Summary
Background
Previous studies show that Asians have an impaired blood flow response (BFR) to occlusion after a single high fat (HF) meal. The mechanism is believed to be the presence and susceptibility to high free radicals in their blood. The free radical concentration after a HF meal has not been examined in Asians. Further the BFR to heat after a single HF meal in Koreans has not been measured.
Material/Methods
This study evaluated postprandial endothelial function by measuring the BFR to vascular occlusion and local heat before and after a HF meal and the interventional effects of anti-oxidant vitamins on improving endothelial function in young Korean-Asians (K) compared to Caucasians (C) with these assessments. Ten C and ten K participated in the study (mean age 25.3±3.6 years old). BFR to vascular occlusion and local heat and oxidative stress were assessed after a single low fat (LF) and HF meal at 2 hours compared to baseline. After administration of vitamins (1000 mg of vitamin C, 800 IU of vitamin E, and 300 mg of Coenzyme Q-10) for 14 days, the same measurements were made.
Results
This study showed that the skin BFR to vascular occlusion and local heat following a HF meal significantly decreased and free radicals significantly increased at 2 hours compared to baseline in K (p<.001), but not in C. When vitamins were given, the BFR to vascular occlusion and local heat before and after HF meal were not significantly different in K and C.
Conclusions
These findings suggest that even a single HF meal can reduce endothelial response to stress through an oxidative stress mechanism but can be blocked by antioxidants, probably through scavenging free radicals in K. Since endothelial function improved even before a HF meal in K, endothelial damage from an Americanized diet may be reduced in K by antioxidants.
Keywords: antioxidant, Asian, endothelial function, heat, occlusion
Background
The vascular endothelium plays an integral role in vascular growth, vasoregulation, and vasoprotection. [1]. A balance between endothelial derived relaxing and contracting factors maintains vascular homeostasis [2]. Studies show that different ethnic populations have different genes that can alter endothelial function [3,4]. For example, people from Korea and other Asians have a “thrifty” gene that was developed in populations where food supply was limited due to famine, thus allowing fat to be stored easily when only limited foods are available [5,6]. Today, Koreans are eating more high fat (HF) foods than a decade ago and the mortality rate due to diabetes mellitus (DM) has doubled during the last decade, increasing from 17.2 per 100,000 persons in 1995 to 24.5 per 100,000 persons in 2005 [7]. “Thrifty” genes seem to heighten the susceptibility to endothelial dysfunction when eating HF foods or gaining body weight [3,4,6]. A standard test of endothelial function is the response to vascular occlusion for 4 minutes. A recent study in this lab showed that Koreans had a lower blood flow response (BFR) to 4 minutes of vascular occlusion and 6 minutes of local heating than Caucasians. This finding suggests that the Korean population has lower endothelial response to stress than Caucasians [8]. In another study, it was found that blood flow was reduced even further in Asians with a single HF meal, again implying reduced endothelial response to stressors due to fats in the diet [6].
The mechanisms of postprandial HTG-induced endothelial dysfunction have been suggested that HTG significantly stimulates leukocytes to produce free radicals increased oxidative stress [6,9]. The detrimental effect of free radicals inducing potential biological damage is termed oxidative stress [10]. High free radical concentration in the body increases cellular oxidation and can biodegrade nitric oxide (NO) and prostacyclin into inactive forms [11]. Both compounds are released by vascular endothelial cells to relax blood vessels. Free radicals bioconvert NO into peroxynitrite, thereby reducing the bioavailability of NO as a vasodilator [12]. This is believed to be one of the mechanisms associated with reduced circulation in older people and diabetics [11].
One purpose of the present investigation was to quantify the effect of a HF meal on the BFR to heat and occlusion in Koreans compared to Caucasians. Unlike other studies measuring flow mediated vasodilatation at rest and after a HF meal, we wanted to measure free radicals in the blood to see how this correlated to flow mediated vasodilatation and the BFR of the skin to heat. In addition, we wanted to assess the effects of vitamins on these vascular responses.
Numerous studies have examined the benefits of vitamins in reducing free radicals in the body [11,13,14]. However, little is known about measuring the effects of a HF meal and vitamins on the level of free radicals and endothelial function. A standard method of assessing endothelial function is post ischemic reactive hyperemia by measuring the rise in blood flow after occlusion [15–17]. Another method of assessing endothelial function is the skin BFR to local heat mediated by the endothelial cells by the increase in skin blood flow [18,19]. High concentrations of free radicals neutralize nitric oxide and prostacyclin and should reduce blood flow response to vascular occlusion and local heat. Malondialdehyde (MDA) can be used to assess the degree of feeding-induced oxidative stress. MDA is a highly reactive three carbon chain aldehyde produced as a byproduct of the decomposition of a lipid hydroperoxide and is commonly used as an indicator of lipid peroxidation [20].
The purpose of this study was to evaluate postprandial endothelial function as related to enhanced oxidative stress in a single HF and LF meal and more importantly the interventional effects of vitamins on scavenging free radicals and improving endothelial function in Korean-Asians. These data were compared to Caucasians. This was done by measuring skin BFR to vascular occlusion and local heat and analyzing MDA levels in both groups after ingestion of a LF and HF meal before and after vitamin intervention.
Material and Methods
Subjects
Healthy young subjects were recruited by word of mouth and assigned to one of two groups based on self-reported ethnicity. Subjects did not have diagnosed cardiovascular disease, hypertension (>140/90 mmHg) or diabetes, were non-smokers, were not taking any medications that would affect the cardiovascular system, and did not have any known peripheral circulatory diseases. The subjects included 10 Koreans and 10 Caucasians. They were in the age range of 20–35 years old and with a BMI of less than 40. The general characteristics of the subjects are shown in Table 1. All protocols and procedures were approved by the Institutional Review Board of Loma Linda University and all subjects signed a statement of informed consent.
Table 1.
