Summary
Ocular ischemic syndrome is a rare condition, which is caused by ocular hypoperfusion due to stenosis or occlusion of the common or internal carotid arteries. Atherosclerosis is the major cause of changes in the carotid arteries. Ocular ischemic syndrome is manifested as visual loss, orbital pain and, frequently, changes of the visual field, and various anterior and posterior segment signs. Anterior segment signs include iris neovascularization and secondary neovascular glaucoma, iridocyclitis, asymmetric cataract, iris atrophy and sluggish reaction to light. Posterior eye segment changes are the most characteristic, such as narrowed retinal arteries, perifoveal telangiectasias, dilated retinal veins, mid-peripheral retinal hemorrhages, microaneurysms, neovascularization at the optic disk and in the retina, a cherry-red spot, cotton-wool spots, vitreous hemorrhage and normal-tension glaucoma. Differential diagnosis of ocular ischemic syndrome includes diabetic retinopathy and moderate central retinal vein occlusion. Carotid artery imaging and fundus fluorescein angiography help to establish the diagnosis of ocular ischemic syndrome. The treatment can be local, for example, ocular (conservative, laser and surgical) or systemic (conservative and surgical treatment of the carotid artery). Since the condition does not affect the eyes alone, patients with ocular ischemic syndrome should be referred for consultation to the neurologist, vascular surgeon and cardiologist.
Keywords: ocular ischemic syndrome, carotid artery occlusion, chronic ocular hypoperfusion
Background
Ocular ischemic syndrome is a rare condition, which is caused by ocular hypoperfusion due to stenosis or occlusion of the common or internal carotid arteries. Atherosclerosis is the major cause of changes in the carotid arteries.
In 1963, Hedges described a case of a 48-year-old patient with complete obstruction of the left internal carotid artery on whose eye fundus he observed peripheral blot hemorrhages and dilated retinal veins. He associated these findings with retinal hypoxia due to carotid artery stenosis [1]. In the same year, Kearnst and Hollenhorst reported ocular symptoms and signs in association with advanced carotid artery stenosis. They named the condition venous stasis retinopathy and found it in 5% of their patients with unilateral stenosis or occlusion of the internal carotid artery [2]. In the following years, many other terms were introduced to describe ocular changes secondary to carotid artery stenosis, such as ischemic ocular inflammation, ischemic coagulopathy and ischemic ophthalmopathy. It was observed that the signs of ischemia are present in both the anterior and the posterior segments of the eye, and eventually the term ocular ischemic syndrome has been coined for the sum of ocular symptoms and signs that may accompany carotid artery occlusive disease [3,4].
In this paper we discuss the clinical findings and diagnostic and therapeutic options of ocular ischemic syndrome.
Epidemiology
Ocular ischemic syndrome (OIS) occurs mostly in the elderly. The mean age of patients is 65 years and the condition is rare before age 50 (3). Men are affected twice as often as women, which is related to the higher incidence of cardiovascular disease, the underlying morbidity, in males. The incidence of OIS does not seem to be related to race. In 20% of cases the involvement is bilateral [4]. The exact incidence of OIS is not known but it is estimated at 7.5 cases per million every year. Since OIS may be misdiagnosed in many patients, this figure may be an underestimation [4].
Pathogenesis
In patients with OIS, a stenosis of 90% or more of the common or internal carotid arteries on the same side is usually found [5]. In 50% of the cases, the affected artery is completely obstructed [5]. It has been estimated that such stenosis reduces the perfusion pressure within the central retinal artery by approximately 50% [4]. Rarely, the occlusion of the ophthalmic artery is responsible for OIS [5]. OIS occurs mostly in patients with poor collateral circulation between the internal and external carotid arteries or between the two internal carotid arteries. Patients with well-developed collateral circulation may not develop OIS in spite of total occlusion of the internal carotid artery. On the other hand, in patients without well-developed collateral circulation, stenosis of the carotid artery by even 50% may lead to the development of OIS [3,6].
In patients with OIS, decreased blood flow in the retrobulbar vessels and reversal of blood flow in the ophthalmic artery are found. In these cases, blood flow is shunted away from the eye to the low-resistance intracranial circuit, with further reduction of retrobulbar blood flow [3].
