Summary
Background
The prevalence of hypertension is growing at an alarming rate. Increasing attention is being focussed on the oxidative stress accompanying this disease. In this study we examined the impact of this disease on some parameters of erythrocytes and human blood plasma.
Material/Methods
We examined the impact of hypertension on some parameters of erythrocytes and human plasma. The study involved 13 patients with hypertension and 19 healthy subjects. We determined lipid peroxidation, SH groups concentration, antioxidants enzymes activity, ATPase activity, total antioxidant capacity, total cholesterol level and erythrocyte membrane fluidity.
Results
We found an increased level of lipid peroxidation and the concentration of SH groups in membrane proteins in patients with hypertension, and a decrease in the activity of catalase and superoxide dysmutase. No changes were observed in glutathione peroxidase and ATPase activity, level of total antioxidant capacity, total cholesterol level and fluidity of erythrocyte membranes.
Conclusions
These results suggest the existence of an impaired oxidative balance in hypertensive human erythrocytes.
Keywords: lipid peroxidation, SH groups, antioxidant system, Na+/K+ ATPase, total antioxidant capacity, membranes fluidity
Background
Hypertension is the main risk factor of cardiovascular disease, including stroke and myocardial infarction [1]. The world-wide prevalence of hypertension is very high and is growing at an alarming rate. It is estimated that the number of people suffering from hypertension in 2025 will be about 1.56 billion [2]. The reasons for the high incidence of this disease are very diverse, and its pathophysiology is still not fully elucidated. Over 90% of cases of hypertension have no obvious cause. In research on the causes of hypertension, increasing attention is being paid to oxidative stress and the possible participation of reactive oxygen species (ROS) in the pathogenesis of this disease. ROS released in physiological quantities operate as mediators and regulators of many cellular processes, but in higher concentrations also react with non-specific cellular components such as proteins, nucleic acids and lipids, modifying and damaging them [3]. To counter these changes, the body has developed the antioxidant system, which is responsible for maintaining oxygen free radicals (OFR) in their inactive forms or inhibiting their formation. The system consists of antioxidant enzymes such as catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD) and glutathione reductase (GSSGR), as well as small-molecule oxidants like glutathione, plasma proteins and vitamins A, C and E [4]. Disorders of the activity of the antioxidant system caused by an imbalance between prooxidant and antioxidant factors may contribute to impaired neutralization of OFR and the consequent reduction of their deleterious functions.
In hypertension, increased levels of superoxide radical (O2−•) and hydrogen peroxide (H2O2) are observed [5,6]. The main source of OFR in the blood vessels are oxidases (i.e., NADH/NADPH oxidase located in the cell membrane of myocytes and endothelial xanthine oxidase present in endothelial cells and plasma) [5]. The activity of these oxidases is increased in people with hypertension [7]. In vitro studies have shown that endothelial nitric oxide synthase (NOS III) is the source of O2−•[8]. NOS III produces both NO and O2−•. NOS III cofactor is tetrahydrobiopterin (BH4). This compound in reduced form interacts with the NOS III, thus promoting the production of NO, a potent antioxidant, and deficiency of reduced biopterin leads to increased production of O2−•[9]. A decrease in NO bioavailability is observed in patients with hypertension [10], which may be due to inactivation of excessive amounts of O2−• or reduction of its synthesis as a result of endothelial cell damage (e.g., through the action of OFR) [11]. To counteract the harmful effects of superoxide radicals and hydrogen peroxide, the body’s defense strategy is directed at a weak point of both OFR. O2−• and H2O2 undergo dismutation reaction catalyzed by the enzymes of the antioxidant system (i.e., superoxide dismutase and catalase). Because of the widespread interest in hypertension in recent years, in this paper attempts were made to examine the impact of this disease on some parameters of erythrocytes and human blood plasma. The parameters were chosen based on reports on the occurrence of antioxidant system disturbances in hypertension, as well as on the demand to discover the impact of hypertension on the structure of erythrocyte membranes.
Material and Methods
Patients
The study materials were erythrocytes and plasma, which were isolated from peripheral blood of patients with hypertension (n=13). Characteristic of patients are presented in Table 1. Inclusion criteria were mild/moderate hypertension, untreated antihypertensive drugs, newly discovered hypertension, and non-smoking patients. Blood was collected in anticoagulant. Research was performed in cooperation with the Department of Internal Diseases and Clinical Pharmacology, Medical University of Lodz. The controls were the blood of healthy subjects (n=19) obtained from the Centre for Blood Donation and Blood Treatment in Lodz. The control group of healthy subjects included 10 women and 9 men, between the ages of 44 and 65 years. Inclusion criteria were absence of hypertension and non-smokers. The testing was approved by the Bioethics Committee of the Medical University of Lodz, No. 241/06/KB.
Table 1.