Mean (SD) of general characteristics of the 5 men and 5 women in each ethnic group.
| Koreans | Caucasians | p-value | |
|---|---|---|---|
| Age (years) | 25.4 (4.2) | 27.8 (2.4) | 0.14 |
| Height (cm) | 168.8 (9.4) | 176.1 (11.3) | 0.14 |
| Weight (kg) | 70.5 (16.7) | 73.6 (8.3) | 0.60 |
| BMI (kg/m2) | 24.5 (3.9) | 23.8 (1.8) | 0.60 |
| Skin thickness (cm) | 0.05 (0.01) | 0.05 (0.01) | 0.51 |
| Fat thickness (cm) | 0.10 (0.01) | 0.11 (0.01) | 0.15 |
| Skin moisture (mg/cm2) | 41.4 (6.3) | 40.8 (12.2) | 0.89 |
Methods
Measurement of skin blood flow
Skin blood flow was measured with a MOOR Laser Doppler Imager (Moor LTD, Oxford, England). The Laser was used in a single spot mode. Blood flow in a Laser Doppler Imager was expressed in units called flux. The Laser was warmed for 30 minutes prior to measurements. The reported reliability from the manufacturer is ±5% from day to day. The Laser’s calibration was checked just before, in the middle and at the end of the study; there was no calibration drift observed.
Measurement of skin temperature
Skin temperature was measured with a thermistor manufactured by BioPac Systems (BioPac Inc., Goleta, CA). The thermistor output was amplified with an SKT 100 Thermistor amplifier (BioPac Systems, Goleta, CA) and the output was then digitized at 1000 samples per second on a BioPac MP150 data collection system at 24 bits of resolution (BioPac Systems, Goleta, CA).
Control of skin temperature
Skin temperature was controlled with a thermode. The thermode consisted of a plastic box with a port on each end so that water could move through the box. The box was approximately 5×2.5×2.5 cm in size. Further details on the technique and the reliability and validity are published elsewhere [21,22].
Test meals
Isocaloric LF and HF meals (726 kcal) were given to the subjects at the study site in the morning after an overnight fast. The nutritional composition of HF meal was 50.1 g total fat, 14 g saturated fat, 443 mg cholesterol, 22.3 g protein, 43.8 g carbohydrates. The nutritional composition of isocaloric LF meal was 5.1 g total fat, 1g saturated fat, 0 mg cholesterol, 31.3 g protein, 135.8 g carbohydrates. To assure consistency across subjects, meals were ordered from the same commercial restaurant.
Measurement of Malondialdehyde
Malondialdehyde (MDA) was measured using the 2-ThioBarbituric Acid Reactive Substances (TBARS) assay kit (Oxford Biomedical Research, Inc., Oxford, MI, USA). This assay is based on the reaction of a chromogenic reagent, 2-thiobarbituric acid, with MDA at 95°C. One molecule of MDA reacts with 2 molecules of 2-thiobarbituric acid via a Knoevenagel-type condensation to yield a chromophore with absorbance maximum at 532 nm.
Procedures
All subjects were asked to abstain from dietary supplements for 1 week prior to starting the study and avoid engaging in physical exercise 48 hours prior to the experimental meal. Subjects fasted 12 hours prior to participating in the study. The order of HF and LF breakfast meals was randomly assigned and separated with a one week wash out period. Subjects began their experiment between 8–10 am and entered a thermally neutral room (22°C) and rested comfortably for 20 minutes. In the first experiment, the test breakfast meal was given to subjects and endothelial function was assessed by two methods at baseline and after 2 hours following the meal. The first was occlusion of the circulation on upper arm for 4 minutes followed by release and measurement of skin blood flow for 2 minutes. The second was assessment of the skin blood flow response to local heat by applying a thermode of 42°C to the forearm. Each procedure was performed on different arms. Since the metabolic effect of a meal peaks at 2 hours on endothelial function, the measurements were repeated after 2 hours following the meal. A 10 mL blood sample was taken at baseline and after 2 hours following the meal for measuring oxidative stress. After a wash out period of 7 days, the same procedure was performed but the subjects were given the other test meal and data was collected at baseline and after 2 hours following the meal. A 10 mL blood sample was taken after 2 hours following the meal. Finally, to see if antioxidants can reduce the damage to endothelial cells from a HF meal, after a wash out period of 7 days from the last previous meal, a final series of experiments had the subjects ingest anti-oxidant supplements daily of 1000 mg of vitamin C, 800 IU of vitamin E, and 300 mg of Coenzyme Q-10 for 14 days. After 14 days following anti-oxidant supplements intake, on the eighth day, measurements were repeated in baseline and followed by ingestion of a HF meal. A 10 mL blood sample was taken at baseline and after 2 hours following a HF meal. The blood sample was not drawn more than 2 times per week and was assayed for oxidative stress.
Statistical analysis
Data was summarized as Means and standard deviations. Baseline characteristics of Caucasians and Koreans were compared using an independent t-test (Table 1). A mixed factorial ANOVA was conducted to compare the BFRse to 4 minutes of vascular occlusion and 6minutes of local heat before and after two different meals with or without vitamins in Koreans and Caucasians. Also, a paired t-test was conducted to compare the MDA concentration before versus after the meals with or without vitamins. The level of significance was set at p=0.05. Effect sizes were calculated to account for group variability. A post power analysis with an effect size of 1.6 according to the change in skin blood flow between Korean and Caucasian groups, an alpha level of.05, and a sample size of 20 indicated that the power was.91.
Results
Data is presented as baseline measurements and the results after a LF and HF meal and pre and post vitamins intervention as given below.