Atherosclerosis is the main cause of OIS [5]. Other causes include dissecting aneurysm of the carotid artery, giant cell arteritis, fibrovascular dysplasia, Takayasu arteritis, aortic arch syndrome, Behçet’s disease, trauma or inflammation causing stenosis of the carotid arteries and complications after intravitreal anti-VEGF injections and after radiotherapy for nasopharyngeal carcinoma [3,4,7–9]. Since OIS is associated with atherosclerosis, patients usually have other related co-morbidities. Hypertension is found in 73% of the patients and diabetes mellitus in 56% [10]. Myocardial infarction occurs in approximately 4% of patients with OIS [10]. The mortality rate is as high as 40% within 5 years of onset [10]. Cardiovascular disease is the main cause of death (approximately 66%), followed by stroke as the second leading cause of death [4], which is why patients with OIS should be referred to the cardiologist, for imaging studies of the carotid arteries, and to the vascular surgeon.
Changes in the retinal arteries, usually asymptomatic, are present in 29% of patients with a symptomatic carotid occlusion and 1.5% per year progress to symptomatic OIS [12]. On the other hand, OIS is the first observed manifestation of internal carotid stenosis in as many as 69% of patients [6].
Clinical presentation
Visual loss in the affected eye is present in over 90% of patients with OIS [10]. It is usually related to chronic or acute retinal ischemia or damage to the optic nerve due to secondary glaucoma. In 67% of patients visual loss occurs gradually over a few weeks or months, in 12% it occurs over a period of days, and in another 12% the loss is sudden over a period of minutes or seconds [3]. In patients with a sudden visual loss a cherry-red spot is usually observed at the fundus, related to embolization of the central retinal artery [4].
The visual acuity in patients with a recently diagnosed OIS ranges from 0.4 to 1.0 (assessed by a Snellen chart) in 43% of patients to counting fingers or worse in 37% of patients [10]. At 12-month follow-up, the rates are 24% and 58%, respectively. Loss of light perception is usually not observed at onset, but it may develop in later stages of OIS, usually as a result of neovascular glaucoma [4].
Recovery of visual function after exposure to bright light is usually delayed in patients with severe carotid artery stenosis; the phenomenon may be accounted for by macular ischemia [4]. Transient visual loss occurs in approximately 10% of patients with OIS and lasts from a few seconds to a few minutes [5]. It is usually caused by transient embolization of the central retinal artery, although in some patients vasospasm may be the causative factor [13]. It must be remembered, however, that most patients with transient visual loss do not have OIS.
Visual fields in patients with OIS may vary from normal, to central scotoma, to centrocecal defects, to nasal defects, to the presence only of a central or temporal island [6].
Pain in the affected eye or in the periorbital area may be present in 40% of patients [5] and is usually due to neovascular glaucoma, but in patients with normal intraocular pressure (IOP) it may be caused by hypoxia of the eyeball and/or dura mater [3]. Ischemic pain is described as dull; it develops gradually over a period of hours or days and is relieved when the patient lies down [3].
Anterior segment signs may be the single manifestation of OIS [3]. In approximately 66% of patients, neovascularization of the iris and at the iridocorneal angle is found, which results in impaired outflow of aqueous humor from the eyeball. However, increased IOP and neovascular glaucoma are noted in only 50% of patients, while in some ocular hypotony may be observed, in spite of fibrovascular tissue secondary to neovascularization closing the angle. This is due to ischemia of the ciliary body and reduced production of aqueous humor [4,5]. In most patients opalescence of the aqueous humor is noted and in 20% inflammatory cells are seen in the anterior chamber [5]. They are not numerous, are rarely above Grade 2, and are usually related to iritis. Even more unusual are precipitates on the corneal endothelium, which occur in cases of clinically silent iridocyclitis. Inflammatory conditions in the anterior segment may result in the posterior synechiae. In patients with unilateral OIS, the lens in the affected eye is usually more opaque [4]. As a result of ischemia and atrophy of the sphincter muscle of the pupil, the pupil is fixed and semi-dilated. There is a sluggish reaction to light, which also may be due to retinal ischemia [3,4]. The other signs of OIS may include dilatation of conjunctival and episcleral vessels [14] and corneal edema, which may lead to bullous keratopathy. In very rare cases, liquefactive necrosis of the cornea may develop [3,14].