Characteristic of patients with hypertension.
| Parameter | Mean ±SD |
|---|---|
| Age (year) | 60.17±6.09 |
| RRs (mm Hg) | 160.67±12.39 |
| RRr (mm Hg) | 92±4.94 |
| Body mass (kg) | 83.83±11.83 |
| BMI (kg/m2) | 30.23±3.50 |
| Waist size (cm) | 98.50±7.27 |
| Glucose (mg/dl) | 91.67±14.71 |
| TG (mg/dl) | 158.92±33.63 |
| TC (mg/dl) | 230.17±24.23 |
| LDL-C (mg/dl) | 153.83±21.10 |
| HDL (mg/dl) | 44.53±5.21 |
| Uric acids (mg/dl) | 5.80±1.28 |
| Fb (μmol/l) | 237.75±21.73 |
| hsCRP (mg/l) | 3.09±1.03 |
| TNF-α (pg/ml) | 6.30±2.71 |
RRs – systolic blood preasure; RRr – diastolic blood preasure; BMI – body mass index; TG –triglycerides; TC – total cholesterol; LDL-C – low-density lipoprotein cholesterol; HDL – high-density lipoprotein; Fb – plasma free fatty acids; hsCRP – high-sensitivity C-reactive protein; TNF-α – tumor necrosis factor.
Statistical analyses were performed with STATISTICA 9. Statistical significance was determined using the Mann-Whitney U test and Student’s T test.
Isolation of erythrocytes
Peripheral blood was collected into tubes with anticoagulant (23 mM citric acid, 45.1 mM sodium citrate, 45 mM glucose), then centrifuged for 10 min at 600× g at 4°C. After removal of plasma and leukocyte layers, samples were washed 3 times with 0.9% NaCl solution after each washing, and spinning in the same conditions. The erythrocytes were suspended in 0.9% NaCl solution to obtain a final hematocrit of 50%.
Isolation of erythrocyte membranes
Plasma membranes were prepared using the hypotonic hemolysis according to the modified method of Dodge et al. [12]. Isolated erythrocytes were centrifuged at 12000× g for 5 min at 4°C until purified erythrocyte membranes were obtained. Each time, the membranes were washed in 20 mmol/l TRIS-HCl buffer at pH 7.4.
Lipid peroxidation
The concentration of substances reacting with thiobarbituric acid (TBA) followed the method described by Stocks and Dormandy [13] in isolated erythrocytes. Erythrocyte solution of 50% hematocrit were incubated in the presence of 20% TCA and H2O at 4°C for 1 h, and then centrifuged at 1000× g for 5 min. The obtained supernatant in the presence of 0.26 M TBA was heated for 15 min at 100°C. Absorbance measurement was performed at λ=532 nm.
Concentration of hemoglobin
Concentration of hemoglobin was performed by the method described by Drabkin [14]. Hemolysate of erythrocyte solution of 50% hematocrit were centrifuged for 5 min. The obtained supernatant was incubated for 15 min at room temperature in the presence of Drabkin reagent. Absorbance measurement was performed at λ=540 nm.
Concentration of –SH groups
Concentration of thiol groups in the membranes was performed by using the method of Ellman [15]. Isolated erythrocyte membranes were diluted in SDS and samples were incubated in the presence of Na2HPO4 and 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) for 30 min at room temperature. Absorbance measurement was performed at λ=412 nm.
Total cholesterol
Total cholesterol was determined using the Liebermann-Burchard reagent. Lipids were extracted by the method of Rodriguez-Vico et al. [16] using solvents with low toxicity. Lipid residue was dissolved in a mixture of ethanol and chloroform. Cholesterol level was determined on the basis of the Liebermann-Burchard reaction. Absorbance measurement was performed at λ=660 nm.
Activity of ATPases
The isolated erythrocyte membranes were incubated in incubation medium with and without ouabain for 30 min at 37°C and 0°C. A level of orthophosphate released was measured by the method van Veldhoven and Mannaerst [17] with malachite green in a solution of polyvinyl alcohol, based on the calibration curve as a model for KH2PO4. Absorbance measurement was performed at λ=610 nm.
Activity of antioxidant enzymes
Catalase activity was determined spectrophotometrically by the method of Bartosz [18] in 1% hemolysate.
Glutathione peroxidase activity was determined spectrophotometrically in reaction of enzyme from 1% hemolysate with GSH and NADPH. The catalase activity was inhibited by solution of sodium azide, KCN and potassium ferricyanide.
Superoxide dismutase activity was determined spectrophotometrically by using the method of Misra and Fridovich [19] in 10% hemolysate.
Total plasma antioxidant capacity
Total plasma antioxidant capacity was determined by means of reduction of ABTS cation as described by Re et al. in modification of the method of Bartosz [18]. Absorbance measurement was performed at λ=414 nm.
Concentration of protein
Determination of protein concentration in preparations of erythrocyte membranes was performed by using the method of Lowry et al. [20], with bovine serum albumin used as a standard.