Baseline
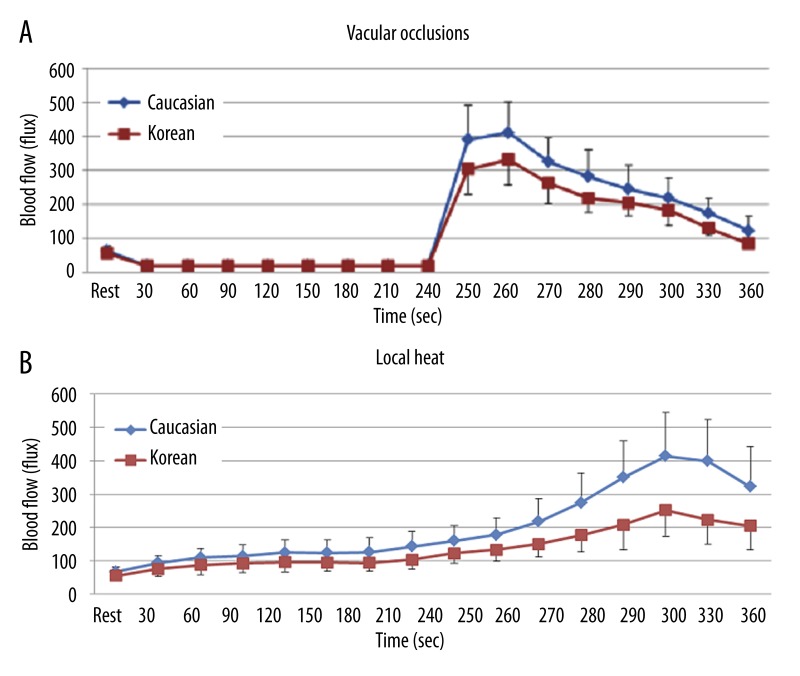
The results of skin blood flow during 4 minutes of vascular occlusion and then the first 2 minutes following occlusion at baseline are shown in Figure 1A. There was a significant difference in the skin BFR after 4 minutes between Koreans and Caucasians (p=0.016). The mean blood flow rapidly increased to a peak of 332.4±75.8 flux in Koreans compared to 411.9±88.9 flux in Caucasians at 20 seconds after the release of the four minutes of vascular occlusion. The blood flow then decreased to a final value of 84.8±15.5 flux in Koreans compared to 122.3±42.4 flux in Caucasians at the end of the 2 minute period.
Figure 1.
Mean ±SD of baseline blood flow (flux) measured during the 4 minutes period of vascular occlusion and the 2 minute period following the release of the occlusion cuff (A) and 6 minutes period of local heat (B) in 10 Koreans and 10 Caucasians.
The results of BFR to local heat during 6 minutes at baseline are shown in Figure 1B. The skin blood flow was significantly lower in Koreans than in Caucasians after 60 seconds of heat exposure (p=0.003). The peak blood flow after heat exposure was 251±77.9 flux in Koreans and 413.7±132.1 flux in Caucasians at 240 seconds, respectively.
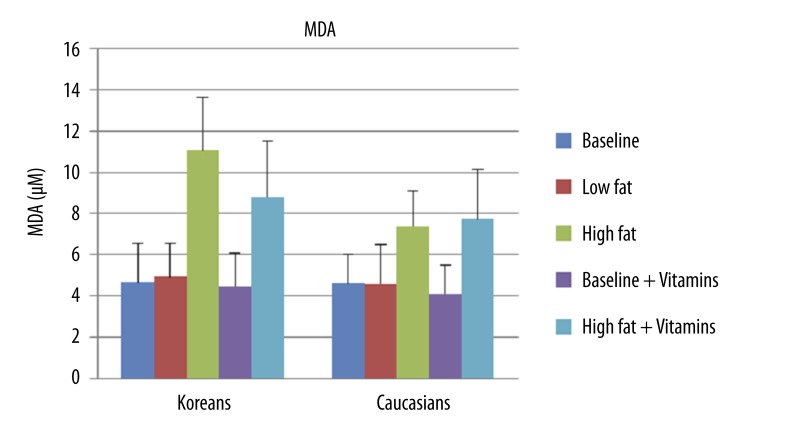
To determine the level of lipid peroxidation and oxidative stress following meals, MDA concentration was measured using TBARS assay. When comparing the concentration of MDA at baseline, there were no significant differences between Koreans (4.6±1.9 μM) and Caucasians (4.6±1.4 μM) (Figure 2).
Figure 2.
Mean ±SD of MDA concentration at baseline, after 2 hours of low fat meal, after 2 hours of high fat meal, at baseline with vitamins, and after 2 hours of high fat meal with vitamins in 10 Koreans and 10 Caucasians. * p<0.01 compared to Caucasians.
Results after two meals
Low fat meal
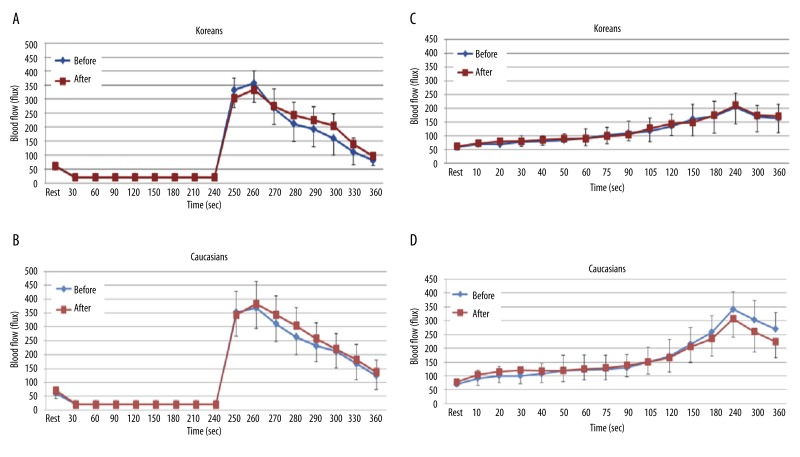
The results of skin blood flow during 4 minutes of vascular occlusion and then the first 2 minutes following occlusion at baseline and at 2 hours after the ingestion of the LF meal in both Koreans and Caucasians are shown in Figure 3A and B. When comparing blood flow at baseline and at 2 hours, the skin blood flow was not significantly different in both Koreans and Caucasians.
Figure 3.
Mean ±SD of blood flow (flux) after a low fat meal measured during the 4 minutes period of vascular occlusion and the 2 minute period following the release of the occlusion cuff (A, B) and 6 minutes period of local heat (C, D) at baseline and after 2 hours in 10 Koreans and 10 Caucasians.
The results of skin BFR to local heat during 6 minutes at baseline and at 2 hours after the ingestion of the LF meal in both Koreans and Caucasians are shown in Figure 3C and D. There were no significant differences in the skin blood flow in both Caucasians and Koreans.