Posterior segment signs are more frequent than anterior segment signs. On ophthalmoscopy, the retinal arteries are narrowed and the retinal veins are dilated. In some cases, both arteries and veins may be narrowed. Occasionally, spontaneous retinal arterial pulsations are observed. Retinal hemorrhages are very characteristic and are seen in about 80% of affected eyes. Hemorrhages are mostly located in the external retinal layers, at the mid-periphery (Figure 1); they are not numerous and are almost never confluent. They are most probably due to leakage of blood from the small retinal vessels or from ruptured capillary microaneurysms [3,4]. Microaneurysms are very frequent in OIS and may be located both in the macula and at the mid-periphery [3,4,15]. Additionally, one of the first manifestations observed in the fundus are diffuse macular capillary telangiectasias, which, together with microaneurysms, may cause macular edema [3,16] (Figure 2). Small branches of the central retinal artery may be occluded spontaneously, or the occlusion may be caused by cholesterol emboli with the resulting areas of retinal hypoperfusion. Occasionally, retinal arterio-venous communications proximal to areas of avascular retina may be present [3]. A cherry-red spot, characteristic of retinal ischemia, mostly macular, is seen in 12% of eyes, predominantly when IOP exceeds the perfusion pressure within the central retinal artery in the eyes with neovascular glaucoma or in some cases as a result of embolic occlusion of the central retinal artery [3,5]. Neovascular glaucoma usually produces quickly progressing damage to the optic disk. In some patients, reduction of the retrobulbar blood flow and ischemia of the optic disk may lead to the nerve atrophy in the course of normal-tension glaucoma [17]. In OIS, retinal neovascularization may also occur, as a result of increased production of the vascular endothelial growth factor (VEGF). New vessels are formed more often at the optic disk than in the retina. New vessels can bleed, with resulting hemorrhages into the vitreous body and, rarely, fibrovascular proliferation [3]. Further signs of OIS include anterior and posterior ischemic optic neuropathy, choroidal neovascular membrane, cotton-wool spots in the retina (reflecting local ischemia), edema of optic axons in the optic nerve layer of the retina, and areas of chorio-retinal atrophy resulting from choroidal ischemia [3,4,15].
Figure 1.
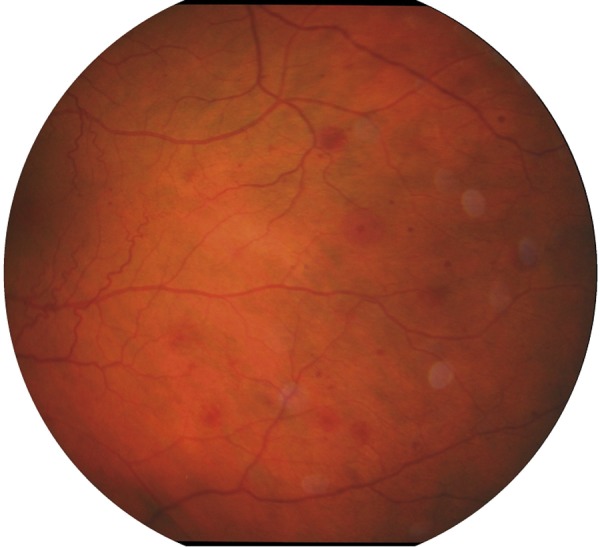
Fundus photograph of the left eye: retinal haemorrhages at the mid-periphery from the patient with identified considerable stenosis of the left internal carotid artery (autor of the photograph: Barbara Terelak-Borys).
Figure 2.
