Summary
Background
Inflammatory myofibroblastic tumor (IMT) is a rare borderline tumor. The nomenclature of this disease is confused in the literature.
Case Report
In this report, the case of a 62-year-old man with IMT recurrence of bladder secondary to prostate is presented. The possible etiology of IMT is discussed, along with its clinical manifestation and pathological features. The patient received a laparoscopic bladder radical resection. The pathology finding demonstrated the diagnosis of IMT and no regional lymph node involvement.
Conclusions
IMT is a borderline tumor and unlikely to metastasize to regional lymph nodes. The patient has been observed for 2 years without recurrence.
Keywords: inflammatory myofibroblastic tumor, bladder, prostate
Background
IMT is a rare condition of unknown etiology and pathogenesis. It can affect any part of the body, but is most commonly seen in the lungs, mesentery and omentum, and usually follows a benign clinical course [1]. IMT is very rare in the genitourinary tract. Most are benign and the patients receive transurethral resection or partial cystectomy. In spite of the evidence of its histologically benign features, there have been reports of local recurrences, metastases, medical invasion and sarcomatous degeneration [2]. Recurrence after local resection is more frequent than distant metastases [3], necessitating radical resection in occasional cases like ours. This report presents a special case with IMT recurrence of bladder secondary to prostate and discusses the clinical presentations, diagnosis and management of this rare disease.
Case Report
A 62-year-old man was initially referred to the Department of Urology with a 1-month history of dysuria. The prostate specific antigen (PSA) level was 2.3 ng/ml (normal <4 ng/ml) and maximum outflow rate was 5.7 ml/s (normal >15 ml/s). Digital rectal examination disclosed an enlarged, round, soft prostate. Cystoscopy also revealed an intumescence of the prostate, extending from the verumontanum and obstructing the lumen of the urethra. A non-radical transurethral resection of the prostate (TURP) was performed. The pathological report was benign prostatic hyperplasia with infection, and many lymphocytes were seen in the stroma.
The patient was free of symptoms during the 10 months after surgery, unless he underwent another TURP because the symptom of dysuria recurred. This time the pathological report was that the submitted samples were fibrous connective tissues and blood clots. In the fibrous connective tissue there are diffusive inflammatory infiltrates of plasma cells and lymphocytes. Final diagnosis was hematoma, infection and inflammatory polyps of the posterior urethra after TURP. Unfortunately, after the procedure, the symptom was relieved for only 3 days, so the patient received a computed tomographic (CT) scan, which found a mass in the right lobe of the prostate (Figure 1A). The patient underwent additional TURP and this time the pathological report was spindle cells tumor with local inflammatory infiltrate and edema. Immunohistochemical finding was desmin (+), S-100 (−), Myogenin (±), CD117 (+), CD34 (−), dog-1 (−). The final diagnosis was IMT of the prostate.
Figure 1.
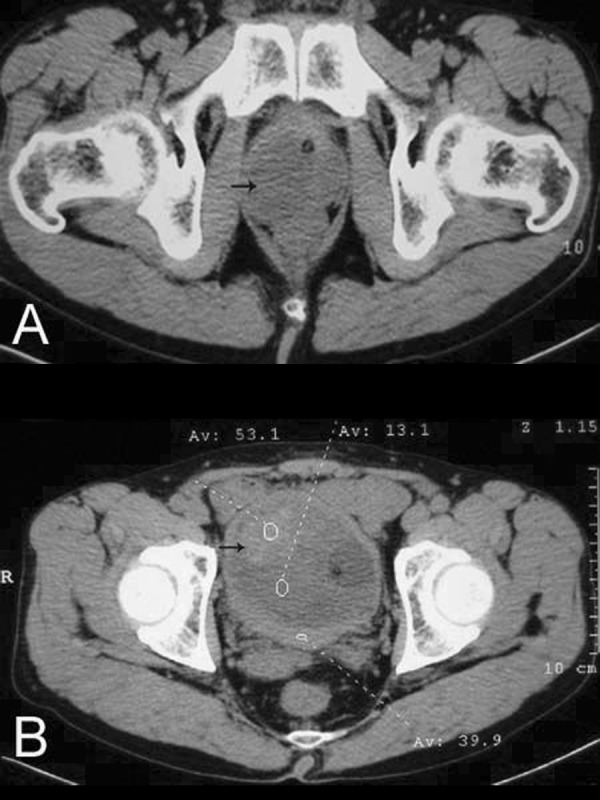
Image of computed tomography showing masses locating at the site of right prostatic lobe (A) and bladder (B) respectively (black arrows).