When comparing the concentrations of MDA at baseline and at 2 hours, there was no significant difference in both Koreans (4.6±1.9 μM vs. 4.9±1.6 μM) and Caucasians (4.6±1.4 μM vs. 4.6±1.9 μM) (Figure 2).
High fat meal
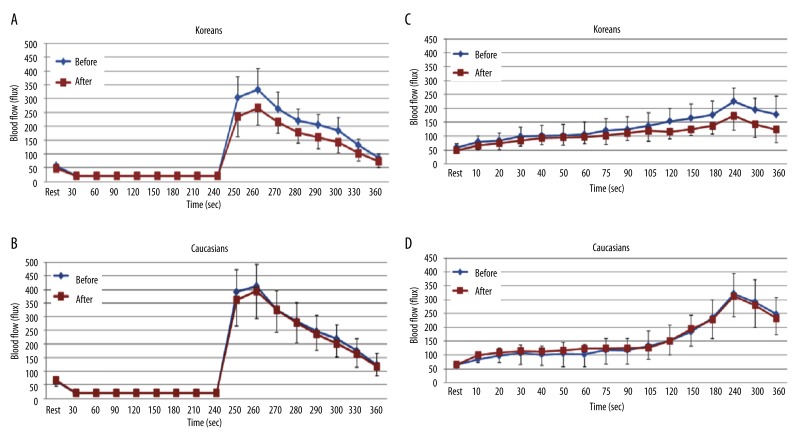
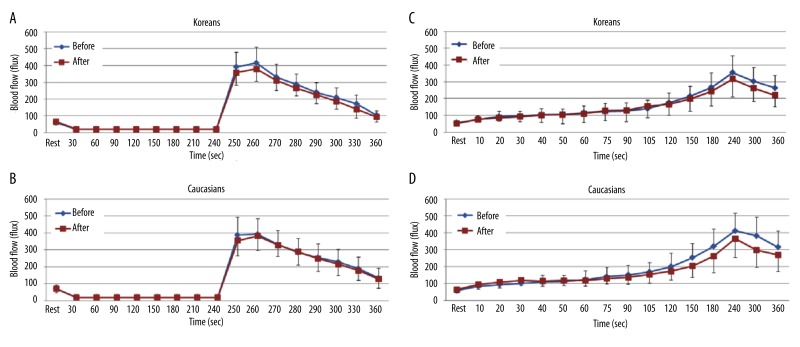
When comparing BFR after 4 minutes of vascular occlusion at baseline and at 2 hours after the ingestion of the HF meal, the total skin blood flow significantly decreased at 2 hours compared to baseline in Koreans (p<0.001). The peak blood flow after occlusion was 332.4±75.8 flux at baseline and 266.2±61.7 flux at 2 hours at 260 seconds respectively (Figure 4A). However, there were no significant differences in Caucasians after 2 hours compared to baseline (Figure 4B).
Figure 4.
Mean ±SD of blood flow (flux) after a high fat meal measured during the 4 minutes period of vascular occlusion and the 2 minute period following the release of the occlusion cuff (A, B) and 6 minutes period of local heat (C, D) at baseline and after 2 hours in 10 Koreans and 10 Caucasians.
When applying 6 minutes of local heat at baseline and at 2 hours after the ingestion of the HF meal, the skin blood flow after 90 seconds of heat exposure significantly decreased at 2 hours compared to baseline in Koreans (p<0.001). The peak blood flow after heat exposure was 224.9±48.5 flux at baseline and 173.5±52.3 flux at 2 hours at 240 seconds respectively (Figure 4C). However, there were no significant differences in Caucasians after 2 hours compared to baseline (Figure 4D).
In the MDA measurements, there was a significantly higher mean MDA concentration at 2 hours than at baseline in both Koreans (4.6±1.9 μM vs. 11.1±2.6 μM) (p<0.001) and Caucasians (4.6±1.4 μM vs. 7.4±1.7 μM) (p=0.002) (Figure 2). Koreans had a 138.15% increase and Caucasians had a 60.22% increase from baseline.
Results after the intake of vitamins for two weeks
In contrast, when comparing the BFR after 4 minutes of vascular occlusion at baseline and at 2 hours after the ingestion of the HF meal after the intake of vitamins for 14 days, there were no significant differences in both Koreans and Caucasians (Figure 5A and B).
Figure 5.
Mean ±SD of blood flow (flux) measured during the 4 minutes period of vascular occlusion and the 2 minute period following the release of the occlusion cuff (A, B) and 6 minutes period of local heat (C, D) at baseline and at 2 hours after the ingestion of the high fat meal after the intake of vitamins for 14 days in 10 Koreans and 10 Caucasians.
Also, when comparing BFR to 6 minutes of local heat at baseline and at 2 hours after the ingestion of the HF meal after the intake of vitamins for 14 days, the skin blood flow was not significantly different in both Koreans and Caucasians (Figure 5C and D).
In MDA concentration, however, there were significant differences in both Koreans (4.5±1.6 μM vs. 8.8±2.7 μM) (p=0.001) and Caucasians (4.1±1.4 μM vs. 7.7±2.4 μM) (p=0.001) (Figure 2).
Comparison of baseline with vitamins and without vitamins
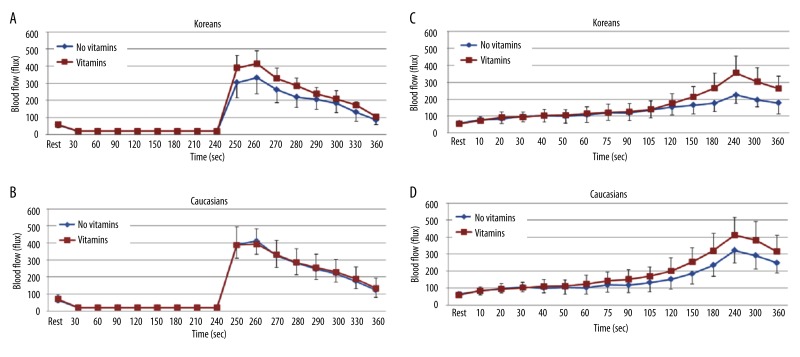
When comparing the baseline BFR to 4 minutes of vascular occlusion between 14 days of vitamins and no vitamins in both ethnic groups, the baseline blood flow was significantly higher when vitamins were given compared to no vitamins in Koreans (p=0.024). The peak blood flow after occlusion was 414.7±92.9 flux with vitamins compared to 332.4±75.8 flux without vitamins at 260 seconds, respectively (Figure 6A). However, no significant differences were observed in Caucasians (Figure 6B).