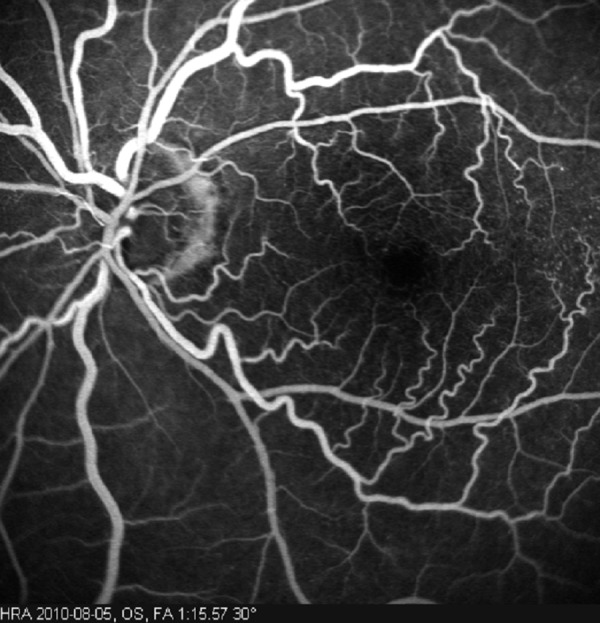
Fluorescein angiography of the left fundus: macular capillary telangiectasias with microaneurysms from the patient identified considerable stenosis of the left internal carotid artery (autor of the photograph: Barbara Terelak-Borys).
Orbital infarction syndrome can be rarely observed in ocular ischemic syndrome. In this syndrome, ischemia of intraorbital and intraocular structures may be observed. The syndrome consists of anterior and posterior segment ischemia, ophthalmoplegia, orbital pain, hypotony and ptosis [3,18].
Clinical manifestations of ocular ischemic syndrome are summarized in Table 1.
Table 1.
| Anterior segment |
| Rubeosis iridis and neovascular glaucoma |
| Uveitis |
| Anterior and posterior synechia |
| Spontaneous hyphema |
| Asymmetric cataract |
| Atrophy of sphincter pupillae and semi-dilated pupil |
| Sluggish reaction to light |
| Conjunctival and episcleral injection |
| Corneal edema with Descemet’s folds (sometimes with bullous keratopathy) |
| Scleral melting |
|
|
| Posterior segment |
| Narrowed retinal arteries |
| Spontaneous retinal arteries pulsations |
| Dilated retinal veins |
| Retinal hemorrhages |
| Microaneurysms |
| Retinal teleangiectasia |
| Cherry-red spot |
| Cholesterol emboli |
| Glaucoma (neovascular glaucoma, normal tension glaucoma) |
| Neovascularization (optic disc, retina) |
| Vitreous hemorrhage |
| Anterior and posterior ischemic optic neuropathy |
| Cotton-wool spots |
| Choroidal neovascular membrane |
| Areas of chorio-retinal atrophy |
|
|
| Orbital infarction syndrome |
| Anterior and posterior segment ischemia |
| Ophthalmoplegia |
| Orbital pain |
| Hypotony |
| Ptosis |
Diagnosis
Imaging studies of the carotid arteries are the essential diagnostic test in OIS. The most commonly used methods include non-invasive tests such as Doppler ultrasound and ocular plethysmography and invasive techniques such as carotid arteriography. Non-invasive tests allow detection of carotid artery stenosis in at least 75% of cases. When OIS is suspected, but Doppler ultrasound scan of the carotid arteries is within normal limits, Doppler imaging of retrobulbar vessels, especially of the ophthalmic artery, should be performed. Carotid arteriography is performed only in very advanced cases before planned surgery on the carotid arteries [4]. New minimally invasive methods such as computed tomographic angiography and magnetic resonance angiography can be also used as the second-line test in carotid artery stenosis [3].
Fluorescein angiography of the fundus is a test commonly used in the diagnosis of OIS. The prolonged arm-to-choroid and arm-to-retina circulation time is a frequent sign. Irregular and/or prolonged retinal filling time is present in approximately 60% of patients with OIS. The normal retinal filling time is approximately 5 seconds, but in the affected eyes it may be 1 minute or longer. This is the most specific (but not the most sensitive) fluorescein angiography sign of OIS [3–5]. The most sensitive angiographic sign of OIS is prolonged retinal arteriovenous time, which is present in up to 95% of cases, but it is not OIS-specific [3–5]. In 85% of affected eyes, staining of the major retinal vessels (mostly arteries) and their branches may be observed at the late phase of the test and is attributed to the increased permeability of the vessels. It may be accounted for by endothelial cell damage due to chronic ischemia [4]. Macular edema is seen in 17% of eyes with OIS and is often accompanied by hyperfluorescence of the optic disk [4,15], caused by leakage from disk capillaries [19]. In some cases, retinal capillary non-perfusion can be seen, mostly located at the mid-periphery. It is related to the loss of endothelial cells and pericytes in these vessels and obliteration of their lumen [4,20].