After this operation, the patient was free of symptoms for 6 months, and then he received a CT scan that found a large mass in the bladder lumen (Figure 1B). In view of his long history of disease, this time we performed a laparoscopic bladder radical resection simultaneously with a pelvic lymph node dissection. The tumor showed a polypoid and sarcomatoid appearance protruding into the lumen of the bladder (Figure 2), about 7×5 cm in size. The pathological report was spindle cells tumor with inflammatory infiltrate. Immunohistochemical finding was smooth muscle actin (+), pan cytokeratin (±), HHF35 (−), S-100 (−), Myogenin (−), and Vimentin (+) (Figure 3). Final diagnosis was IMT of bladder without regional lymph node involvement. This patient has been followed up for 2 years to date without any evidence of local recurrence and distant metastasis (Figure 4).
Figure 2.
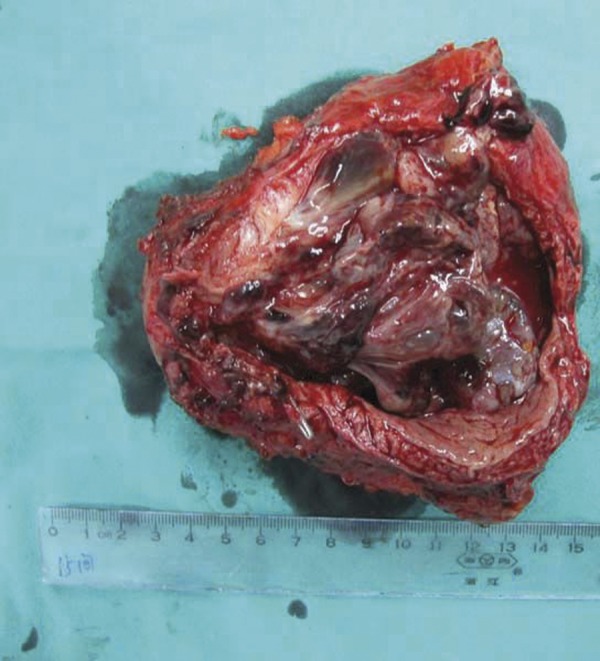
The tumor showed a polypoid and sarcomatoid appearance with a size of 8 cm ×5 cm, extending into the bladder lumen.
Figure 3.
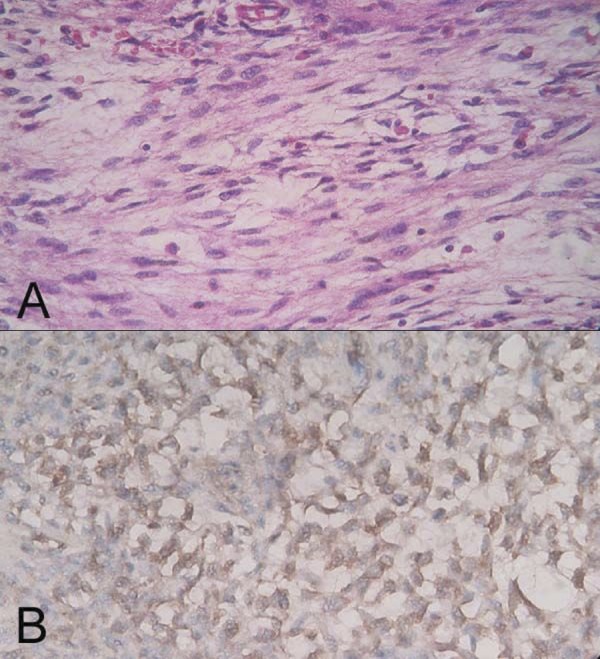
Micrographs of IMT in the bladder. The visual field is filled with many spindle cells and dispersal inflammatory infiltrate (A). The cells were positive for vimentin (B). Original magnification ×400.