Figure 6.
Mean ±SD of baseline blood flow (flux) measured during the 4 minutes period of vascular occlusion and the 2 minute period following the release of the occlusion cuff (A, B) and 6 minutes period of local heat (C, D) before and after the intake of vitamins for 14 days in 10 Koreans and 10 Caucasians.
In regards to the baseline BFR to 6 minutes of local heat, the skin blood flow after 90 seconds of heat exposure significantly increased with vitamins compared to no vitamins in Koreans (p=0.029). The peak blood flow after heat exposure was 355.2±128.6 flux with vitamins versus 224.9±48.5 flux without vitamins at 240 seconds, respectively (Figure 6C). However, there were no significant differences in the skin blood flow in Caucasians (Figure 6D).
For the MDA concentration, there were no significant differences in both Koreans (4.6±1.9 μM vs. 4.5±1.6 μM) and Caucasians (4.6±1.4 μM vs. 4.1±1.4 μM). MDA concentration decreased by 3.88% in Koreans and decreased by 10.86% in Caucasians (Figure 2).
Comparison of high fat meal and high fat meal with vitamins
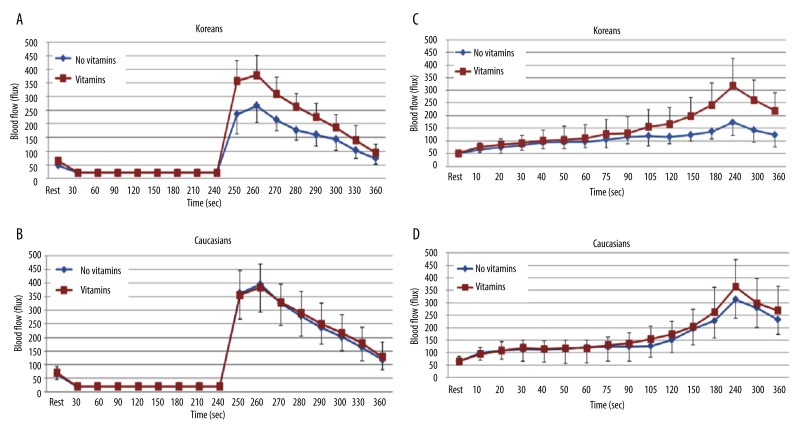
When comparing the BFR to 4 minutes of vascular occlusion at 2 hours after a HF meal with a previous intake of vitamins for 14 days to no vitamins in both ethnic groups, the skin blood flow was significantly higher when vitamins were given compared to no vitamins in Koreans (p=0.001). The peak blood flow after occlusion was 378.5±73.7 flux with vitamins and 266.2±61.7 flux without vitamins at 260 seconds, respectively (Figure 7A). However, there were no significant differences in the skin blood flow in Caucasians (Figure 7B).
Figure 7.
Mean ±SD of blood flow (flux) after a high fat meal measured during the 4 minutes period of vascular occlusion and the 2 minute period following the release of the occlusion cuff (A, B) and 6 minutes period of local heat (C, D) before and after the intake of vitamins for 14 days in 10 Koreans and 10 Caucasians.
When comparing the BFR to 6 minutes of local heat at 2 hours after HF meal with a previous intake of vitamins for 14 days to no vitamins in both ethnic groups, the skin blood flow significantly increased with vitamins compared to no vitamins in Koreans (p=0.024). The peak blood flow after heat exposure was 317.2±137.3 flux with vitamins and 173.5±52.3 flux without vitamins at 240 seconds, respectively (Figure 7C). However, there were no significant differences in the skin blood flow in Caucasians (Figure 7D).
For the MDA concentration, there were no significant differences in both Koreans (11.1±2.6 μM vs. 8.8±2.7 μM) and Caucasians (7.4±1.7 μM vs. 7.7±2.4 μM). However, MDA concentration decreased by 20.36% in Koreans and increased by 4.75% in Caucasians (Figure 2).
Comparison of Koreans and Caucasians with high fat meal
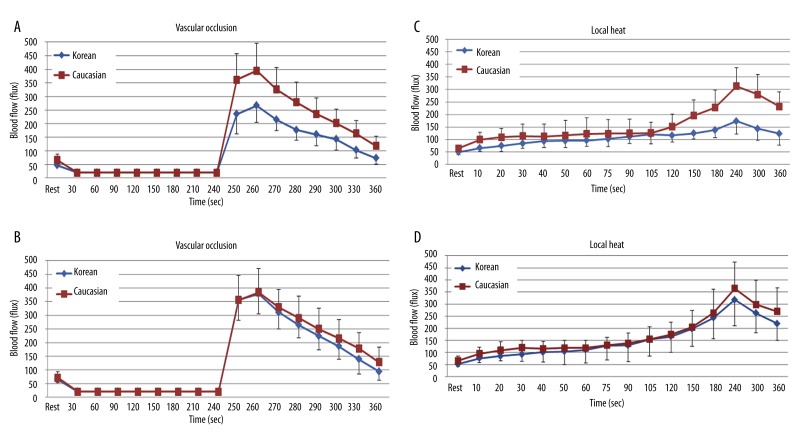
When comparing the BFR to 4 minutes of vascular occlusion at 2 hours after a HF meal, the skin blood flow was significantly lower in Koreans compared to Caucasians (p=0.001). The peak blood flow after occlusion was 266.2±61.6 flux in Koreans and 394.3±100.7 flux in Caucasians, respectively (Figure 8A). However, there were no significant differences in the skin blood flow at 2 hours after a HF meal with a previous intake of vitamins for 14 days between Koreans and Caucasians (Figure 8B).