Erythrocyte membrane fluidity was determined using fluorescent probes by means of a PERKIN ELMER LS 50B fluorometer. The study used 1,6-diphenylo-1,3,5-hexatrien (DPH), which gives information about the microviscosity of lipid bilayer to a depth of 4 carbon alkyl chains of membrane phospholipids and (1-(4-trimetyloaminofenylo)-6-phenyl-1,3,5 heksatrien) p-toluene sulfonate (TMA-DPH), which gives information about the structure of membranes at a depth of less than 4 carbon alkyl chains of membrane phospholipids. DPH probes were added to 1 ml of membranes at a concentration of 0.1 mg protein/ml DPH or TMA-. The obtained samples were incubated for 10 min at 37°C. Then, after excitation with wavelength of λ=348 nm, fluorescence anisotropy was measured at wavelengths of λ =426 nm.
Results
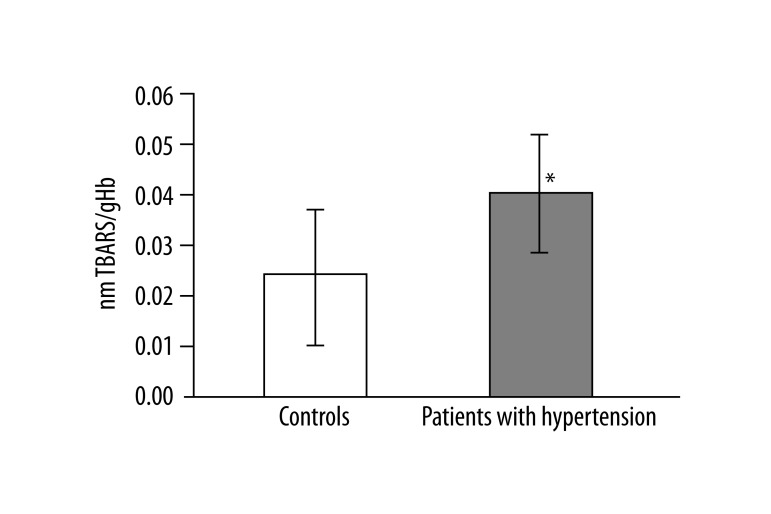
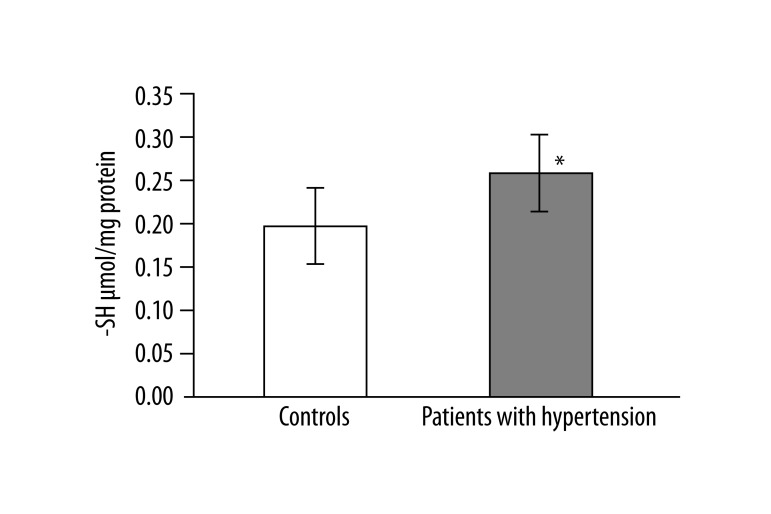
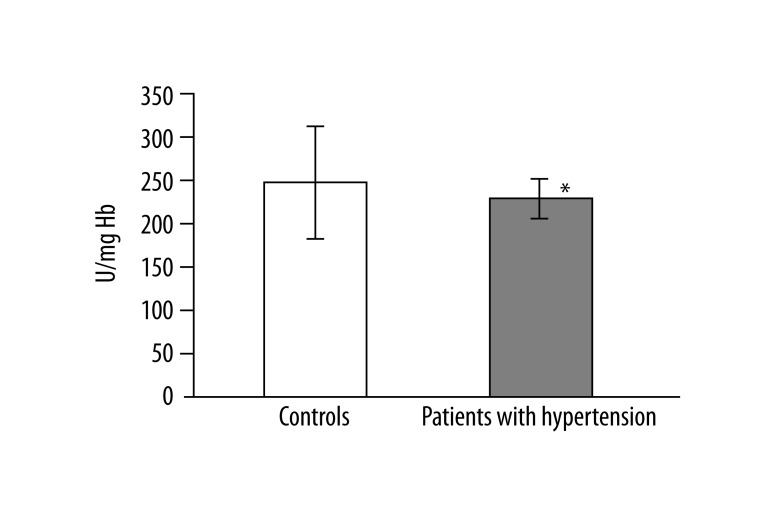
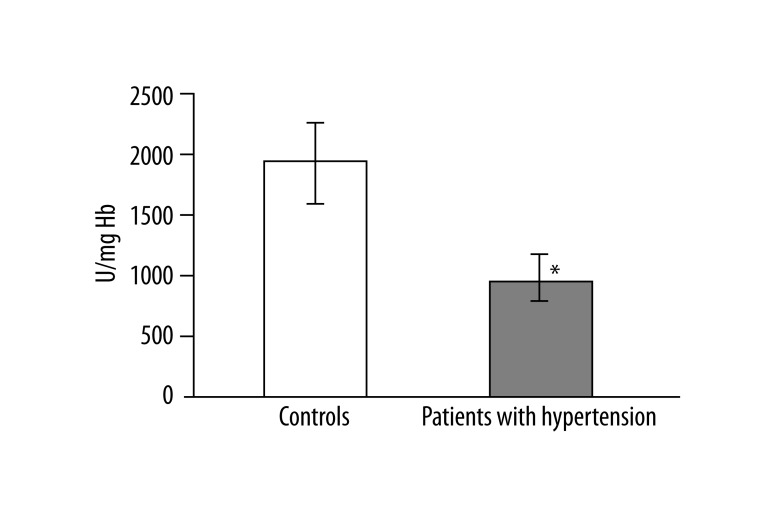
