: group 1 (91 patients) receiving fresh frozen autograft bone, group 2 (23 patients) with PMMA prosthesis molded intra-operatively, and group 3 (17 patients) receiving a custom-made prefabricated PMMA prosthesis manufactured based on CT exam. Group 2 patients required significantly more operating time than both group 1 and group 3 patients, but the operating time did not differ significantly between groups 1 and 3. Mean intra-operative blood loss was significantly higher in group 2 than in group 1, but did not differ significantly between group 1 and group 3. The infection rate in group 3 was lower than that in group 2 and was comparable to that of group 1.
D’Urso et al. [22] used CT data and stereolithography (SL) technology to manufacture cranioplasty acrylic implants in 30 patients. Such customized implants reduced operating time, afforded excellent cosmesis and were cost-effective. The patients reported that the opportunity to see the biomodel and implant preoperatively improved their understanding of the procedure.
In our study the neurosurgeons found reverse engineering/rapid prototyping-based cranioplasty particularly useful in more complicated bone deficits. Individually prefabricated and profiled prostheses were much better fitted in patients with different curvatures in various planes, resulting in a much better cosmetic effect.
Dean et al. [24], using CT data for manufacturing PMMA or pre-bent titanium cranioplasty implants, also found them to be better fitting and more cosmetically suitable compared to manually prepared implants. They also concluded that such well-fitting implants were more likely to protect the brain from trauma and infection.
According to Goh et al. [25], using customized implants fabricated by computer-aided design helps to avoid unsatisfactory outcomes of the surgery and should be the preferred method in patients after previously failed cranioplasty.
The additional cost of manufacturing the rapid prototyping model in our research was about $300. It is difficult to directly compare it with the other authors’ values (for example approximately $1300 per case in D’Urso’s [22] research) because of the different techniques used. However, some authors have investigated some feasible technical solutions for minimizing the implant cost based on the availability of production technologies in a region. Hieu et al. [26] used CNC milling techniques for making molds to fabricate PMMA implants and found the cost acceptable for the Association of South-East Asian Nations (ASEAN) region.
The future application of computer-aided systems for cranioplasty will probably not be limited to customized implant fabrication. In patients with skull bone tumors, the robot-guided bone resection based on CT data will be possible. Weihe et al. [27] constructed a prototype system of single-step skull bone resection and reconstruction based on CT data and tested it on an animal cadaver model.
Finally, it should be emphasized that reverse engineering and rapid prototyping technologies may be helpful not only in cranioplasty but also in many other surgical reconstruction procedures, including craniofacial bones [28].
Conclusions
Reverse engineering and rapid prototyping may reduce the time needed for cranioplasty neurosurgery and improve the fitting of the prosthesis.
Such technologies may utilize data obtained by commonly used spiral CT scanners.
The manufacturing of individually-adjusted prostheses using reverse engineering and rapid prototyping technologies should be commonly used in patients planned for cranioplasty with synthetic material.
Footnotes
Source of support: Departmental sources
References
- 1.Sanan A, Haines SJ. Repairing holes in the head: a history of cranioplasty. Neurosurgery. 1997;40:588–603. doi: 10.1097/00006123-199703000-00033. [DOI] [PubMed] [Google Scholar]
- 2.Dujovny M, Aviles A, Agner C, et al. Cranioplasty: cosmetic or therapeutic? Surg Neurol. 1997;47:238–41. doi: 10.1016/s0090-3019(96)00013-4. [DOI] [PubMed] [Google Scholar]
- 3.Winkler PA, Stummer W, Linke R, et al. Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J Neurosurg. 2000;93:53–61. doi: 10.3171/jns.2000.93.1.0053. [DOI] [PubMed] [Google Scholar]
- 4.Kuo JR, Wang CC, Chio CC, et al. Neurological improvement after cranioplasty – analysis by transcranial Doppler ultrasonography. J Clin Neurosci. 2004;11:486–89. doi: 10.1016/j.jocn.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Isago T, Nozaki M, Kikuchi Y, et al. Sinking skin flap syndrome. A case of improved cerebral blood flow after cranioplasty. Ann Plast Surg. 2004;53:288–92. doi: 10.1097/01.sap.0000106433.89983.72. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto S, Eguchi K, Kiura Y, et al. CT perfusion imaging in the syndrome of the sinking skin flap before and after cranioplasty. Clin Neurol Neurosurg. 2006;108:583–85. doi: 10.1016/j.clineuro.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Iwama T, Yamada J, Imai S, et al. The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery. 2003;52:591–95. doi: 10.1227/01.neu.0000047891.86938.46. [DOI] [PubMed] [Google Scholar]