Indocyanine green angiography can also help to establish the diagnosis of OIS. The test shows abnormalities of the choroidal circulation. As in fluorescein angiography, arm-to-choroid circulation time and intrachoroidal circulation time are prolonged. Choroidal hypoperfusion is mainly manifested as areas of vascular filling defects in the posterior pole of the eye [3]. Another characteristic angiographic finding is slow filling of the watershed zones (areas between zones supplied by 2 different vessels). This may be observed (eg, between the macula and the optic disk) [3].
Ophthalmologic studies such as visual-evoked potentials (VEP), electroretinography (ERG) and ophthalmotonometry are rarely used to establish the diagnosis of OIS [4,21].
Differential Diagnosis
The differential diagnosis of OIS should definitely include diabetic retinopathy and moderately advanced central retinal vein occlusion (CRVO). The main sign that helps to differentiate OIS from CRVO is absence of tortuous retinal veins in OIS. Additionally, retinal arterial pulsations are difficult to elicit in CRVO [4]. In OIS, intraretinal hemorrhages are less numerous than in diabetic retinopathy. While cotton-wool spots, a typical finding in diabetic retinopathy, may be also present in OIS, the presence of hard exudates suggests changes in the fundus due to diabetes [4]. Diabetic retinopathy may coexist with OIS; patients with marked asymmetry of retinopathy should be examined for possible carotid artery stenosis since approximately 20% of such patients have hemodynamically significant carotid artery stenosis [3].
Absence of retinal arterial stasis and choroidal filling defects detected by fluorescein angiography in diabetic retinopathy and CRVO is an important feature distinguishing these 2 conditions from OIS [4].
The differential diagnosis of OIS should further include the hyperviscosity syndromes and autoimmune uveitis, especially when manifestations of anterior choroiditis predominate [3].
The differential diagnosis of ocular ischemic syndrome, diabetic retinopathy and central retinal vein occlusion is summarized in Table 2.
Table 2.
The differential diagnosis of ocular ischemic syndrome, diabetic retinopathy and central retinal vein occlusion [3,4].
| Ocular ischemic syndrome | Diabetic retinopathy | Central retinal vein occlusion | |
|---|---|---|---|
| Age | 50s to 80s | Variable | 50s to 80s |
| Laterality | 80% unilateral | Bilateral | Usually unilateral |
| Posterior segment signs | |||
| Retinal veins | Dilated but not tortuous | Dilated and beaded | Dilated and tortuous |
| Hemorrhages | Dot and blot, mid-periphery, in deeper retina layers | Dot, blot in deeper retina layers and flamme-shaped in in nerve fiber layer | Flamme-shaped in in nerve fiber layer |
| Microaneurysms | In midperiphery | In posterior pole | Variable |
| Hard exudates | Absent | Common | Rare |
| Optic disk | Normal | Diabetic papillopathy (rarely) | Swollen |
| Retinal arteria perfussion pressure | Decreased | Normal | Normal |
| Fluorescein angiography | |||
| Arterio-venous transie time | Prolonged | Usually normal | Prolonged |
| Choroidal filling | Delayed, patchy | Normal | Normal |
| Retinal vessel staining | Arteries > veins | Usually absent | Veins > arteries |
Treatment
OIS should be treated not only by an ophthalmologist, but also (and, in some cases above all) by a multidisciplinary team of other specialists, including vascular surgeons, cardiologists, neurologists and primary care physicians. The treatment can be ocular (conservative, laser and surgical) and systemic (conservative and surgical).