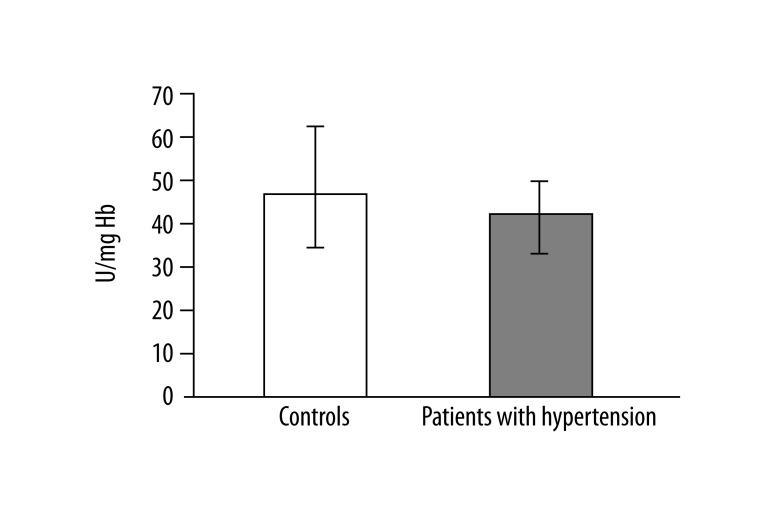
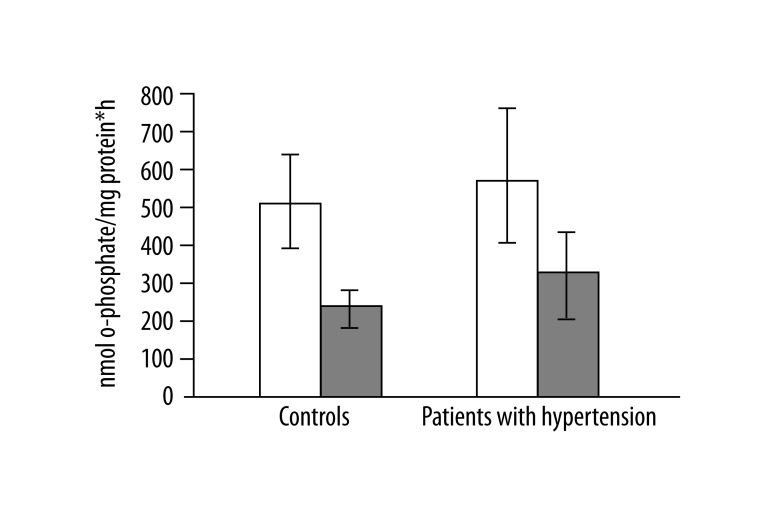
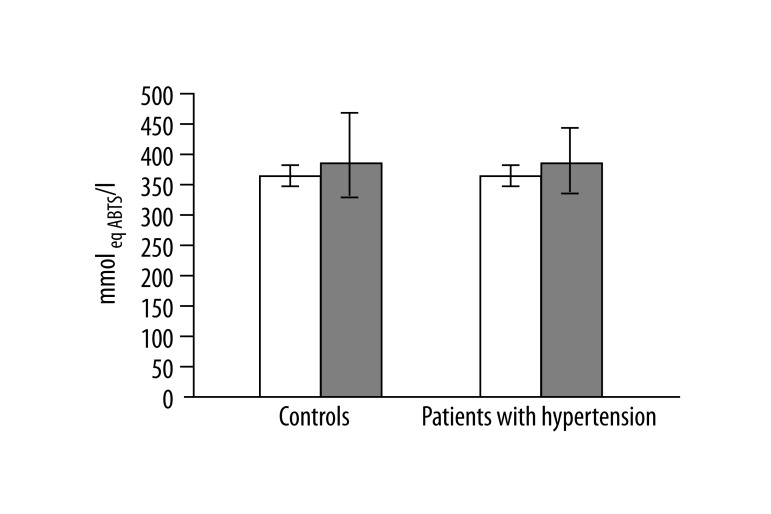
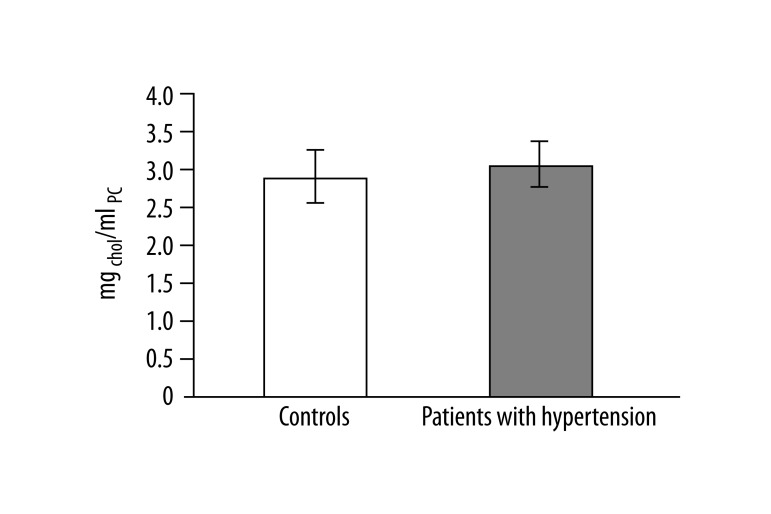
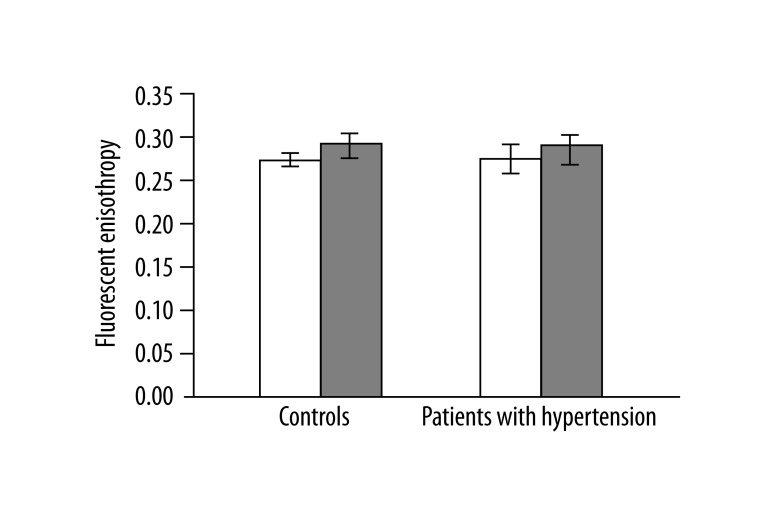
In 13 studied patients with hypertension, changes in the structure of membranes of erythrocytes and activity of antioxidant system were observed. Significantly higher (p<0.001) lipid peroxidation was noted in patients with hypertension than in healthy subjects. TBARS level in healthy subjects was 0.023±0.012, while in patients with hypertension it was 0.038±0.013 (Figure 1). Similarly, significant differences were found in the concentration of SH groups in membrane proteins. In healthy subjects the value was 0.195±0.042 –SH μmol/mg protein, whereas in patients with hypertension it was 0.258±0.043 –SH μmol/mg protein (p<0.02) (Figure 2). A statistically significant decrease was found in the activity of antioxidant enzymes (ie, catalase and superoxide dismutase). Catalase activity in healthy subjects was 256.99±60.96 U/mg Hb, whereas in hypertensive patients it was 214.06±18.11 U/mg Hb (p<0.05) (Figure 3). A much greater decrease was found in the activity of superoxide dismutase. In the control group SOD activity was 1904.67±369.44 U/mg Hb, while in the study group it was 871.84±173.42 U/mg Hb (p<0.001) (Figure 4). Statistically significant differences were not observed in glutathione peroxidase activity (Figure 5), ATP-ases total activity (Figure 6), Na+/K+ ATPase activity (Figure 6), total antioxidant capacity dependent rapid antioxidants (Figure 7) and the content of antioxidants (Figure 7). In addition, no differences were observed in level of cholesterol in erythrocyte membranes (Figure 8) and in membrane fluidity of erythrocytes at surface layers, at depths of up to 4 carbons of alkyl chains of membrane phospholipids (DPH), or at a depth of less than 4 carbons of alkyl chains of membrane phospholipids (TMA-DPH) (Figure 9).