Figure 8.
Mean ±SD of blood flow (flux) after a high fat meal measured during the 4 minutes period of vascular occlusion and the 2 minute period following the release of the occlusion cuff and 6 minutes period of local heat before (A, C) and after (B, D) the intake of vitamins for 14 days between 10 Koreans and 10 Caucasians.
When comparing the BFR to 6 minutes of local heat at 2 hours after high fat meal, the skin blood flow significantly lower in Koreans compared to Caucasians (p=0.003). The peak blood flow after heat exposure was 173.5±52.3 flux in Koreans and 312.8±74.2 flux in Caucasians at 240 seconds, respectively (Figure 8C). However, there were no significant differences in the skin blood flow at 2 hours after a HF meal with a previous intake of vitamins for 14 days between Koreans and Caucasians (Figure 8D).
For the MDA concentration, there were significant differences between Koreans and Caucasians (7.4±1.7 μM vs. 11.1±2.6 μM) at 2 hours after a HF meal (p=0.004). However, there were no significant differences between Koreans and Caucasians at 2 hours after a HF meal with a previous intake of vitamins for 14 days (Figure 2).
Discussion
Hypertriglyceridemia (HTG) due to a HF diet is an independent risk factor of CVD and DM [23]. Furthermore, postprandial HTG induces endothelial dysfunction by enhanced oxidative stress [24]. Asians have a “thrifty” gene that heightens their susceptibility to a reduced endothelial response to stressors when eating HF foods or gaining body weight [3,4,6]. According to a recent study, even a single HF meal impaired endothelial response to stress in Asians but not in Caucasians [6]. A possible mechanism of endothelial dysfunction after the ingestion of a HF meal may be related to an increase in free radicals which affect NO production and reduce NO bioavailability [11,25].
In the present investigation, we have found that Koreans have a lower baseline BFR to vascular occlusion and local heat compared to Caucasians. We further showed that just one single HF meal decreased the BFR to vascular occlusion and local heat in Koreans, but not in Caucasians and also increased oxidative stress, as measured by MDA in both Koreans and Caucasians. In addition, pre-treatment with antioxidant vitamins (vitamin C, vitamin E, and Coenzyme Q-10) for 14 days improved baseline BFR to vascular occlusion and local heat significantly in Koreans but not in Caucasians. Also, the vitamins markedly eliminated the decrease in the BFR to vascular occlusion and local heat following the HF meal in Koreans, but did not increase vasodilation in Caucasians. There are several intriguing findings in these data. First, the fact that the vascular response to 2 stressors, heat and occlusion were increased before a HF meal simply by taking vitamins for 2 weeks (antioxidants) in Koreans but not Caucasians implies higher levels of harmful free radicals in the Korean group than the Caucasian group. This was confirmed in the MDA measurements. Since the Korean group had lower BMIs than the Caucasian group, it is not linked to obesity but must be linked to heredity of diet. The fact that endothelial function improved with antioxidants would seem to imply high free radicals due to their diet, which, as cited in the introduction, has been westernized in recent years. The higher concentrations of free radicals in the blood of the Koreans would not be surprising since, these Koreans had been in the United States for an average of 7 months and, unlike Caucasians, do not have the genetic makeup to allow for a westernized diet. With vitamin administration, free radicals fell and the BFR to the stressors examined here matched that of Caucasians. Thus there seems to be no permanent damage to endothelial cells that could be reversed by vitamins or perhaps diet. And yet these were young subjects. Older subjects, after years of HF meals may have permanent damage that cannot be reversed by vitamins.
A second finding is that in Koreans, but not in Caucasians, a single HF meal can impair endothelial response to stressors and can be reversed by antioxidant vitamins. This finding in Koreans, but not in Caucasians, may also due to the influence of “thrifty” genes. This “thrifty” genotype is a genetic difference regulating lipid metabolism and fat storage, and differs depending on ethnicity [5,26–28]. For example, one of the “thrifty” SNPs related to lipid metabolism, fatty acid-binding protein 2 (FABP2), has been associated with obesity because it enhances fat absorption. The allelic frequency of FABP2 is 55% in Asians and 27.1% in Caucasians. Thus, if Asians consume the same amount of fat, a higher body fat deposit at a lower or the same BMI will be observed in Asians [26,27]. South Korea is one of the countries where the socioeconomic environment has changed rapidly to reflect more westernization as well as its negative health consequences. For the adoption of a more westernized lifestyle, higher dietary fat consumption and less physical activity are common in Korea, and hence, these “thrifty” genes heighten the susceptibility to insulin insensitivity and CVD in relation to increased body fat and dyslipidemia in Koreans [6,27,28]. Increased body fat and dyslipidemia stimulate leukocytes to induce free radicals. The high concentration of free radicals neutralizes NO and prostacyclin, the 2 principal vasodilators, and reduces BFR to vascular occlusion and local heat [6,11]. These findings may explain why Koreans had a lower BFR to vascular occlusion and local heat and higher MDA concentration after ingestion of a single HF meal compared to Caucasians in this study. It should be pointed out that international studies among different Asian national populations in China, Korea, Philippines, Singapore, and Taiwan show increased risk of Type 2 DM and CVD at lower BMI than European populations [29].
Numerous studies have demonstrated that antioxidant vitamins play an important role in increasing endothelial function and decreasing oxidative stress [11,30,31]. To our knowledge, no study has examined the effects of antioxidant vitamins on endothelial function by ethnicity. In the present study, antioxidant vitamins of 1000 mg of vitamin C, 800 IU of vitamin E, and 300 mg of Coenzyme Q-10 were given to Korean and Caucasian subjects for 14 days. Most investigators use 400–800 IU of vitamin E, 500–1000 mg of vitamin C, and 60–300 mg of Coenzyme Q-10. This pre-treatment of vitamins restored decreased endothelial response to stressors following a HF meal in Koreans but didn’t improve endothelial response to stressors in Caucasians. Moreover, vitamins restore impaired endothelial response to stressors by scavenging free radicals, but may not improve normal endothelial response to stressors [6,32]. Several studies showed that antioxidant vitamins improved endothelial function in people with DM, coronary artery disease, and smokers but not in healthy control groups [33,34]. In some studies antioxidant vitamins did not change oxidative stress status in healthy athletes [32]. However, in a study of young health men and women, the response to heat, unlike the response to occlusion, was increased by administration of large doses of vitamins in young, fit students at similar dosages to the present investigation and also for 2 weeks [11].