Ocular treatment
The most important aim of ocular management is to treat complications of OIS, especially in the posterior segment, as they are associated with the highest risk of vision loss. Unfortunately, some of these conditions are acute and irreversible, as those due to embolization of the central retinal artery or occlusion of vessels supplying the optic disk, and result in anterior or, less frequently, posterior ischemic optic neuropathy. These conditions are usually refractory to such treatment options as intravenous infusion of vasodilating or anticoagulant drugs or agents improving tissue blood supply and metabolism. Thus far thrombolytic agents are not part of standard ophthalmologic management, while surgery cannot be used due to the very small size of the retinal vessels and the optic nerve.
The most dangerous consequence of chronic retinal and choroidal ischemia is excessive production of vascular endothelial growth factor (VEGF), which causes neovascularization in the iris and at the irido-corneal angle in the anterior segment and in the retina and at the optic disk in the posterior segment. In the anterior segment, this complication may lead to the development of neovascular secondary glaucoma, which is usually refractory to medical treatment. One of the treatment options employed in retinal ischemia is reduction of retinal oxygen demand by ablation of the peripheral, optically non-functional part of the retina. This inhibits neovascularization at the irido-corneal angle and prevents development of secondary glaucoma. Panretinal photocoagulation is indicated in patients with iris and posterior segment neovascularization to prevent development of secondary neovascular glaucoma and intraocular hemorrhages. However, it is effective in only 36% of treated eyes with OIS because choroidal ischemia alone with no retinal ischemia may be sufficient to induce neovascularization [3]. If the fundus is not visible due to media opacities or poor dilation of the pupil, transconjunctival cryotherapy in the mid-peripheral and peripheral retina and trans-scleral diode laser retinopexy should be considered as alternative treatment modalities [3,22].
In the early stage of neovascular glaucoma, medical treatment may be used to lower intraocular pressure. It consists mainly of topical β-adrenergic blockers or α-2-agonists along with topical or oral carbonic anhydrase inhibitors. Since neovascularization is accompanied by inflammation, prostaglandin analogues should be avoided because of their possible pro-inflammatory effect [3]. Pilocarpine is contraindicated because it may be conductive to the development of posterior synechiae and secondary miosis, which increases closure of the irido-corneal angle and is another factor raising IOP. However, application of mydriatics is indicated.
When neovascular glaucoma, refractory to medical treatment, develops, which is very frequent, trabeculectomy (surgical creation of a fistula) may be considered in patients with preserved visual acuity and limited angle neovascularization. However, with this surgical approach the risk for intra- and post-operative complications and a low success rate (even when antimetabolites are used) must be taken into consideration. When trabeculectomy fails or if marked neovascularization is present in the anterior segment, aqueous shunt implants are a recommended treatment option. In patients with ocular pain and no useful vision due to glaucoma, partial cycloablation by cryosurgery or using a diode laser is preferred. If the pain is not relieved by the procedure, retrobulbar injection of alcohol should be considered for sensory denervation of the eyeball. If this again does not relieve the pain in the blind eye, enucleation or evisceration should be considered.
In cases of normal-tension glaucoma, topical agents are used to decrease IOP, including prostaglandin analogues, which are the most potent ocular hypotensive agents [23]. This form of glaucomatous neuropathy develops because IOP is relatively too high in relation to the optic nerve resistance, which is due to decreased ocular blood flow and low perfusion pressure in the vessels supplying the optic disk.
There have been attempts to treat macular edema in the course of OIS with intravitreal injections of steroids (eg, triamcinolone acetonide) and VEGF inhibitors, but to date there is not enough data to confirm their safety and efficacy [3]. In one isolated case report, Kofoed et al noted that a patient with ocular ischemic syndrome demonstrated vision loss, retinal vessel calibre constriction and profound retinal ischaemia after intravitreal anti-VEGF injection [24].
In cases of anterior uveititis, treatments include steroidal and non-steroidal anti-inflammatory drugs in the form of drops, mydriatic agents to prevent posterior synechiae, and cytoplegic agents to stabilize the blood-aqueous barrier and prevent spontaneous hyphema.
Systemic treatment
Causal treatment (ie, restoration of the carotid artery patency and prevention of its stenosis) seem to be the most appropriate approach to OIS.