Figure 1.
Lipid peroxidation level in erythrocytes (*p<0.001).
Figure 2.
Concentration of group –SH in proteins of erythrocyte membranes (*p<0.02).
Figure 3.
Activity of catalase (*p<0.02).
Figure 4.
Activity of superoxide dismutase (*p<0.001).
Figure 5.
Activity of glutathione peroxidase.
Figure 6.
Activity of ATPases. Empty bars – total activity of ATPases, filled bars – activity of Na+/K+ ATPase.
Figure 7.
Total antioxidant capacity of plasma dependent rapid antioxidants. Empty bars – total antioxidant capacity of plasma dependent rapid antioxidant, filled bars – total antioxidant capacity of plasma dependent slow antioxidant.
Figure 8.
Level of cholesterol in erythrocyte membranes.
Figure 9.
Membrane fluidity of erythrocytes. Empty bars – DPH, filled bars – TMA-DPH.
Discussion
The results of our experiments showed the involvement of antioxidant system disorders in patients with hypertension. The increase of the level of free radicals, which was observed in hypertensive patients, has an impact on the statistically significant increase of lipid peroxidation observed in our study. Elevated MDA shows an increased level of lipid peroxidation in hypertension, which was observed in the study of van Marke de Lumen et al. [21] and Russo et al. [22]. Cracowski et al. studied the level of 15-F2t-ISOP, a biomarker of lipid peroxidation in the early stages of hypertension present in the urine. The results of these studies showed no change in the level of the above biomarker, indicating no dependence of early stages of hypertension and changes in the level of lipid peroxidation. However, results of these studies do not preclude changes in more advanced stages of disease [23]. Instead, Digiesi et al. conducted investigations among people with hypertension and observed an increase of lipid peroxidation. The increase in lipid peroxidation was observed only in the elderly and more severely hypertensive patients [24]. Comparison with the results of Cracowski et al. suggests that the observed increase of lipid peroxidation, along with the stage of hypertension, corresponds with the increase of the level of free radicals, along with the severity of this disease.
Our study also found an increased concentration of thiol groups in membrane proteins, whereas reports in the literature indicate a decrease in the level of thiol groups. Voevod et al. [25] conducted research on rats with induced chronic venous hypertension and showed a decreased SH level. Lower levels of SH groups of human plasma proteins were also observed by Simic et al. [26]. The increasing concentration of thiol groups in erythrocyte membranes may be explained by the increase of lipid peroxidation. The increase of lipid peroxidation of membrane proteins may cause changes in their conformation. We observed slightly elevated total cholesterol level and level of cholesterol in erythrocyte membranes, but did not reveal significant changes in fluidity of erythrocyte membranes. On the other hand, Tsuda showed a decrease in membrane fluidity [27]. Wang et al. observed the impact of modified protein thiol groups that resulted from oxidative stress on membrane fluidity [28]. In our study, we observed an increase in thiol group concentration, which can be explained by changes in erythrocyte membrane fluidity.
Our study also showed no changes in the Na+/K+ ATPase activity. Similar results were obtained by Chan et al. in healthy rats and rats with induced hypertension [29]. The lack of changes in the Na+/K+ ATPase activity obtained in our study may be explained by changes in erythrocyte membrane fluidity.
Among people with hypertension, reduction in the activity of antioxidative enzymes such as catalase and superoxide dismutase is observed. However, literature reports concerning the activity of antioxidant enzymes differ in the results, as the authors maintain the relationship between antioxidant stress and hypertension. The decrease of superoxide dismutase activity was observed by Russo et al. [22]. A slight drop in the activity of this enzyme in hypertension patients was observed also in the work of Assadpoor-Piranfar et al. [30] and van Marke de Lumen [21]. Similarly, lower catalase activity was shown by Amirikhizi et al. [31] in women with hypertension. On the other hand, no differences in catalase activity between healthy and hypertension patients was observed by Kedziora-Kornatowska et al. [32] and Rozwadowska et al. [33]. In these works, there were also no statistically significant changes in the activity of glutathione peroxidase, which was also shown in this work. Decreased activity of antioxidant enzymes indicates the presence of increased oxidative stress, and thus can explain the harmful effects of free radicals, the increased level of which level is usually observed in hypertension. ROS interacts with enzymatic proteins by changing their structure or conformation, and thus causing their inactivation. In hypertension, a higher level of H2O2 exists, which is also responsible for SOD activity inhibition. Moreover, decreased SOD activity may also contribute to the increase of O2−• level, which is observed in hypertension [6]. Simic et al. [26], in a study of blood plasma of patients with hypertension, observed a 2-fold increase in glutathione peroxidase activity, explained by an adaptive phenomenon related to increased free radical production in hypertension. Extracellular GPx is produced in the proximal renal tubules. In vitro, GPx reduces organic hydroperoxides of phospholipids. Studies on mice have shown protective antioxidant effect, but high accumulation of lipid peroxyl radicals overwhelms its antioxidant capacity.