Figure 4.
The patient has been followed up for 2 years without recurrence after the surgery.
Discussion
IMTs of the urinary tract are uncommon borderline lesions, which can involve at any site, including in the bladder and prostate. Given its various histologic features, it might be confused, even by the experienced urologist and pathologist. This tumor has been described in the literature with many synonyms (eg, inflammatory pseudotumor, atypical myofibroblastic tumor, pseudosarcomatous myofibroblastic proliferation, pseudosarcoma, and plasma cell granuloma), and is commonly known as an inflammatory pseudotumor [4]. In 1994, the World Health Organization defined IMT as an intermediate soft tissue tumor that is composed of myofibroblast-differentiated spindle cells and accompanied by numerous inflammatory cells, plasma cells, and/or lymphocytes [5]. The exact etiology of IMT is unknown, although it has been considered by some scholars as an abnormal reparative response to trauma, surgery or infections such as Epstein-Barr virus, herpes virus 8 and bacteria [6,7]. It is now reported that 40%–60% of IMTs are anaplastic lymphoma kinase (ALK)-positive with a common 2p23 rearrangement [8], which supports the concept of IMT as a neoplasm.
IMT can occur in various age groups, but is more common in youths, with a sex predilection for females [9]. It is rare in the genitourinary tract, with the most common site being the urinary bladder [9]. IMT of the bladder and prostate were first described by Roth [10] in 1980 and Hafiz et al [11] in 1984. The principal presenting complaint is painless hematuria with or without clots [12]. Others include frequency, dysuria, or lower abdominal discomfort. The imaging characteristics and locations of IMT in the genitourinary tract vary widely, but are nonspecific. Thus, diagnosis of IMT is often difficult and mainly depends on histological examination. The essential histological patterns should include myofibroblastic mesenchymal spindle cells accompanied by an inflammatory infiltrate [9]. Immunohistochemistry can contribute to the differential diagnosis of IMT. Immunoreactivity is often positive for ALK, vimentin, smooth muscle actin, cytokeratin, desmin, but not S-100 [9,13]. In the present case, the immunohistochemical finding was vimentin (+), smooth muscle actin (±), pan cytokeratin (+), desmin(+), myogenin (−) and S-100 (−). Finally, the tumor was demonstrated to be an IMT of the bladder.
At present, although significant positive response to anti-inflammatory drugs, radiotherapy or chemotherapy have been reported in a few cases [14,15], surgery is still the principal treatment. Management of IMT should entail complete surgical resection in the form of complete transurethral resection or partial cystectomy [12]. The difficulty of completely excising the tumor in most of the cases is related to its size, location and its invasion degree. In the present case, high suspicion of IMT had been considered, because the patient had a series of surgical history and was once diagnosed as IMT of the prostate. In view of the large size of the tumor, complete transurethral resection was not possible, so a radical cystectomy was performed. Thus, we implemented a laparoscopic surgical procedure, which differed from other reported cases. Laparoscopic approach could accomplish complete removal in a deep and narrow pelvic cavity, with minimal trauma to the normal structures, because it presents a good surgical view with an excellent exposure of all surrounding structures, due to the magnification of the surgical field. The bladder, the prostate and the seminal vesicle were totally resected, accompanied with a pelvic lymph node dissection. Finally, the tumor was demonstrated to be an inflammatory IMT of the bladder, without any lymph node metastasis.
Conclusions
IMT is a very unusual borderline lesion in the urinary tract. Most patients with IMT have a good prognosis. However, it can locally recur but rarely metastasizes, which manifested significantly in our present case. From a retrospective view, the IMT recurred in the bladder secondary to the prostate, and the pathological feature of this case originally presented with atypical inflammatory infiltrate, then with typical spindle cells tumor accompanied by inflammatory infiltrate. Thus, it is important for urologists and pathologists to recognize it since it can easily be mistaken for a malignancy. Histological examination and immunohistochemistry are very important to confirm the diagnosis. Considering its histological resemblance to certain malignant tumors, close follow-up of these patients is strongly suggested.