Carotid artery endarterectomy (CEA) is a surgical method used in the treatment of carotid artery stenosis [25]. It is effective in symptomatic carotid artery stenosis of 70–90% and in asymptomatic stenosis of at least 60%. Thus this treatment option should be considered in all patients with OIS and severe stenosis of the carotid artery. Carotid artery stenting can be the alternative treatment to CEA [3]; however, in cases of a complete occlusion, the procedure is not effective, as the thrombus usually travels distally to the large arteries, in which case arterial by-pass surgery should be performed. Such procedures, which restore normal blood flow in the eyeball, are thought to stabilize or restore visual acuity, especially if they are performed before iris neovascularization and secondary glaucoma develop. Performed at the early stages of neovascular glaucoma, by-pass surgery may result in regression of neovascularization at the angle and resolution of glaucoma. It should be noted, however, that IOP may increase after endarterectomy due to improved oxygen supply and restoration of normal production of aqueous humor by the ciliary body. This phenomenon is observed mostly in eyes with well-developed fibrous tissue at the iridocorneal angle, and ablation of the ciliary body or antiglaucoma surgery should be considered [4].
Because of numerous co-morbidities in patients with OIS and consequently the high mortality rate, referral of the patients for assessment by specialists in internal medicine, cardiologists and neurologists is mandatory. Systemic treatment usually includes antiplatelet agents, treatment of hypertension, atherosclerosis, diabetes mellitus and coronary heart disease. Appropriate diet, cessation of smoking, and physical activity play a very important role.
Conclusions
Although ocular ischemic syndrome is a rare condition, its complications may lead to irreversible vision loss. Considering that signs of severe carotid artery stenosis may be first observed in the eye before they are manifested in the cerebrovascular system, the ophthalmologist has a very important role in the proper diagnosis and referral for further investigations. Collaboration between the ophthalmologist, vascular surgeon, cardiologist, neurologist and primary care physician is essential for appropriate management of the OIS patient.
Method of literature search
To prepare this article, literature searches were conducted in Web of Knowledge (search based on MeSH and keywords) with no date restrictions. Search words used included: ocular ischemic syndrome, carotid artery occlusion, chronic ocular hypoperfusion, neovascular glaucoma, carotid artery disease.
Footnotes
Source of support: Self financing
References
- 1.Hedges TR., Jr Ophthalmoscopic findings in internal carotid artery occlusion. Am J Ophthalmol. 1963;55:1007–12. [PubMed] [Google Scholar]
- 2.Kearns TP, Hollenhorst RW. Venous stasis retinopathy of occlusive disease of the carotid artery. Mayo Clin Proc. 1963;38:304–12. [PubMed] [Google Scholar]
- 3.Mendrinos E, Machinie TG, Pournaras CJ. Ocular Ischemic Syndrome. Surv Ophthalmol. 2010;55(1):2–34. doi: 10.1016/j.survophthal.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S, Brown GC. In: Ocular Ischemic Syndrome. Ryan SJ, Hinton DR, Schachat AP, et al., editors. Elsevier; 2006. pp. 1491–502. [Google Scholar]
- 5.Brown GC, Magargal LE. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. Int Ophthalmol. 1988;11(4):239–51. doi: 10.1007/BF00131023. [DOI] [PubMed] [Google Scholar]
- 6.Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology. 1997;104(5):859–64. doi: 10.1016/s0161-6420(97)30221-8. [DOI] [PubMed] [Google Scholar]
- 7.Peter J, David S, Danda D, et al. Ocular manifestations of Takayasu arteritis: a cross-sectional study. Retina. 2011;31(6):1170–78. doi: 10.1097/IAE.0b013e3181fe540b. [DOI] [PubMed] [Google Scholar]