We observed no statistically significant differences in the total antioxidant capacity of plasma. Similar results were obtained by Digiesi et al. [24]. The studies on rats conducted by Mantle et al. showed no changes in total antioxidant capacity [34]. There were no changes in total antioxidant capacity of plasma that indicated any impact of hypertension on the changes in the antioxidant system in plasma.
Patients analyzed in our study also showed elevated BMI index and TNF-α level, which lowers the concentration of NO. Both of these factors favor the development of hypertension.
Conclusions
In summary, the results of these studies suggest a negative effect of oxidative stress, accompanying hypertension, on the erythrocytes and the antioxidant system of erythrocytes. Damage of erythrocytes is noticeable in the changes of lipid peroxidation level in erythrocytes and in the level of –SH groups, present in the membrane of erythrocytes. Disorders of the antioxidant system indicate the strong reduction in SOD activity and a smaller decrease of catalase activity.
Abbreviations
- RRs
systolic blood pressure
- RRr
diastolic blood pressure
- BMI
body mass index
- TG
triglycerides
- TC
total cholesterol
- LDL-C
low-density lipoprotein cholesterol
- HDL
high-density lipoprotein
- Fb
plasma free fatty acids
- hsCRP
high-sensitivity C-reactive protein
- TNF-α
tumor necrosis factor
- TBA
thiobarbituric acid
- TBARS
thiobarbituric acid-reacting substances
- TCA
trichloroacetic acid
- ROS
reactive oxygen species
- OFR
oxygen free radicals
- CAT
catalase
- GPx
glutathione peroxidase
- SOD
superoxide dismutase
- GSSGR
glutathione reductase
- NOS III
endothelial nitric oxide synthase
Footnotes
Source of support: University of Lodz, Grant 506/982 and by the Medical University of Lodz, Grant 503/5-165-01/503-01
References
- 1.Kawecka-Jaszcz K, Pośnik-Urbańska A, Jankowski P. Prevalence of arterial hypertension in Poland – impact of gender. Arterial Hypertension. 2007;11(5):377–73. [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions andhuman disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Zabłocka A, Janusz M. Two faces of reactive oxygen species. Postepy Hig Med Dosw. 2008;62:118–24. [PubMed] [Google Scholar]
- 5.Lacy F, O’Connor DT, Schmid-Schönbein GW. Plasma hydrogen peroxide production in hypertensives and normotensive subject at genetic risk of hypertension. J Hypertens. 1998;16(3):291–303. doi: 10.1097/00004872-199816030-00006. [DOI] [PubMed] [Google Scholar]
- 6.Kumar KV, Das UN. Are free radicals involved in the pathobiology of human essential hypertension. Free Radic Res Commun. 1993;19:59–66. doi: 10.3109/10715769309056499. [DOI] [PubMed] [Google Scholar]
- 7.Newaz MA, Adeeb NN, Muslim N, et al. Uric acid, xanthine oxidase and other risk factors of hypertension in normotensive subject. Clin Exp Hypertens. 1996;18:1035–50. doi: 10.3109/10641969609081033. [DOI] [PubMed] [Google Scholar]
- 8.Ohara Y, Peterson TE, Harrison GD. Hypercholesterolemia. Increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–51. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Förstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol. 2000;190:244–54. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Brovkovych V, Dobrucki LW, Brovkovych S, et al. Nitric oxide release from normal and dysfunctional endothelium. J Physiol Pharmacol. 1999;50(4):575–86. [PubMed] [Google Scholar]
- 11.John S, Schmieder RE. Impaired endothelial function in arterial hypertension and hypercholesterolemia: potential mechanism and differences. J Hypertens. 2000;18:363–74. doi: 10.1097/00004872-200018040-00002. [DOI] [PubMed] [Google Scholar]
- 12.Dodge JT, Mitchell C, Hanahan DJ. The preparation and chemical characteristics of hemoglobin – free ghost of human erythrocytes. Arch Biochem Biophys. 1963;100:119–30. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- 13.Stocks J, Dormandy TLJ. The autooxidation of red cell lipids induced by hydrogen peroxide. B J Haematol. 1971;20:95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 14.Drabkin DL. The crystallographic and optical properties of the hemoglobin of men in comparison with those of other species. J Biol Chem. 1946;164:703–23. [PubMed] [Google Scholar]