Previous studies have suggested that antioxidant vitamins C and E improve vascular defense against oxidative stress by reducing free radicals and protecting NO from inactivation, thereby exerting beneficial effects on vascular function and structure [11,31]. Especially, combined administration of vitamins C and E significantly increases endothelium-dependent vasodilation, while monotherapy with vitamin C alone is ineffective [30,31,35]. It is known that vitamins C and E have synergistic antioxidant actions, since vitamin E can have pro-oxidant properties and appropriate concentrations of vitamin C are necessary for the regeneration of vitamin E, thus increasing its antioxidant capacity [31]. These previous findings support the results from this study.
Baseline BFR was significantly lower in Koreans than Caucasians by 17.54%. After stress with heat, the blood flow response was 44.54% less in Koreans and with occlusion it was 32.49% less than that seen for Caucasians. Certainly, some of the difference between the blood flow response to stress may be due to a lower baseline blood flow in Koreans. However, the difference with stress was proportionally much higher after heat and occlusion. Thus it would suggest that not only is the baseline BFR causing this difference but certainly some other factors are also involved to increase the difference with stress between Koreans and Caucasians. Perhaps the most telling evidence was the baseline blood flow after vitamin ingestion in the two groups of subjects. After taking the vitamins, there was no significant difference in baseline blood flow between the two groups. This shows that the difference in the blood flow response to stress is not only due to the difference in baseline blood flow but also due to higher oxidative level in Koreans, corrected by vitamin administration.
In this study, ten young Korean subjects had been living in the United States, rather than in Korea. Although the mean period of stay in US was only 7.1±2.3 months in the Korean subjects, results may be different in Koreans who did not reside in the US. In addition, due to different diets especially HF diet and environment, Koreans who have been in the US for longer periods may have more reduced endothelial response to stressors than those with shorter residing periods. Therefore, further studies need to be conducted with Koreans for those who have been in the US for long periods and those who have not been to US. Moreover, in the present investigation, the reduced endothelial response to stressors was reversed by vitamins at baseline and even after a single HF meal in this young population. However, with longer periods of HF intake among young subjects or in the elderly, the recovery effects of vitamins on reduced endothelial response to stressors might be different. Prolonged high free radicals will probably cause permanent endothelial dysfunction.
Conclusions
This study evaluated postprandial endothelial function by measuring the BFR to vascular occlusion and local heat before and after a HF meal and the interventional effects of anti-oxidant vitamins on improving endothelial function in young Korean-Asians compared to Caucasians. The skin BFR to vascular occlusion and local heat following a HF meal significantly decreased and free radicals significantly increased at 2 hours compared to baseline in Koreans, but not in Caucasians. When vitamins were given, BFR to vascular occlusion and local heat before and after HF meal were not significantly lower in Koreans than Caucasians. These findings suggest that even a single HF meal can reduce endothelial response to stress through an oxidative stress mechanism but can be blocked by antioxidants, probably through scavenging free radicals in Koreans. Koreans and other Asians historically have a diet which is healthier than Caucasians because of the large number of vegetables and low fat intake. However, the present investigation shows that a westernized diet is causing high oxidative levels in Koreans impairing endothelial function. Since Asians have “thrifty” genes, if they continue consuming a westernized diet, they need to take vitamin supplements or go back to their historic low fat diets. These levels of vitamins can not be achieved by diet alone.
Footnotes
Source of support: Departmental sources
References
- 1.Cuevas AM, Germain AM. Diet and endothelial function. Biol Res. 2004;37(2):225–30. doi: 10.4067/s0716-97602004000200008. [DOI] [PubMed] [Google Scholar]
- 2.Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23(1):7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Petrofsky JS, Alshahmmari F, Lee H, et al. Reduced endothelial function in the skin in Southeast Asians compared to. Caucasians Med Sci Monit. 2011;18(1):CR1–8. doi: 10.12659/MSM.882185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pemberton TJ, Mehta NU, Witonsky D, et al. Prevalence of common disease-associated variants in Asian Indians. BMC Genet. 2008;9:13. doi: 10.1186/1471-2156-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radha V, Vimaleswaran KS, Babu HN, et al. Role of genetic polymorphism peroxisome proliferator-activated receptor-gamma2 Pro12Ala on ethnic susceptibility to diabetes in South-Asian and Caucasian subjects: Evidence for heterogeneity. Diabetes Care. 2006;29(5):1046–51. doi: 10.2337/diacare.2951046. [DOI] [PubMed] [Google Scholar]
- 6.Bui C, Petrofsky J, Berk L, Shavlik D, et al. Acute effect of a single high-fat meal on forearm blood flow, blood pressure and heart rate in healthy male Asians and Caucasians: a pilot study. Southeast Asian J Trop Med Public Health. 2010;41(2):490–500. [PMC free article] [PubMed] [Google Scholar]