Footnotes
Source of support: Departmental sources
References
- 1.Janik JS, Janik JP, Lovell MA, et al. Recurrent inflammatory pseudotumors in children. J Pediatr Surg. 2003;38:1491–95. doi: 10.1016/s0022-3468(03)00501-3. [DOI] [PubMed] [Google Scholar]
- 2.Boloursaz MR, Khalilzadeh S, Dezfoli AA, et al. Inflammatory myofibroblastic tumor of the trachea. Pediatr Surg Int. 2011;27(8):895–97. doi: 10.1007/s00383-011-2851-2. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery EA, Shuster DD, Burkart AL, et al. Inflammatory myofibroblastic tumors of the urinary tract: A clinicopathologic study of 46 cases, including a malignant example inflammatory fibrosarcoma and a subset associated with high-grade urothelial carcinoma. Am J Surg Pathol. 2006;30:1502–12. doi: 10.1097/01.pas.0000213280.35413.1b. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher CDM, Unni KK, Mertens F, et al. Inflammatory myofibroblastic tumor. In: Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization classification of tumours: pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press; 2002. pp. 91–93. [Google Scholar]
- 5.Coindre JM. Histologic classification of soft tissue tumors (WHO, 1994) Ann Pathol. 1994;14:426–27. [PubMed] [Google Scholar]
- 6.Tunuguntla H, Mishra A, Jorda M, et al. Inflammatory Myofibroblastic Tumor of the Epididymis: Case Report and Review of the Literature. Urology. 2011;78(1):183–85. doi: 10.1016/j.urology.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 7.Cheuk W, Woo PC, Yuen KY, et al. Intestinal inflammatory pseudotumour with regional lymph node involvement: identification of a new bacterium as the aetiological agent. J Pathol. 2000;192:289–92. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH767>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Satomi T, Watanabe M, Matsubayashi J, et al. A successfully treated inflammatory myofibroblastic tumor of the mandible with long-term follow-up and review of the literature. Med Mol Morphol. 2010;43:185–91. doi: 10.1007/s00795-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 9.Yagnik V, Chadha A, Chaudhari S, et al. Inflammatory myofibroblastic tumor of the urinary bladder. Urol Ann. 2010;2:78–79. doi: 10.4103/0974-7796.65106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth JA. Reactive pseudosarcomatous response in urinary bladder. Urology. 1980;16:635–37. doi: 10.1016/0090-4295(80)90578-6. [DOI] [PubMed] [Google Scholar]
- 11.Hafiz MA, Toker C, Sutula M. An atypical fibromyxoid tumor of the prostate. Cancer. 1984;54:2500–4. doi: 10.1002/1097-0142(19841201)54:11<2500::aid-cncr2820541131>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Li HB, Xu YM, Yu JJ. Diagnostic Puzzle of Inflammatory Pseudotumor of the Urinary Bladder: A Case Report with Brief Literature Review. South Med J. 2010;103:563–66. doi: 10.1097/SMJ.0b013e3181de0ecb. [DOI] [PubMed] [Google Scholar]
- 13.Iczkowski KA, Shanks JH, Gadaleanu V, et al. Inflammatory pseudotumor and sarcoma of urinary bladder: Differential diagnosis and outcome in thirty-eight spindle cell neoplasms. Mod Pathol. 2001;14:1043–51. doi: 10.1038/modpathol.3880434. [DOI] [PubMed] [Google Scholar]
- 14.Berger A, Kim C, Hagstrom N, et al. Successful preoperative treatment of pediatric bladder inflammatory myofibroblastic tumor with anti-inflammatory therapy. Urology. 2007;70:372. doi: 10.1016/j.urology.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 15.Favini F, Resti AG, Collini P, et al. Inflammatory Myofibroblastic Tumor of the Conjunctiva: Response to Chemotherapy With Low-Dose Methotrexate and Vinorelbine. Pediatr Blood Cancer. 2010;54:483–85. doi: 10.1002/pbc.22342. [DOI] [PubMed] [Google Scholar]