- 8.Taggard DA, Menezes AH. Successful use of rib grafts for cranioplasty in children. Pediatr Neurosurg. 2001;34:149–55. doi: 10.1159/000056010. [DOI] [PubMed] [Google Scholar]
- 9.Joffe J, Harris M, Kahugu F, et al. A prospective study of computer-aided design and manufacture of titanium plate for cranioplasty and its clinical outcome. Brit J Neurosurg. 1999;13:576–80. doi: 10.1080/02688699943088. [DOI] [PubMed] [Google Scholar]
- 10.Joffe JM, Nicoll SR, Richards R, et al. Validation of computer assisted manufacture of titanium plates for cranioplasty. Int J Oral Maxillofac Surg. 1999;28:309–13. [PubMed] [Google Scholar]
- 11.Movassaghi K, Ver Halen J, Ganchi P, et al. Cranioplasty with subcutaneously preserved autologous bone grafts. Plast Reconstr Surg. 2006;117:202–6. doi: 10.1097/01.prs.0000187152.48402.17. [DOI] [PubMed] [Google Scholar]
- 12.Lee SC, Wu CT, Lee ST, et al. Cranioplasty using polymethyl methacrylate prostheses. J Clin Neurosci. 2009;16:56–63. doi: 10.1016/j.jocn.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Fathi AR, Marbacher S, Lukes A. Cost-effective patient-specific intraoperative molded cranioplasty. J Craniofac Surg. 2008;19:777–81. doi: 10.1097/SCS.0b013e31816b1b2a. [DOI] [PubMed] [Google Scholar]
- 14.Rotaru H, Baciut M, Stan H, et al. Silicone rubber mould cast polyethylmethacrylate-hydroxyapatite plate used for repairing a large skull defect. J Craniomaxillofac Surg. 2006;34:242–46. doi: 10.1016/j.jcms.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Saringer W, Nöbauer-Huhmann I, Knosp E. Cranioplasty with individual carbon fibre reinforced polymere (CFRP) medical grade implants based on CAD/CAM technique. Acta Neurochir (Wien) 2002;144:1193–203. doi: 10.1007/s00701-002-0995-5. [DOI] [PubMed] [Google Scholar]
- 16.Andrzejak S, Fortuniak J, Wróbel-Wiśniewska G, et al. Clinical evaluation of the polypropylene-polyester knit used as a cranioplasty material. Acta Neurochir (Wien) 2005;147:973–76. doi: 10.1007/s00701-005-0581-8. [DOI] [PubMed] [Google Scholar]
- 17.Solaro P, Pierangeli E, Pizzoni C, et al. From computerized tomography data processing to rapid manufacturing of custom-made prostheses for cranioplasty. Case report. J Neurosurg Sci. 2008;52:113–16. [PubMed] [Google Scholar]
- 18.Maravelakis E, David K, Antoniadis A, et al. Reverse engineering techniques for cranioplasty: a case study. J Med Eng Technol. 2008;32:115–21. doi: 10.1080/03091900600700749. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann B, Sepehrnia A. Taylored implants for alloplastic cranioplasty – clinical and surgical considerations. Acta Neurochir Suppl. 2005;93:127–29. doi: 10.1007/3-211-27577-0_21. [DOI] [PubMed] [Google Scholar]
- 20.Wulf J, Busch LC, Golz T, et al. CAD generated mold for preoperative implant fabrication in cranioplasty. Stud Health Technol Inform. 2005;111:608–10. [PubMed] [Google Scholar]
- 21.Müller A, Krishnan KG, Uhl E, et al. The application of rapid prototyping techniques in cranial reconstruction and preoperative planning in neurosurgery. J Craniofac Surg. 2003;14:899–914. doi: 10.1097/00001665-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 22.D’Urso PS, Earwaker WJ, Barker TM, et al. Custom cranioplasty using stereolithography and acrylic. Br J Plast Surg. 2000;53:200–4. doi: 10.1054/bjps.1999.3268. [DOI] [PubMed] [Google Scholar]
- 23.Agner C, Dujovny M, Evenhouse R, et al. Stereolithography for posterior fossa cranioplasty. Skull Base Surg. 1998;8:81–86. doi: 10.1055/s-2008-1058580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dean D, Min KJ, Bond A. Computer aided design of large-format prefabricated cranial plates. J Craniofac Surg. 2003;14:819–32. doi: 10.1097/00001665-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Goh RC, Chang CN, Lin CL, et al. Customised fabricated implants after previous failed cranioplasty. J Plast Reconstr Aesthet Surg. 2010;63:1479–84. doi: 10.1016/j.bjps.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Hieu LC, Bohez E, Sloten J, et al. Design and manufacturing of cranioplasty implants by 3-axis cnc milling. Technol Health Care. 2002;10:413–23. doi: 10.1046/j.1365-2524.2002.03834.x. [DOI] [PubMed] [Google Scholar]
- 27.Weihe S, Schiller C, Rasche C, et al. CAD/CAM prefabricated individual skull implants: new aspects in robot resection and guided bone regeneration. International Congress Series. 2004;1268:584–90. [Google Scholar]
- 28.Elgalal M, Kozakiewicz M, Loba P, et al. Patient specific implants, designed using Rapid Prototyping and diagnostic imaging, for the repair of orbital fractures. Med Sci Monit. 2010;16(Suppl 1):75–79. [Google Scholar]