- 7.Choi YJ, Kim HC, Kim HM, et al. Prevalence and management of diabetes in Korean adults: Korea National Health and Nutrition Examination Surveys 1998–2005. Diabetes Care. 2009;32(11):2016–20. doi: 10.2337/dc08-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yim J, Petrofsky J, Berk L, et al. Differences in endothelial function between Korean- Asians and Caucasians Med Sci Monit. 2012;18(6):CR337–43. doi: 10.12659/MSM.882902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81(4A):7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 10.Kovacic P, Jacintho JD. Mechanisms of carcinogenesis: focus on oxidative stress and electron transfer. Curr Med Chem. 2001;8(7):773–96. doi: 10.2174/0929867013373084. [DOI] [PubMed] [Google Scholar]
- 11.Petrofsky JS, Laymon MD, Al-Nakhli H, et al. The Effect of Vitamin D and E and Coenzyme Q-10 on Endothelial Function in a Young Population. Anatom Physiol. 2011;1(1) [Google Scholar]
- 12.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Taddei S, Virdis A, Ghiadoni L, et al. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97(22):2222–29. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- 14.Plotnick GD, Corretti MC, Vogel RA. Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single high-fat meal. JAMA. 1997;278(20):1682–86. [PubMed] [Google Scholar]
- 15.Binggeli C, Spieker LE, Corti R, et al. Statins enhance postischemic hyperemia in the skin circulation of hypercholesterolemic patients: a monitoring test of endothelial dysfunction for clinical practice? J Am Coll Cardiol. 2003;42(1):71–77. doi: 10.1016/s0735-1097(03)00505-9. [DOI] [PubMed] [Google Scholar]
- 16.Medow MS, Taneja I, Stewart JM. Cyclooxygenase and nitric oxide synthase dependence of cutaneous reactive hyperemia in humans. Am J Physiol Heart Circ Physiol. 2007;293(1):H425–32. doi: 10.1152/ajpheart.01217.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrofsky J, Lee S. The effects of type 2 diabetes and aging on vascular endothelial and autonomic function. Med Sci Monit. 2005;11(6):CR247–54. [PubMed] [Google Scholar]
- 18.Fieger SM, Wong BJ. Adenosine receptor inhibition with theophylline attenuates the skin blood flow response to local heating in humans. Exp Physiol. 2010;95(9):946–54. doi: 10.1113/expphysiol.2010.053538. [DOI] [PubMed] [Google Scholar]
- 19.Minson CT, Holowatz LA, Wong BJ, et al. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93(5):1644–49. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 20.Bloomer RJ, Fisher-Wellman KH. Systemic oxidative stress is increased to a greater degree in young, obese women following consumption of a high fat meal. Oxid Med Cell Longev. 2009;2(1):19–25. doi: 10.4161/oxim.2.1.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrofsky J, Paluso D, Anderson D, et al. The contribution of skin blood flow in warming the skin after the application of local heat; the duality of the Pennes heat equation. Med Eng Phys. 2011;33(3):325–29. doi: 10.1016/j.medengphy.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Petrofsky JS. A device to measure heat flow through the skin in people with diabetes. Diabetes Technol Ther. 2010;12(9):737–43. doi: 10.1089/dia.2010.0043. [DOI] [PubMed] [Google Scholar]
- 23.Botero D, Ebbeling CB, Blumberg JB, et al. Acute effects of dietary glycemic index on antioxidant capacity in a nutrient-controlled feeding study. Obesity (Silver Spring) 2009;17(9):1664–70. doi: 10.1038/oby.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae JH, Bassenge E, Kim KB, et al. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis. 2001;155(2):517–23. doi: 10.1016/s0021-9150(00)00601-8. [DOI] [PubMed] [Google Scholar]
- 25.Behrendt D, Ganz P. Endothelial function. From vascular biology to clinical applications. Am J Cardiol. 2002;90(10C):40L–48L. doi: 10.1016/s0002-9149(02)02963-6. [DOI] [PubMed] [Google Scholar]
- 26.Sugimoto K, Ishikawa N, Sugioka T, et al. The importance of disinfection therapy using povidone-iodine solution in atopic dermatitis. Dermatology. 2002;204(Suppl 1):63–69. doi: 10.1159/000057728. [DOI] [PubMed] [Google Scholar]
- 27.Nakanishi S, Yamane K, Kamei N, et al. The effect of polymorphism in the intestinal fatty acid-binding protein 2 gene on fat metabolism is associated with gender and obesity amongst non-diabetic Japanese-Americans. Diabetes Obes Metab. 2004;6(1):45–49. doi: 10.1111/j.1463-1326.2004.00313.x. [DOI] [PubMed] [Google Scholar]
- 28.Murphy C, Kanaganayagam GS, Jiang B, et al. Vascular dysfunction and reduced circulating endothelial progenitor cells in young healthy UK South Asian men. Arterioscler Thromb Vasc Biol. 2007;27(4):936–42. doi: 10.1161/01.ATV.0000258788.11372.d0. [DOI] [PubMed] [Google Scholar]
- 29.Palaniappan LP, Wong EC, Shin JJ, et al. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes (Lond) 2011;35(3):393–400. doi: 10.1038/ijo.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tousoulis D, Antoniades C, Tentolouris C, et al. Effects of combined administration of vitamins C and E on reactive hyperemia and inflammatory process in chronic smokers. Atherosclerosis. 2003;170(2):261–67. doi: 10.1016/s0021-9150(03)00250-8. [DOI] [PubMed] [Google Scholar]
- 31.Plantinga Y, Ghiadoni L, Magagna A, et al. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens. 2007;20(4):392–97. doi: 10.1016/j.amjhyper.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen HG, Skjonsberg OH, Lyberg T. Effect of antioxidant supplementation on leucocyte expression of reactive oxygen species in athletes. Scand J Clin Lab Invest. 2008;68(7):526–33. doi: 10.1080/00365510701864602. [DOI] [PubMed] [Google Scholar]
- 33.Teramoto K, Daimon M, Hasegawa R, et al. Acute effect of oral vitamin C on coronary circulation in young healthy smokers. Am Heart J. 2004;148(2):300–5. doi: 10.1016/j.ahj.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Antoniades C, Tousoulis D, Tountas C, et al. Vascular endothelium and inflammatory process, in patients with combined Type 2 diabetes mellitus and coronary atherosclerosis: the effects of vitamin C. Diabet Med. 2004;21(6):552–58. doi: 10.1111/j.1464-5491.2004.01201.x. [DOI] [PubMed] [Google Scholar]
- 35.Takase B, Etsuda H, Matsushima Y, et al. Effect of chronic oral supplementation with vitamins on the endothelial function in chronic smokers. Angiology. 2004;55(6):653–60. doi: 10.1177/00033197040550i606. [DOI] [PubMed] [Google Scholar]