- 8.Tang Y, Luo D, Peng W, et al. Ocular ischemic syndrome secondary to carotid artery occlusion as a late complication of radiotherapy of nasopharyngeal carcinoma. J Neuroophthalmol. 2010;30(4):315–20. doi: 10.1097/WNO.0b013e3181dee914. [DOI] [PubMed] [Google Scholar]
- 9.Huang ZL, Lin KH, Lee YC, et al. Acute vision loss after intravitreal injection of bevacizumab (avastin) associated with ocular ischemic syndrome. Ophthalmologica. 2010;224(2):86–89. doi: 10.1159/000235726. [DOI] [PubMed] [Google Scholar]
- 10.Sivalingam A, Brown GC, Magargal LE. The ocular ischemic syndrome. III. Visual prognosis and the effect of treatment. Int Ophthalmol. 1991;15(1):15–20. doi: 10.1007/BF00150974. [DOI] [PubMed] [Google Scholar]
- 11.Duker JS, Brown GC, Bosley TM, et al. Asymmetric proliferative diabetic retinopathy and carotid artery disease. Ophthalmology. 1990;97(7):869–74. doi: 10.1016/s0161-6420(90)32488-0. [DOI] [PubMed] [Google Scholar]
- 12.Klijn CJ, Kappelle LJ, van Schooneveld MJ, et al. Venous stasis retinopathy in symptomatic carotid artery occlusion: prevalence, cause, and outcome. Stroke. 2002;33(3):695–701. doi: 10.1161/hs0302.104619. [DOI] [PubMed] [Google Scholar]
- 13.Winterkorn JM, Beckman RL. Recovery from ocular ischemic syndrome after treatment with verapamil. J Neuroophthalmol. 1995;15(4):209–11. [PubMed] [Google Scholar]
- 14.Kerty E, Eide N. Chronic ocular ischaemia. Acta Ophthalmol. 1989;67(4):386–92. doi: 10.1111/j.1755-3768.1989.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen CS, Miller NR. Ocular ischemic syndrome: review of clinical presentations, etiology, investigation, and management. Compr Ophthalmol Update. 2007;8(1):17–28. [PubMed] [Google Scholar]
- 16.Brown GC. Macular edema in association with severe carotid artery obstruction. Am J Ophthalmol. 1986;102(4):442–48. doi: 10.1016/0002-9394(86)90071-1. [DOI] [PubMed] [Google Scholar]
- 17.Berkow JW, Flower RW, Orth DH, Kelley JS. Angiografia fluoresceinowa i indocyjaninowa. Wrocław: Górnicki Wydawnictwo Medyczne; 2004. pp. 66–67. [Google Scholar]
- 18.Bogousslavsky J, Pedrazzi PL, Borruat FX, Regli F. Isolated complete orbital infarction: a common carotid artery occlusion syndrome. Eur Neurol. 1991;31(2):72–76. doi: 10.1159/000116650. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto M, Ohtsuka K, Ohtsuka H, Nakagawa T. Normal-tension glaucoma with reversed ophthalmic artery flow. Am J Ophthalmol. 2000;130(5):670–72. doi: 10.1016/s0002-9394(00)00588-2. [DOI] [PubMed] [Google Scholar]
- 20.Dugan JD, Jr, Green WR. Ophthalmologic manifestations of carotid occlusive disease. Eye. 1991;5(Pt 2):226–38. doi: 10.1038/eye.1991.38. [DOI] [PubMed] [Google Scholar]
- 21.Kofoed PK, Munch IC, Sander B, et al. Arsen, Prolonged multifocal electroretinographic implicit times in the ocular ischemic syndrome. Invest Ophthalmol Vis Sci. 2010;51(4):1806–10. doi: 10.1167/iovs.09-4555. [DOI] [PubMed] [Google Scholar]
- 22.Gross R. Neovascular glaucoma and ocular ischemic syndrome. J Glaucoma. 2000;9(5):409–12. doi: 10.1097/00061198-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Wierzbowska J, Robaszkiewicz J, Figurska M, Stankiewicz A. Future Possibilities in glaucoma therapy. Med Sci Monit. 2010;16(11):RA252–259. [PubMed] [Google Scholar]
- 24.Kofoed PK, Munch IC, Larsen M. Profound retinal ischaemia after ranibizumab administration in an eye with ocular ischaemic syndrome. Acta Ophthalmol. 2010;88(7):808–10. doi: 10.1111/j.1755-3768.2009.01612.x. [DOI] [PubMed] [Google Scholar]
- 25.Dzierwa K, Pieniazek P, Musialek P, et al. Treatment startegies in severe symptomatic carotid and coronary artery disease. Med Sci Monit. 2011;17(8):RA191–97. doi: 10.12659/MSM.881896. [DOI] [PMC free article] [PubMed] [Google Scholar]