- 15.Ellman G. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Vico F, Martinez-Cayuela M, Zafra MF, et al. A procedure for the simultaneous determination of lipid and protein in biomembranes and other biological samples. Lipids. 1991;26:77–80. doi: 10.1007/BF02544029. [DOI] [PubMed] [Google Scholar]
- 17.Van Veldhoven PP, Mannaerts GP. Inorganic and organic phosphate measurements in the nanomolar range. Anal Biochem. 1987;161:45–48. doi: 10.1016/0003-2697(87)90649-x. [DOI] [PubMed] [Google Scholar]
- 18.Bartosz G. Second face oxygen: free radical in nature. 2nd ed. Warszawa: Wydawnictwo Naukowe PWN; 2003. Cookbook for novice researchers, reactive oxygen species; pp. 376–89. [in Polish] [Google Scholar]
- 19.Misra HP, Fridrovich I. The generation of superoxide radical during autooxidation of hemoglobin. J Biol Chem. 1972;247:6960–62. [PubMed] [Google Scholar]
- 20.Lowry OH, Rosebrough A, Ferr L, Randall RJ. Protein measurement with the Folin Phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 21.Van Marke de Lumen K, Kędziora-Kornatowska K, Czuczejko J, et al. Time dependent effect of melatonin administration on lipid peroxidation, superoxide dismutase activity and melatonin concentration in the elderly patients with essential arterial hypertension. Przeg Lek. 2008;65(6):273–76. [PubMed] [Google Scholar]
- 22.Russo C, Olivieri O, Girelli D, et al. Anti-oxidant status and lipid peroxidation in patients with essential hypertension. J Hypertens. 1998;16:1267–71. doi: 10.1097/00004872-199816090-00007. [DOI] [PubMed] [Google Scholar]
- 23.Cracowski JL, Baguet JP, Ormezzano O, et al. Lipid peroxidation is not increased in patients with untreated mild-to-moderate hypertension. Hypertens. 2003;41:286–88. doi: 10.1161/01.hyp.0000050963.16405.e6. [DOI] [PubMed] [Google Scholar]
- 24.Digiesi V, Oliviero C, Giannò V, et al. Reactive metabolites of oxygen, lipid peroxidation, total antioxidant capacity and vitamin E in essential arterial hypertension. Clin Ter. 1997;148(11):515–19. [PubMed] [Google Scholar]
- 25.Voevod CMV, Mocan T, Moldovan R, et al. Oxidative stress in chronic venous hypertension-insights from an animal model. Fizjologia (Physiology) 2010;3(67):17–21. [Google Scholar]
- 26.Simic DV, Mimic-Oka J, Pljesa-Ercegovac M, et al. Byproducts of oxidative protein damage and antioxidant enzyme activities in plasma of patients with different degrees of essential hypertension. J Hum Hypertens. 2006;20:149–55. doi: 10.1038/sj.jhh.1001945. [DOI] [PubMed] [Google Scholar]
- 27.Tsuda K. Roles of adiponectin and oxidative stress in the regulation of membrane microviscosity of red blood cells in hypertensive men – an electron spin resonance study. J Obes. 2011:548140. doi: 10.1155/2011/548140. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Wu Z, Song G, et al. Effects of oxidative damage |of membrane protein thiol groups on erythrocyte membrane viscoelasticities. Clinl Hemorheol Micro. 1999;21:137–46. [PubMed] [Google Scholar]
- 29.Chan TCK, Godin DV, Sutter MC. Erythrocyte membrane abnormalities in hypertension: A comparison between two animal models. Clin Exp Hypertens. 1983;5:691–719. doi: 10.3109/10641968309081802. [DOI] [PubMed] [Google Scholar]
- 30.Assadpoor-Piranfar M, Pordal AH, Beyranvand MR. Measurement of oxidized low-density lipoprotein and superoxide dismutase activity in patients with hypertension. Arch Iranian Med. 2009;12(2):116–20. [PubMed] [Google Scholar]
- 31.Amirkhizi F, Siass F, Djalali M, Foroushani AR. Assessment of antioxidant enzyme activities in erythrocytes of pre-hypertensive and hypertensive women. JRMS. 2010;15(5):270–78. [PMC free article] [PubMed] [Google Scholar]
- 32.Kędziora-Kornatowska K, Czuczejko J, Pawluk H, et al. The markers of oxidative stress and activity of the antioxidant system in the blond of elderly patients with essentials arteria hypertension. Cell Mol Biol Lett. 2004;9:635–41. [PubMed] [Google Scholar]
- 33.Rozwodowska MM, Rozwadowska M, Œwiątkiewicz I, et al. Evaluation of concentration of lipid peroxidation products and of antioxidant enzymes activity in patients with essential hypertension. Arterial Hypertension. 2005;9(3):178–83. [Google Scholar]
- 34.Mantle D, Patel VB, Why HJF, et al. Effects of lisinopril and amlodipine on antioxidant status in experimental hypertension. Clin Chim Acta. 2000;299:1–10. doi: 10.1016/s0009-8981(00)00270-9. [DOI] [PubMed] [Google Scholar]